Preparation and Optoelectronic Characteristics of ZnO/CuO-Cu2O Complex Inverse Heterostructure with GaP Buffer for Solar Cell Applications
Abstract
:1. Introduction
2. Experimental Section
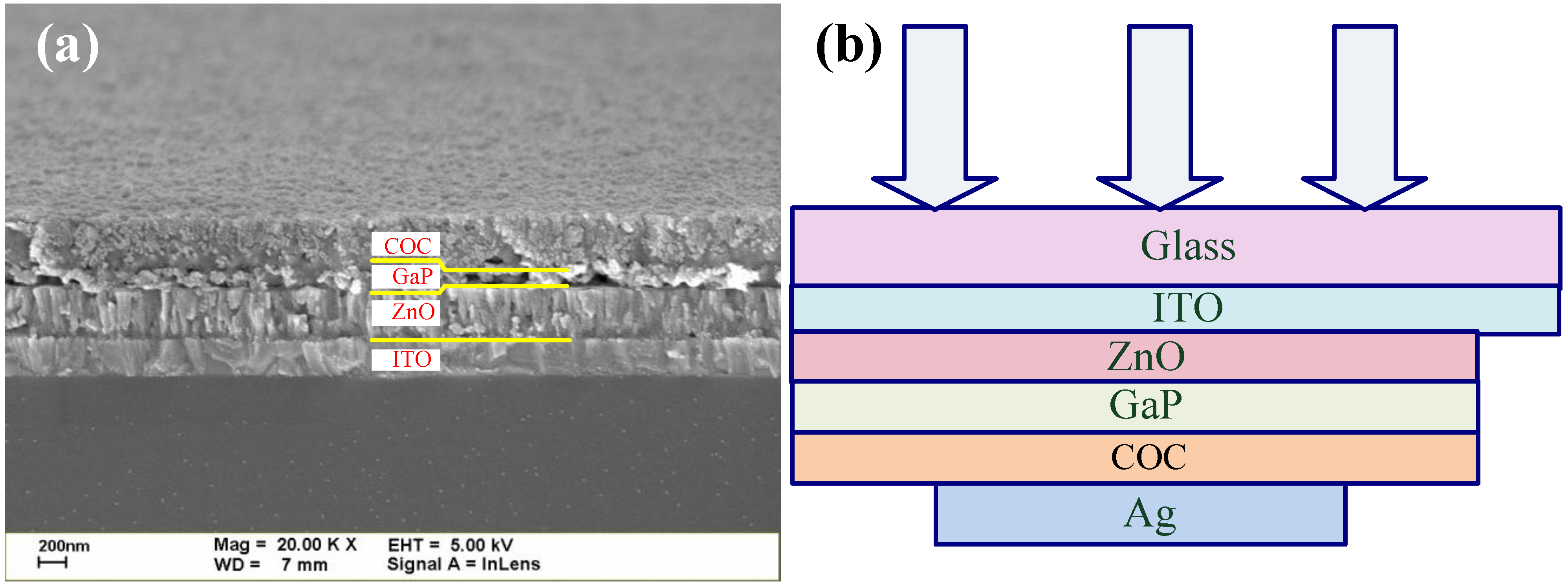
3. Results and Discussion

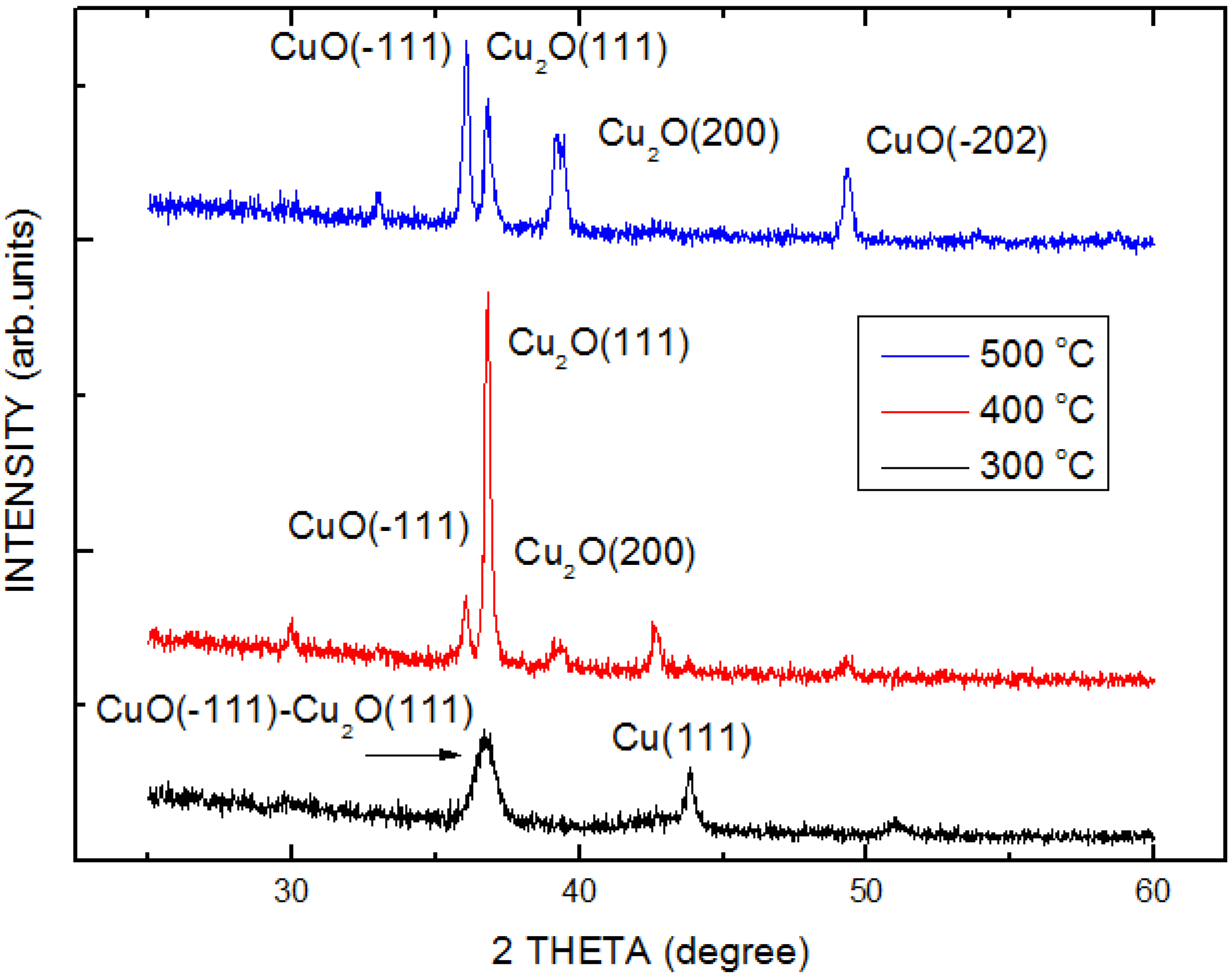
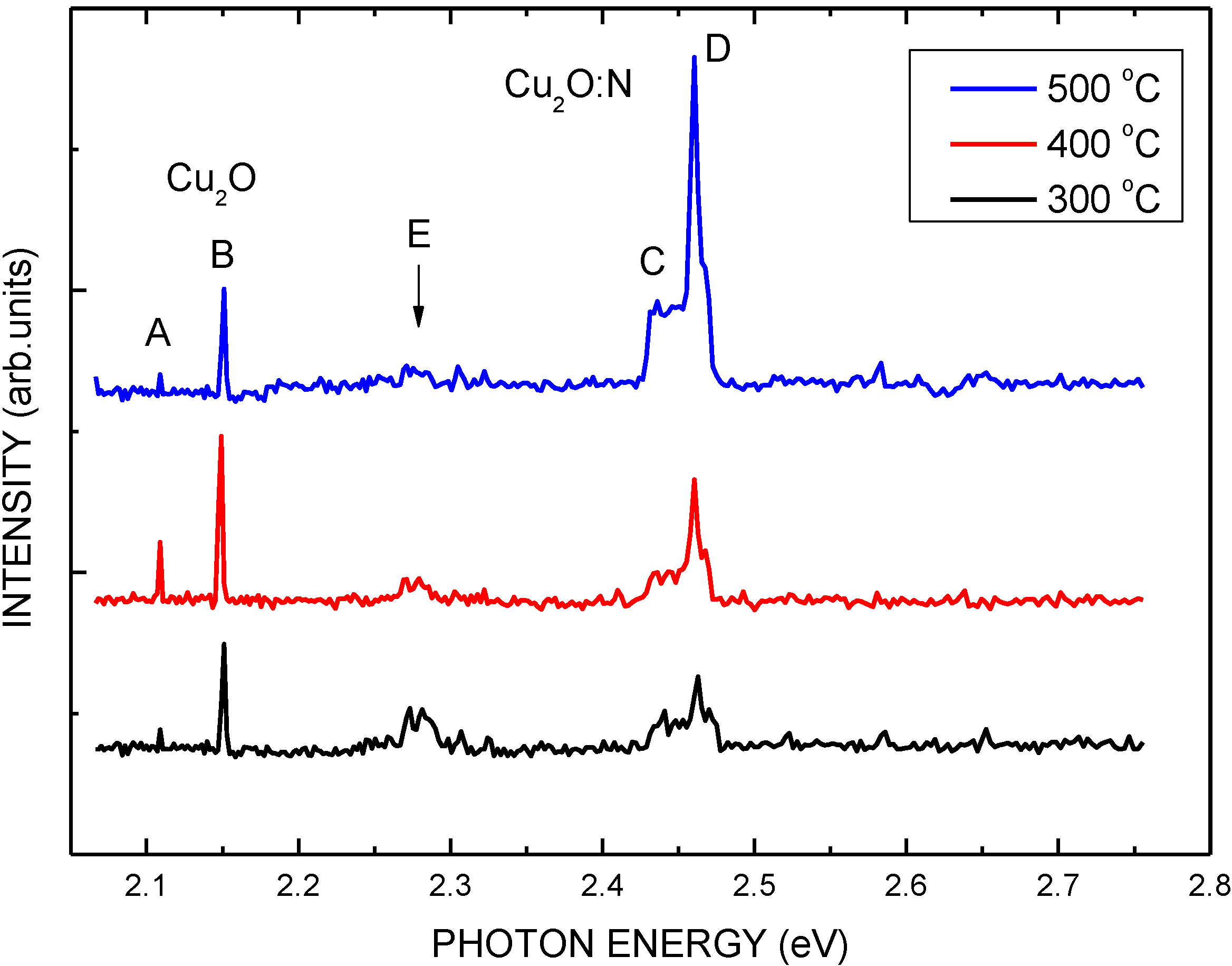
