Simulations and Measurements of Human Middle Ear Vibrations Using Multi-Body Systems and Laser-Doppler Vibrometry with the Floating Mass Transducer
Abstract
:1. Introduction
2. Methods
2.1. Reconstruction of the Middle Ear
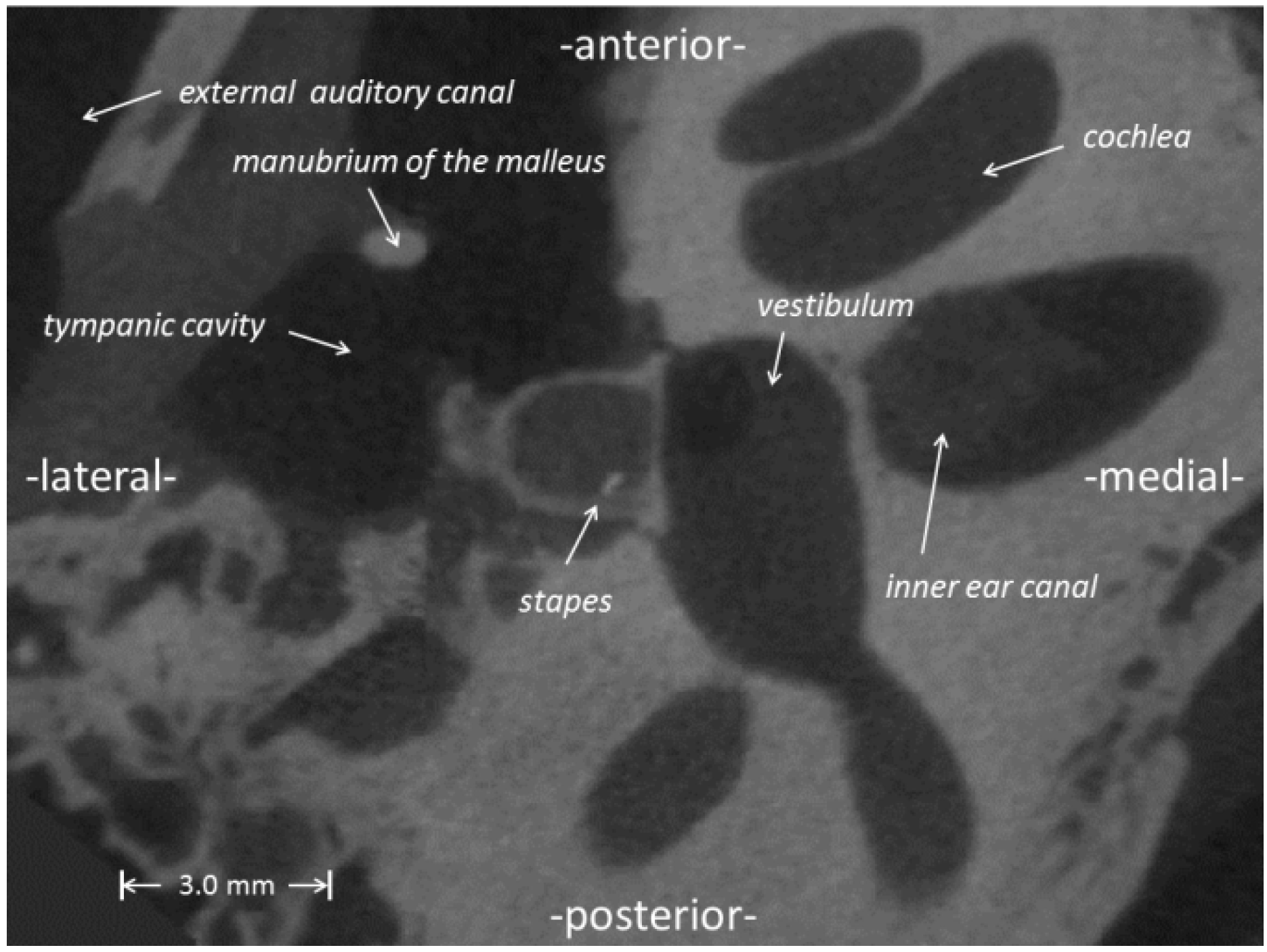
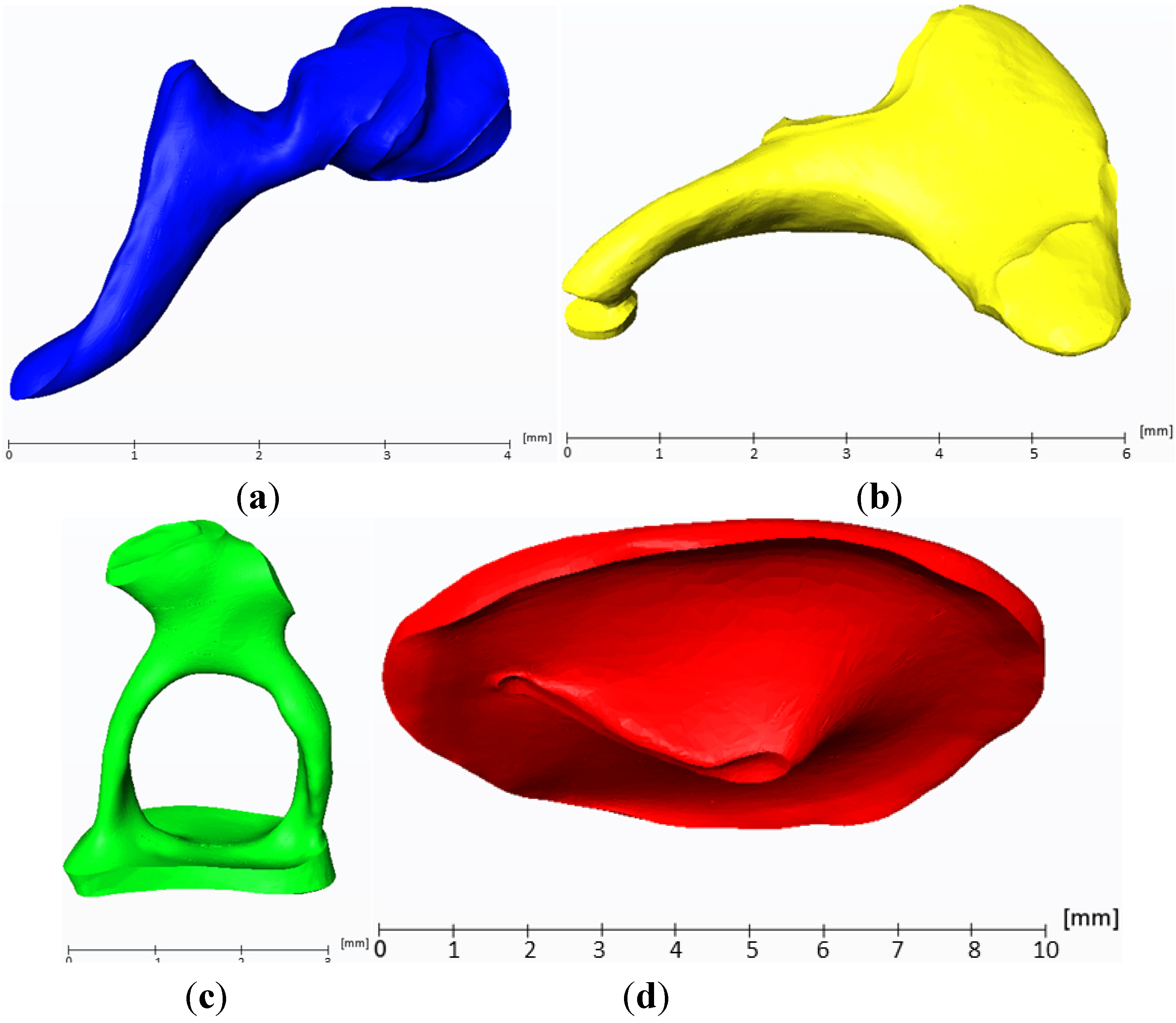
2.2. Multi-Body Systems
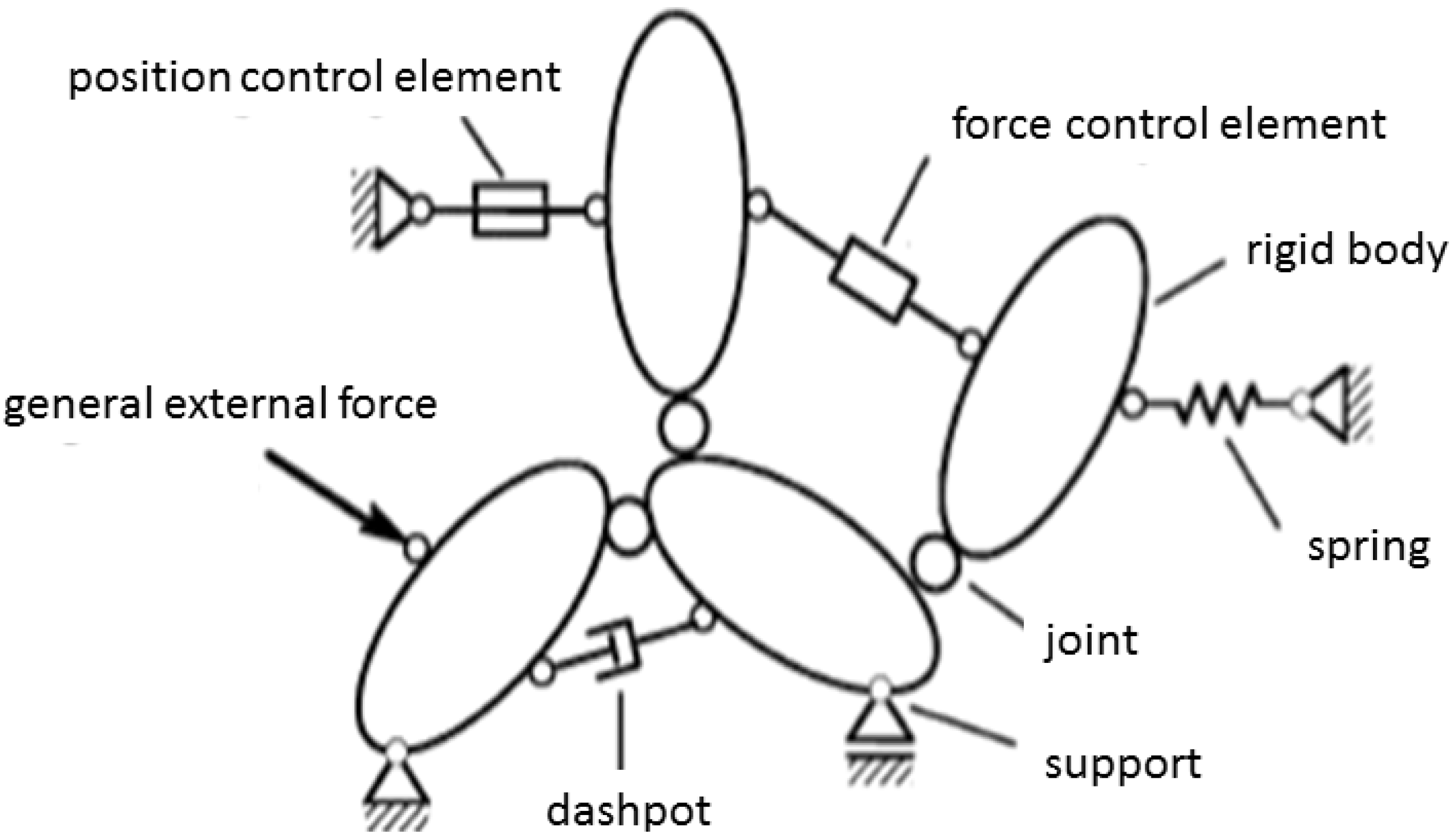
2.3. Multi-Body System (MBS) of the Human Middle Ear
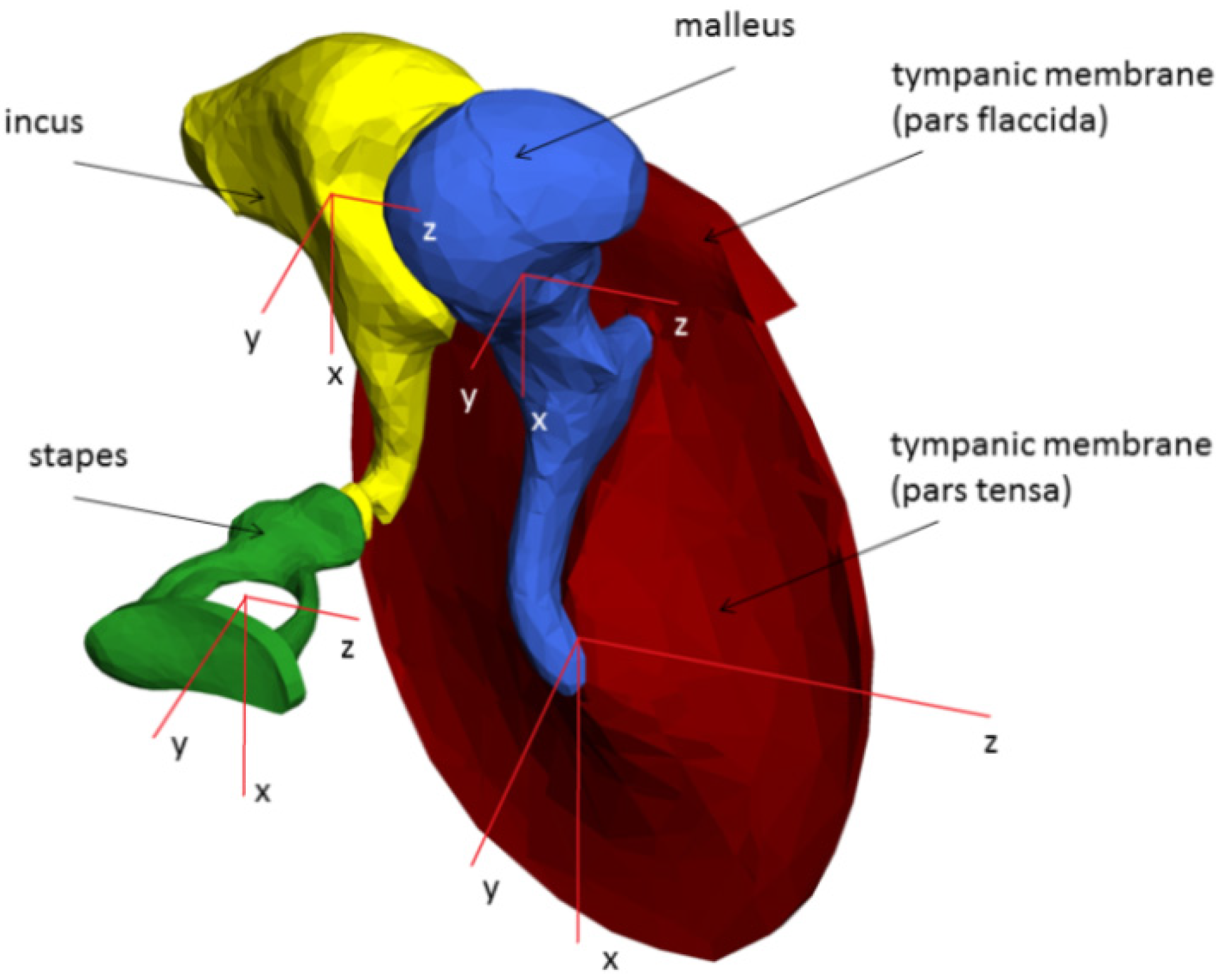