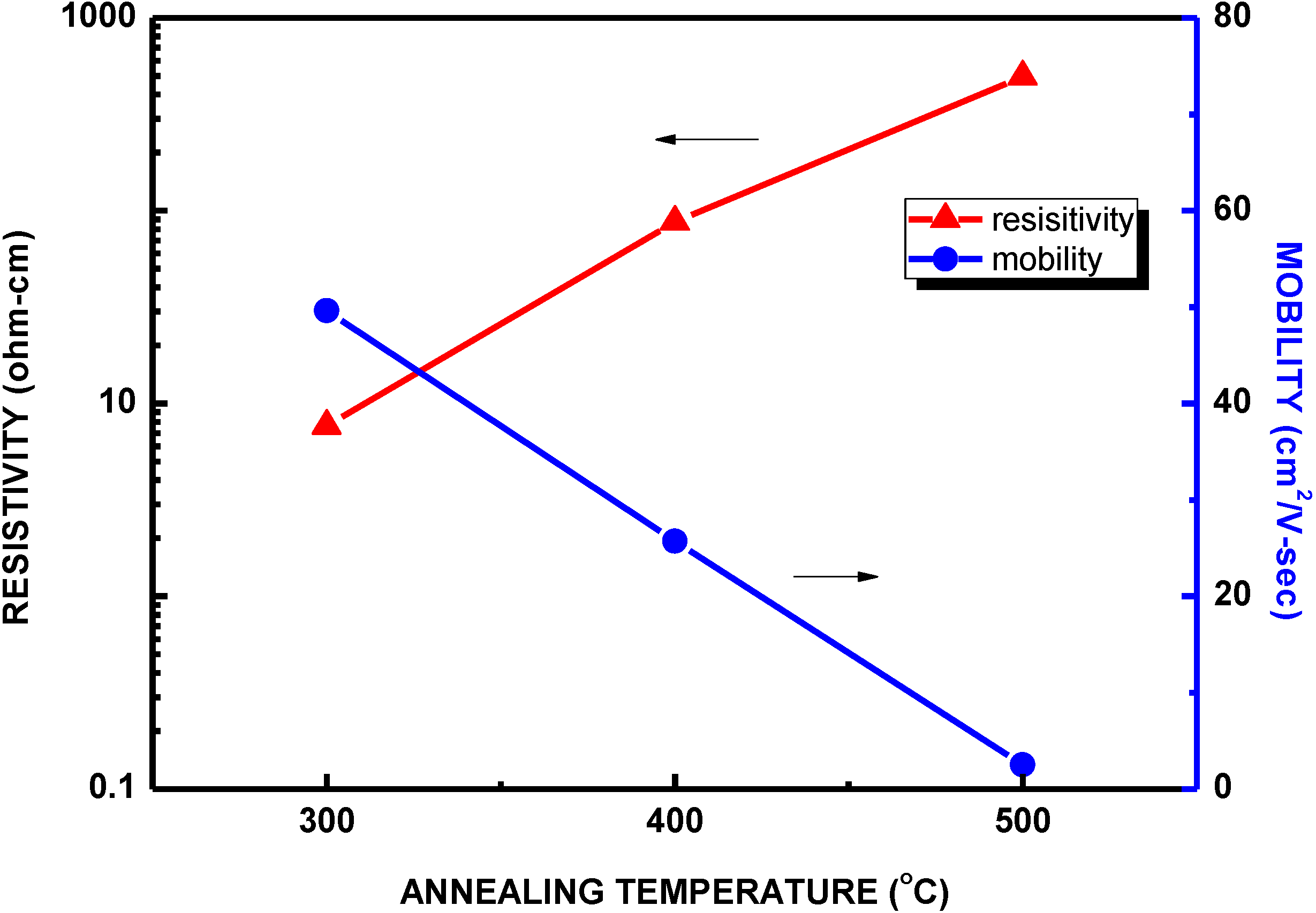
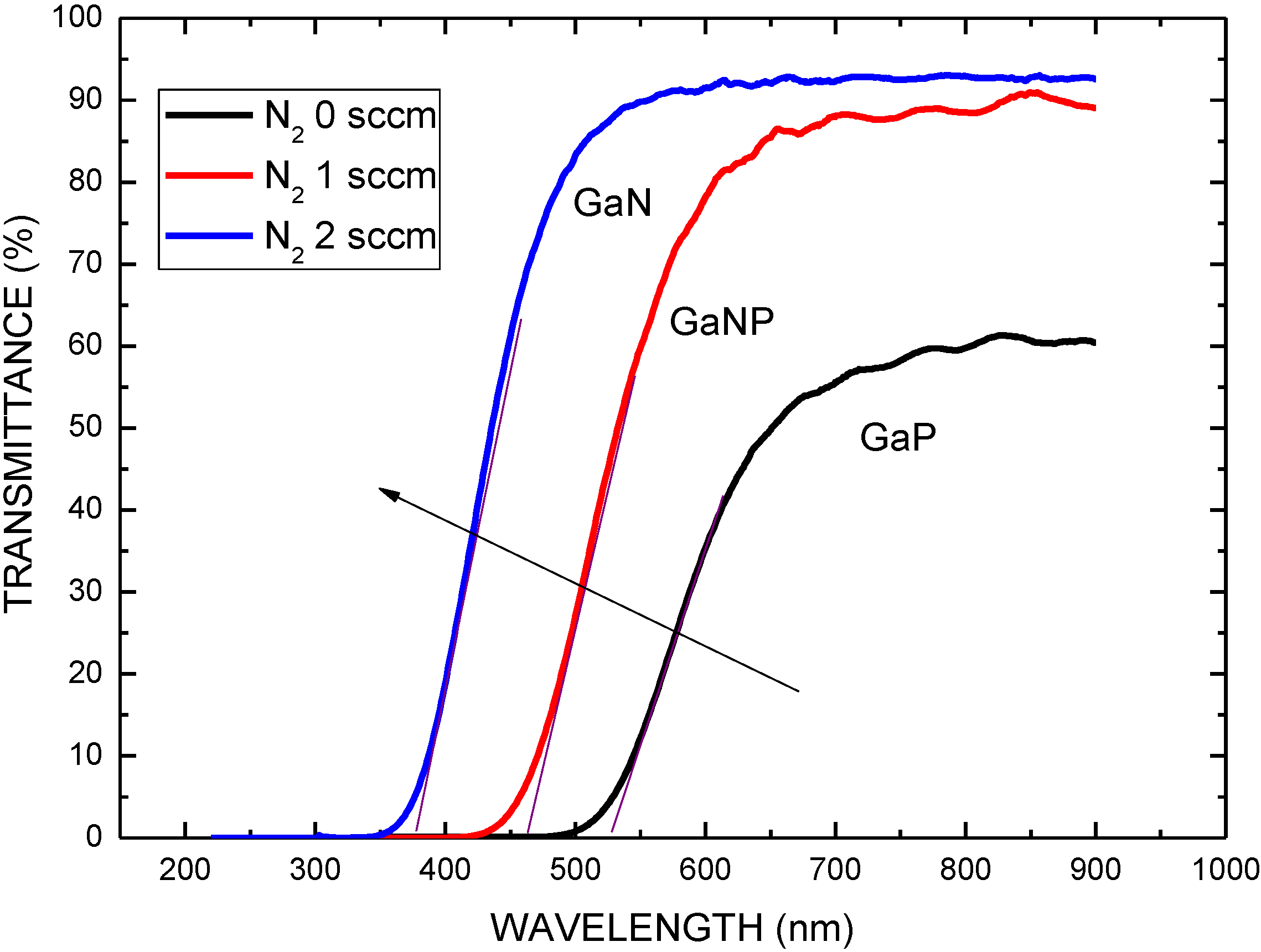
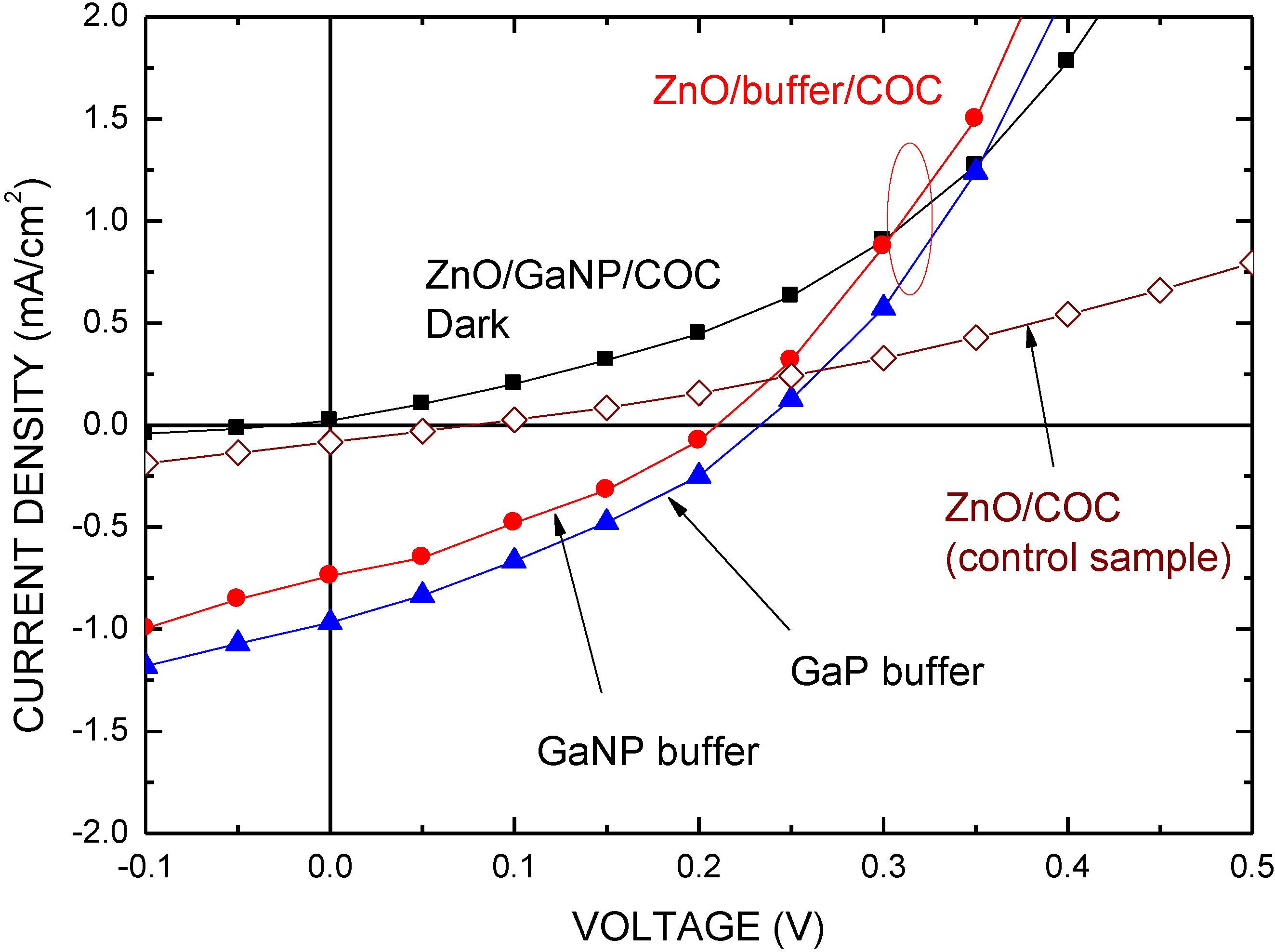
| Structure | Voc (V) | Jsc (mA/cm2) | FF | η (%) | Rs (Ω) | Rsh (Ω) |
|---|---|---|---|---|---|---|
| ZnO/COC | 0.077 | 0.083 | 0.250 | 0.016 | 54 | – |
| ZnO/GaP/COC | 0.234 | 0.968 | 0.316 | 0.720 | 8 | 61 |
| ZnO/GaNP/COC | 0.211 | 0.738 | 0.307 | 0.478 | 6 | 53 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Noguet, C.; Tapiero, M.; Schwab, C.; Zielinger, J.P.; Trivich, D.; Komp, R.J.; Wang, E.Y.; Weng, K. Cuprous Oxide as a Photovoltaic Converter. In Proceedings of the Photovoltaic Solar Energy Conference, Luxemburg, UK, 27–30 September 1977.