| Rigid body | Density (kg/m3) | Calculated mass (mg) | Calculated volume (mm3) | Calculated area (mm2) |
|---|---|---|---|---|
| Pars tensa | 1200 (Koike and Wada) | 10.01 | 8.34 | 131.21 |
| Pars flaccida | 1200 (Koike and Wada) | 1.4 | 1.17 | 15.37 |
| Malleus | 3590 (Lee) | 57.21 | 15.94 | 44.87 |
| Incus | 3230 (Lee) | 46.31 | 14.34 | 42.14 |
| Stapes | 2200 (Lee) | 3.94 | 1.79 | 16.18 |
| FMT Samarium | 7520 | 31.68 | 40.2 | 19.95 |
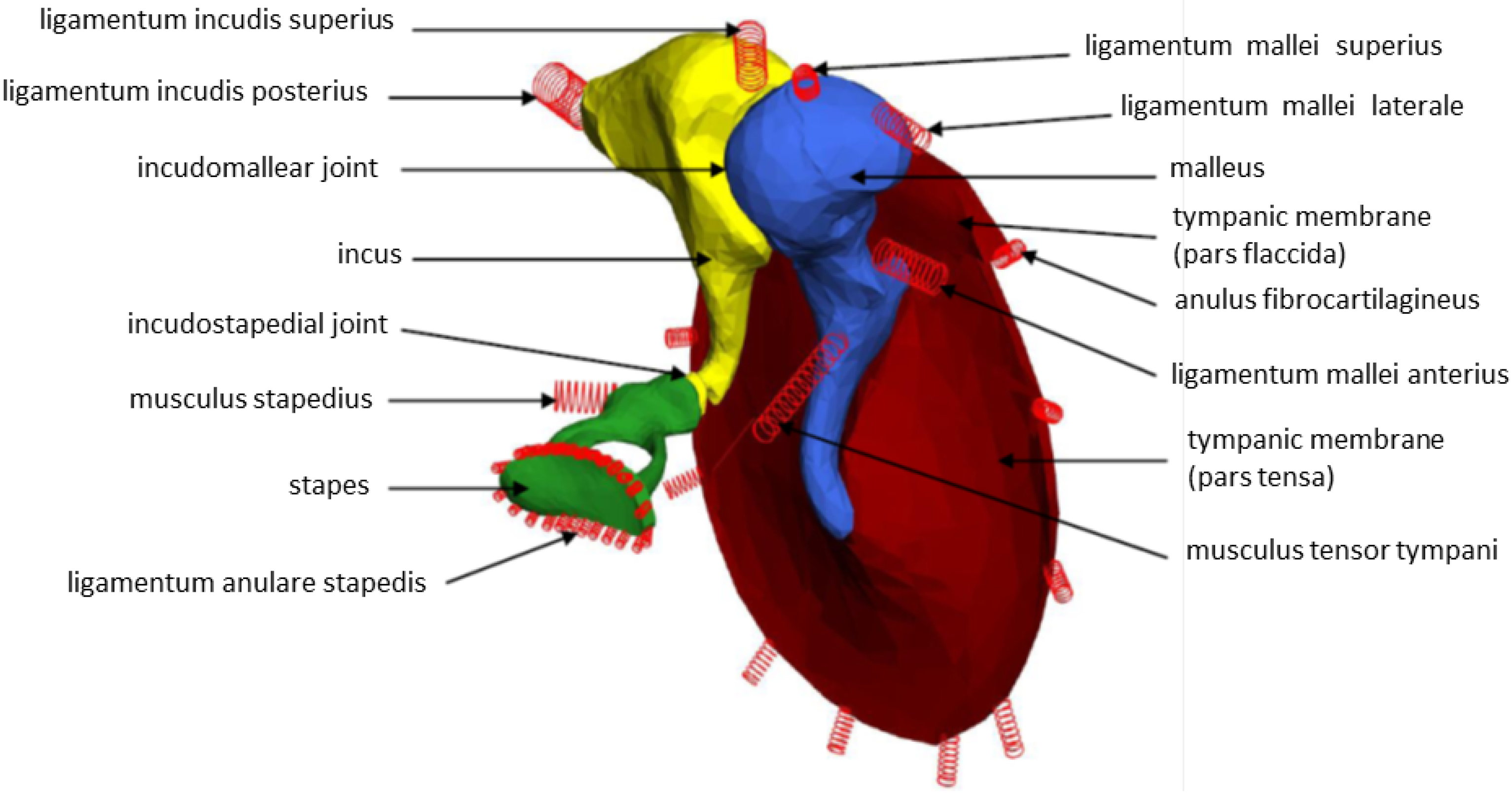