- Berezin, A.A.; Weichman, F.L. Photovoltaic effect in cuprous oxide-copper junctions in relation to the optical absorption spectrum of cuprous oxide. Solid State Commun. 1981, 37, 157–160. [Google Scholar] [CrossRef]
- Tapiero, M.; Noguet, C.; Zielinger, J.P.; Schwab, C.; Pierrat, D. Conversion photovoltaïque dans Cu2O. Rev. Phys. Appl. 1979, 14, 231–236. [Google Scholar] [CrossRef]
- Olsen, L.C.; Addis, F.W.; Bohara, R.C. Investigation of Cu2O Solar Cells. In Proceedings of the 14th IEEE Photovoltaic Specification Conference, San Diego, CA, USA, 7–10 January 1980.
- Wang, E.Y.; Trivich, D.; Sawaiha, H.; Thomas, G. Cuprous Oxide Schottky Photovoltaic Cells as Potential Solar Energy Converters. In Proceedings of the Complex International Conference, Dharan, Saudi Arabia, 2–6 November 1975.
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.S.; Mittiga, A.; Salza, E.; Masci, A.; Passerini, S. Electrodeposited ZnO/Cu2O heterojunction solar cells. Electrochim. Acta 2008, 53, 2226–2231. [Google Scholar] [CrossRef]
- Mittiga, A.; Salza, E.; Sarto, F.; Tucci, M.; Vasanthi, R. Heterojunction solar cell with 2% efficiency based on a Cu2O substrate. Appl. Phys. Lett. 2006, 88. [CrossRef]
- Nakano, Y.; Saeki, S.; Morikawa, T. Optical bandgap widening of p-type Cu2O films by nitrogen doping. Appl. Phys. Lett. 2009, 94, 022111:1–022111:3. [Google Scholar] [CrossRef]
- Tanaka, H.; Shimakawa, T.; Miyata, T.; Satob, H.; Minami, T. Electrical and optical properties of TCO-Cu2O heterojunction devices. Thin Solid Films 2004, 469–470, 80–85. [Google Scholar] [CrossRef]
- Zhang, D.K.; Liu, Y.C.; Liu, Y.L.; Yang, H. The electrical properties and the interfaces of Cu2O/ZnO/ITO p-i-n heterojunction. Phys. B Condens. Matter 2004, 470, 178–183. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Fan, H.; Wang, H.; Peng, B.; Long, C.; Zhai, Y. Electrical charge conductivity behavior of electrodeposited Cu2O/ZnO heterojunction thin films on PET flexible substrates by impedance spectroscopy analysis. J. Mater. Sci. 2013, 48, 3334–3340. [Google Scholar] [CrossRef]
- Musselman, K.P.; Wisnet, A.; Iza, D.C.; Hesse, H.C.; Scheu, C.; MacManus-Driscoll, J.L.; Schmidt-Mende, L. Strong efficiency improvements in Ultra-low-Cost inorganic nanowire solar cells. Adv. Mater. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Musselman, K.P.; Marin, A.; Schmidt-Mende, L.; MacManus-Driscoll, J.L. Incompatible length scales in nanostructured Cu2O solar cells. Adv. Funct. Mater. 2012, 22, 2202–2208. [Google Scholar] [CrossRef]
- Liu, Y.; Turley, H.K.; Tumbleston, J.R.; Samulski, E.T.; Lopez, R. Minority carrier transport length of electrodeposited Cu2O in ZnO/Cu2O heterojunction solar cells. Appl. Phys. Lett. 2011, 98, 162105:1–162105:3. [Google Scholar]
- Fenske, F.; Kliefoth, K.; Elstner, L.; Selle, B. ZnO/c-Si heterojunction interface tuning by interlayers. J. Mater. Sci. 1996, 426, 135–140. [Google Scholar]
- De, A.; Pryor, C.E. Predicted band structures of III-V semiconductors in the wurtzite phase. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef]