2.4. Laser-Doppler Vibrometry (LDV)
2.5. Floating Mass Transducer (FMT)
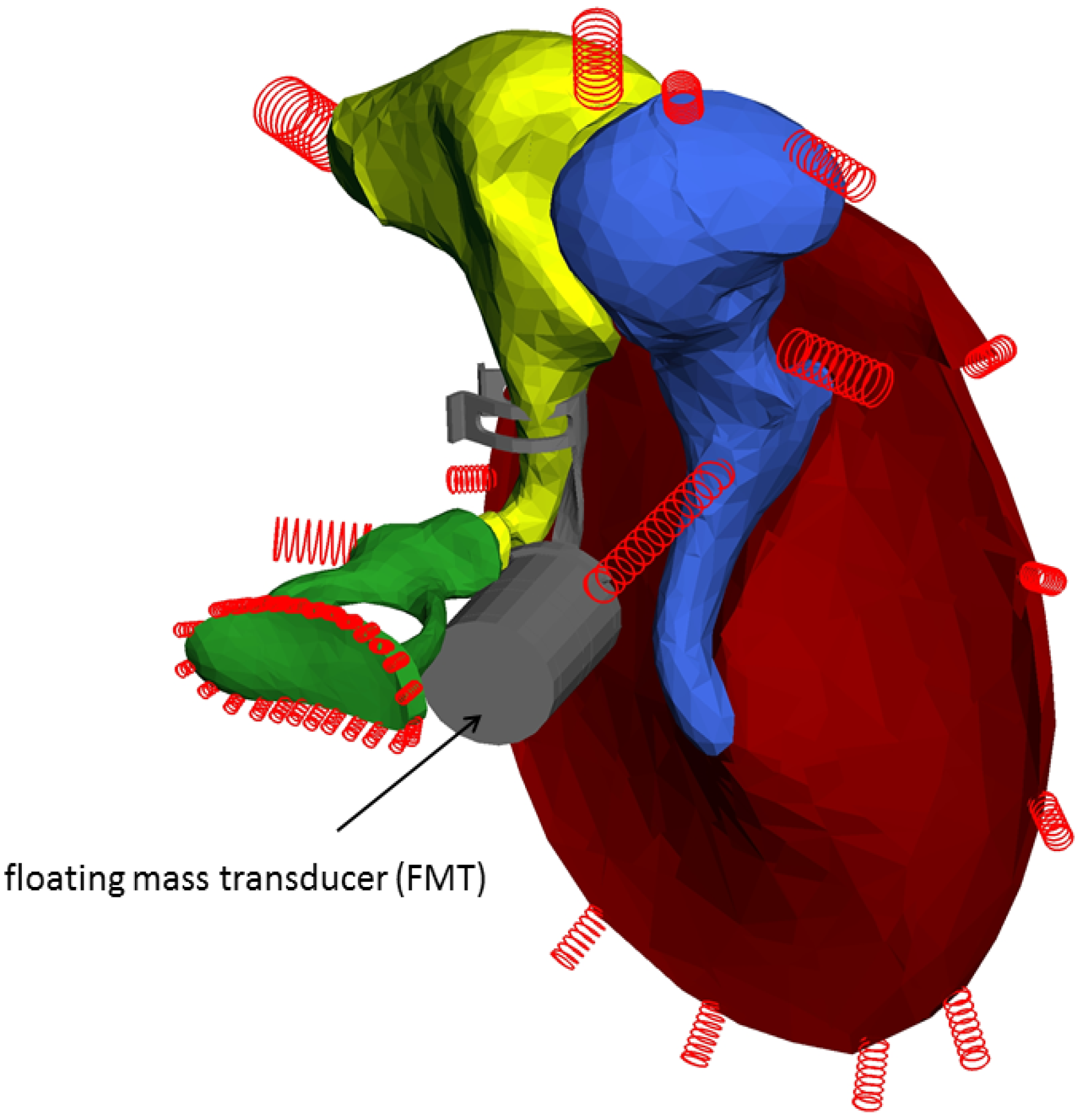
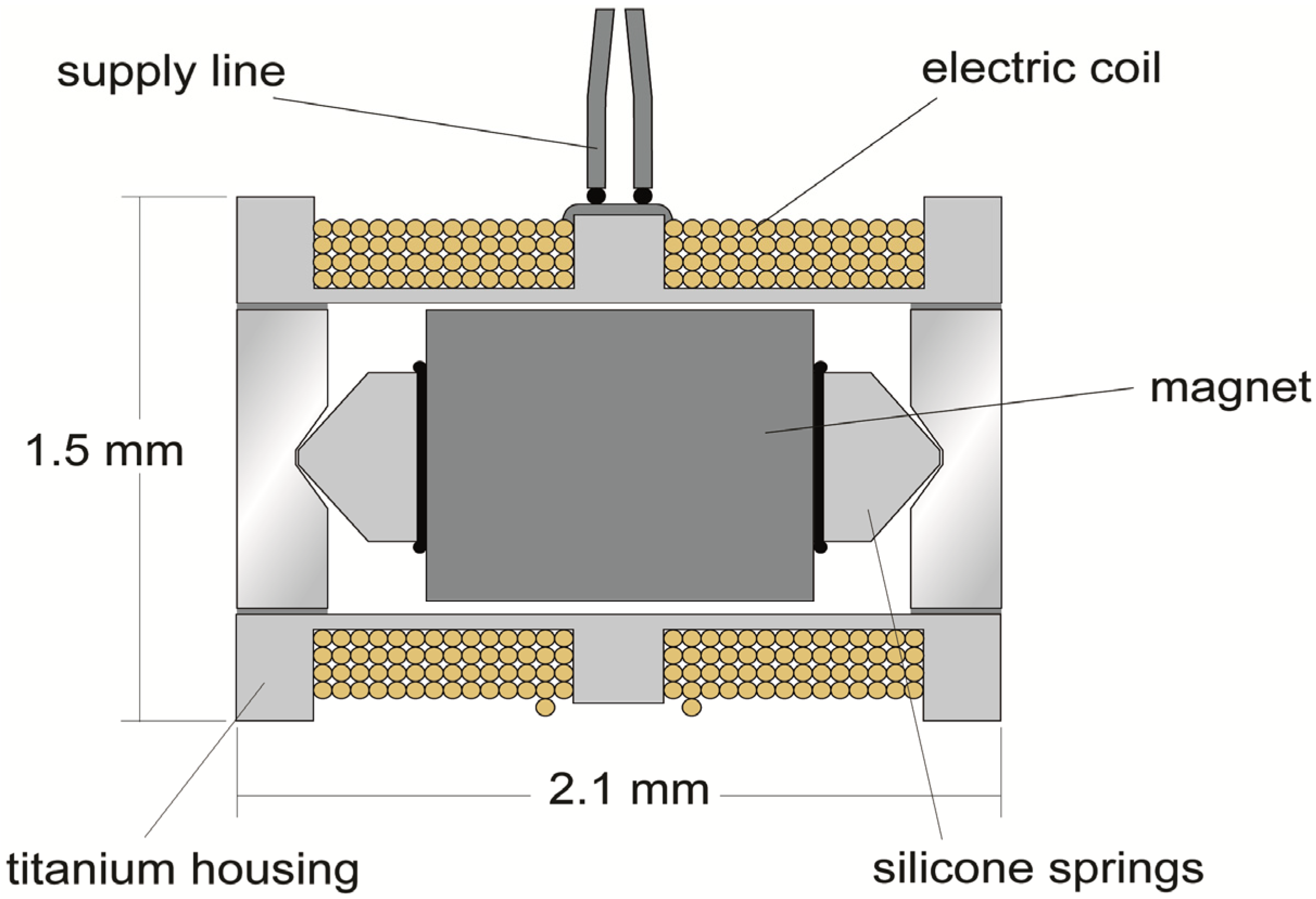
3. Results
3.1. Calculated and Measured Stapes Footplate Displacements (SFD)

3.2. Calculated and Measured Stapes Footplate Phases (SFP)
4. Discussion
5. Conclusions
Abbreviations
| cgs | centimeter, gram, second units |
| DOF | degree of freedom |
| FEM | finite element method |
| FMT | floating mass transducer |
| f | frequency |
| i | imaginary unit |
| j | number of joints (hinges) |
| L | length |
| LDV | laser-Doppler vibrometry |
| MBS | Multi Body System |
| ME | middle ear |
| mks | meter, kilogram, second units |
| μ0 | 4 × π × 10−7 Vs/Am magnet field constant |
| N | number of DOF of joints, Newton (unit) |
| n | number of bodies, including the inertial system |
| μCT | micro-computer-tomography |
| R | radius |
| SFD | stapes footplate displacement |
| SFP | stapes footplate phase |
| TM | tympanic membrane |
| ω | circular frequency |
Acknowledgments
Conflicts of Interest
References
- Zwislocki, J. Analysis of the middle-ear function. Part 1: Input Impedance. J. Acoust. Soc. Am. 1962, 34, 1514–1523. [Google Scholar] [CrossRef]
- Wada, H.; Metoki, T.; Kobayashi, T. Analysis of dynamic behavior of human middle ear using a finite-element method. J. Acoust. Soc. Am. 1992, 92, 3157–3168. [Google Scholar] [CrossRef] [PubMed]
- Kwacz, M.; Wysocki, J.; Krakowian, P. Reconstruction of the 3D geometry of the Ossicular Chain Based on Micro-CT Imaging. Biocybern. Biomed. Eng. 2012, 32, 27–40. [Google Scholar] [CrossRef]
- Buytaert, J.A.N.; Salih, W.H.M.; Dierick, M.; Jacobs, P.; Dircks, J.J. Realistic 3D computer model of the gerbil middle ear, featuring accurate morphology of bone and soft tissue structures. J. Assoc. Res. Otolaryngol. 2011, 12, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Z.; Feng, B.; Sun, Q. Three-dimensional finite element modeling of human ear for sound transmission. Ann. Biomed. Eng. 2004, 32, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Eiber, A. Mechanical modeling and dynamical behavior of the human middle ear. Audiol. Neurootol. 1999, 4, 170–177. [Google Scholar] [CrossRef] [PubMed]
- SIMPACK Home Page. Available online: www.simpack.com (accessed on 22 October 2013).