- Belabbes, A.; Panse, C.; Furthműller, J.; Bechstedt, F. Electronic bands of III-V semiconductor polytypes and their alignment. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Assali, S.; Zardo, I.; Plissard, S.; Kriegner, D.; Verheijen, M.A.; Bauer, G.; Meijerink, A.; Belabbes, A.; Bechstedt, F.; Haverkort, J.E.M.; et al. Direct band gap wurtzite gallium phosphide nanowires. Nano Lett. 2013, 13, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Natesan, K. Oxidation of copper and electronic transport in copper oxides. Oxid. Met. 1993, 39, 411–435. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Mema, R.; Zhou, G. Driving force and growth mechanism for spontaneous oxide nanowire formation during the thermal oxidation of metals. Acta Mater. 2011, 59, 2491–2500. [Google Scholar] [CrossRef]
- Wang, J.P.; Cho, W.D. Oxidation behavior of pure copper in oxygen and/or water vapor at intermediate temperature. ISIJ Int. 2009, 49, 1926–1931. [Google Scholar] [CrossRef]
- Gastev, S.V.; Kaplyanskii, A.A.; Sokolov, N.S. Relaxed excitons in Cu2O. Solid State Commun. 1982, 42, 389–391. [Google Scholar] [CrossRef]
- Akimoto, K.; Ishizuka, S.; Yanagita, M.; Nawa, Y.; Paul, G.K.; Sakurai, T. Thin film deposition of Cu2O and application for solar cells. Solar Energy 2006, 80, 715–722. [Google Scholar] [CrossRef]
- Zang, Z.; Nakamura, A.; Temmyo, J. Single cuprous oxide films synthesized by radical oxidation at low temperature for PV application. Opt. Express 2013, 21, 11448–11456. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.C.; Bohara, R.C.; Urie, M.W. Explanation for low-efficiency Cu2O Schottky-barrier solar cells. Appl. Phys. Lett. 1979, 34, 47–49. [Google Scholar] [CrossRef]
- Olsen, L.C.; Addis, F.W.; Miller, W. Experimental and theoretical studies of Cu2O solar cells. Solar Cells 1982, 7, 247–279. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Sablon, K.A.; Little, J.W.; Sergeev, A.; Vagidov, N.; Mitin, V. Solar cell with built-in charge: Experimental studies of diode model parameters. J. Vac. Sci. Technol. A. 2012, 30. [CrossRef]
- Musselman, K.P.; Marin, A.; Wisnet, A.; Scheu, C.; MacManus-Driscoll, J.L.; Schmidt-Mende, L. A novel buffering technique for aqueous processing of zinc oxide nanostructures and interfaces, and corresponding improvement of electrodeposited ZnO-Cu2O photovoltaics. Adv. Funct. Mater. 2011, 21, 573–582. [Google Scholar] [CrossRef]
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsu, C.-H.; Chen, L.-C.; Lin, Y.-F. Preparation and Optoelectronic Characteristics of ZnO/CuO-Cu2O Complex Inverse Heterostructure with GaP Buffer for Solar Cell Applications. Materials 2013, 6, 4479-4488. https://doi.org/10.3390/ma6104479
Hsu C-H, Chen L-C, Lin Y-F. Preparation and Optoelectronic Characteristics of ZnO/CuO-Cu2O Complex Inverse Heterostructure with GaP Buffer for Solar Cell Applications. Materials. 2013; 6(10):4479-4488. https://doi.org/10.3390/ma6104479
Chicago/Turabian StyleHsu, Chih-Hung, Lung-Chien Chen, and Yi-Feng Lin. 2013. "Preparation and Optoelectronic Characteristics of ZnO/CuO-Cu2O Complex Inverse Heterostructure with GaP Buffer for Solar Cell Applications" Materials 6, no. 10: 4479-4488. https://doi.org/10.3390/ma6104479