- Funnel, W.R.J.; Laszlo, C.A. Modeling of the cat eardrum as a thin shell using the finite-element method. J. Acoust. Soc. Am. 1978, 63, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Röösli, C.; Chatzimichalis, M.; Eiber, A.; Huber, A. Characterization of stapes anatomy: Investigation of human and guinea pig. J. Assoc. Res. Otolaryngol. 2013, 12, 681–696. [Google Scholar]
- Van der Jeught, S.; Dirckx, J.J.; Aerts, J.R.M.; Bradu, A.; Podoleanu, A.G.; Buytaert, J.A.N. Full-Field thickness distribution of human tympanic membrane obtained with optical coherence tomography. J. Assoc. Res. Otolaryngol. 2013, 14, 483–494. [Google Scholar] [CrossRef]
- Schiehlen, W. Technische Dynamik (in German); B.G. Teubner: Stuttgart, Germany, 1986. [Google Scholar]
- Kauf, A. Dynamik des Mittelohres, Fortschrittberichte VDI Reihe 17: Biotechnik/Medizintechnik (in German); Springer VDI-Verlag: Düsseldorf, Germany, 1997. [Google Scholar]
- Freitag, H.G. Zur Dynamik von Mittelohrprothesen. Ph.D. Dissertation, Stuttgart University, Stuttgart, Germany, 1 November 2003. [Google Scholar]
- Hoffstetter, M.; Schardt, F.; Lenarz, T.; Wacker, S.; Wintermantel, E. Parameter study on a finite element model of the middle ear. Biomed. Tech. 2010, 55, 19–26. [Google Scholar] [CrossRef]
- Ball, G. MRI Safe Actuator for Implantable Floating Mass Transducer. U.S. Patent 20,120,219,166 A1, 30 August 2012. [Google Scholar]
- Strenger, T.; Stark, T. Einsatz implantierbarer Hörgeräte am Beispiel der Vibrant Soundbridge. HNO 2012, 60, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Strenger, T. Laservibrometrische Schwingungsmessungen am “Floating Mass Transducer” des teilimplantierbaren Mittelohrhörgerätes “Vibrant Soundbridge” (in German). M.D. Dissertation, University Würzburg Germany, Würzburg, Germany, 29 October 2009. [Google Scholar]
- Strenger, T.; Reitsberger, M.; Stark, T.; Böhnke, F. Laservibrometrische ankopplungsuntersuchungen des FMTs im felsenbein (in German). Ger. Med. Sci. Curr. Posters Otolaryngol. Head Neck Surg. 2011, 7. [Google Scholar] [CrossRef]
- Winter, M.; Weber, B.P.; Lenarz, T. Measurement method for the assessment of transmission properties of implantable hearing aids. Biomed. Tech. 2002, 47, 726–727. [Google Scholar] [CrossRef]
- Aibara, R.; Welsh, J.T.; Puria, S.; Goode, R.L. Human middle-ear sound transfer function and cochlear input impedance. Hear. Res. 2001, 152, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, H.; Stieger, C.; Perriard, Y. Design of a Semi-Implantable Hearing Device for Direct Acoustic Cochlear Stimulation. IEEE Trans. Biomed. Eng. 2011, 58, 420–428. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Böhnke, F.; Bretan, T.; Lehner, S.; Strenger, T. Simulations and Measurements of Human Middle Ear Vibrations Using Multi-Body Systems and Laser-Doppler Vibrometry with the Floating Mass Transducer. Materials 2013, 6, 4675-4688. https://doi.org/10.3390/ma6104675
Böhnke F, Bretan T, Lehner S, Strenger T. Simulations and Measurements of Human Middle Ear Vibrations Using Multi-Body Systems and Laser-Doppler Vibrometry with the Floating Mass Transducer. Materials. 2013; 6(10):4675-4688. https://doi.org/10.3390/ma6104675
Chicago/Turabian StyleBöhnke, Frank, Theodor Bretan, Stefan Lehner, and Tobias Strenger. 2013. "Simulations and Measurements of Human Middle Ear Vibrations Using Multi-Body Systems and Laser-Doppler Vibrometry with the Floating Mass Transducer" Materials 6, no. 10: 4675-4688. https://doi.org/10.3390/ma6104675




