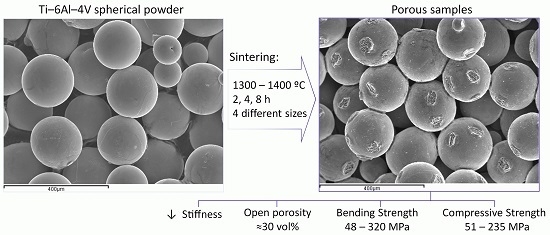
Microstructure and Mechanical Behavior of Porous Ti–6Al–4V Processed by Spherical Powder Sintering
Abstract
:1. Introduction
- (1)
- Minimal stiffness when compared to that of solid Ti–6Al–4V titanium alloy.
- (2)
- Achievement of a strength equivalent (or higher) to that of cortical or cancellous human bone tissue.
- (3)
- Open porosity and interconnected pores.
- (4)
- Porosity in the range of 20% to 80%, as defined by Bansiddhi et al. [18].
- (5)
- Pore size greater than 100 µm to facilitate bone colonization [19].
2. Experimental Procedure
2.1. Raw Material
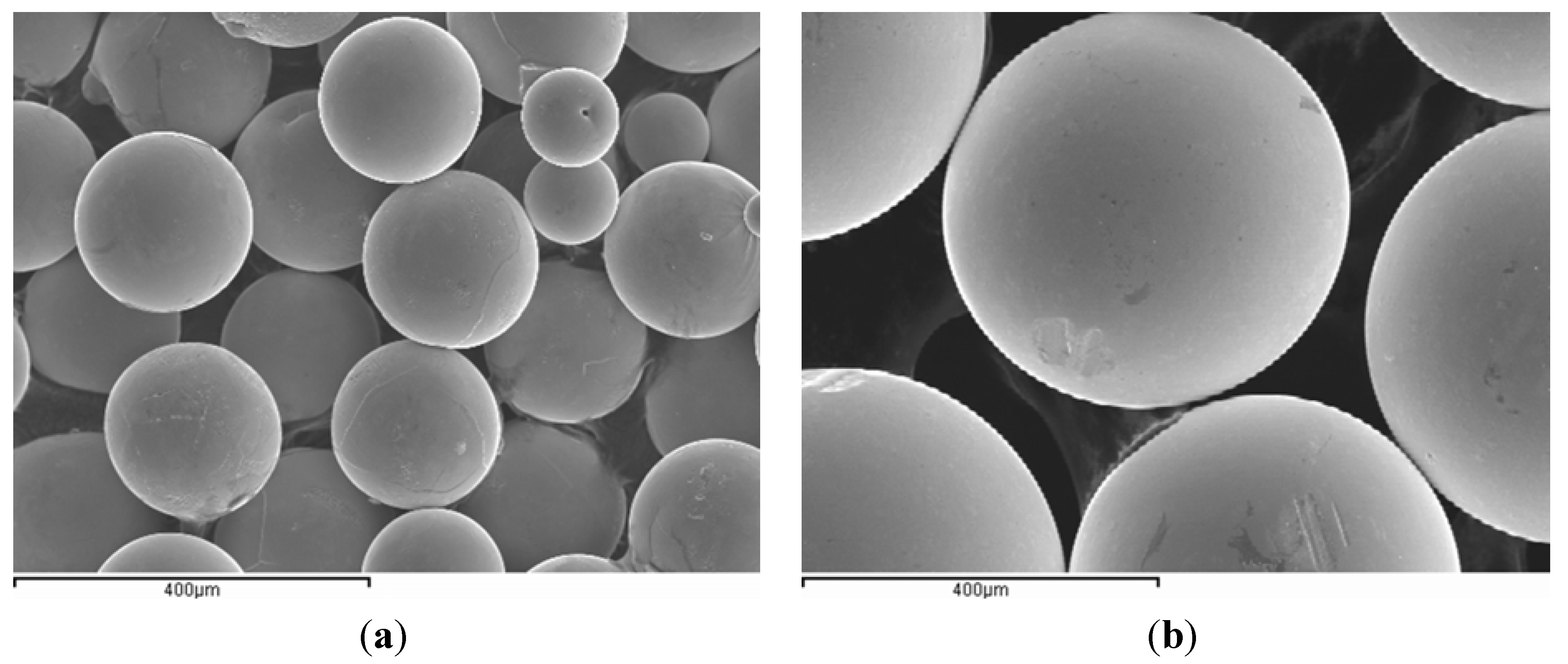
2.2. Equipment and Methods
| Process variables | Values | Unit |
|---|---|---|
| Sintering temperatures | 1300, 1350, 1400 | °C |
| Sintering times | 2, 4, 8 | h |
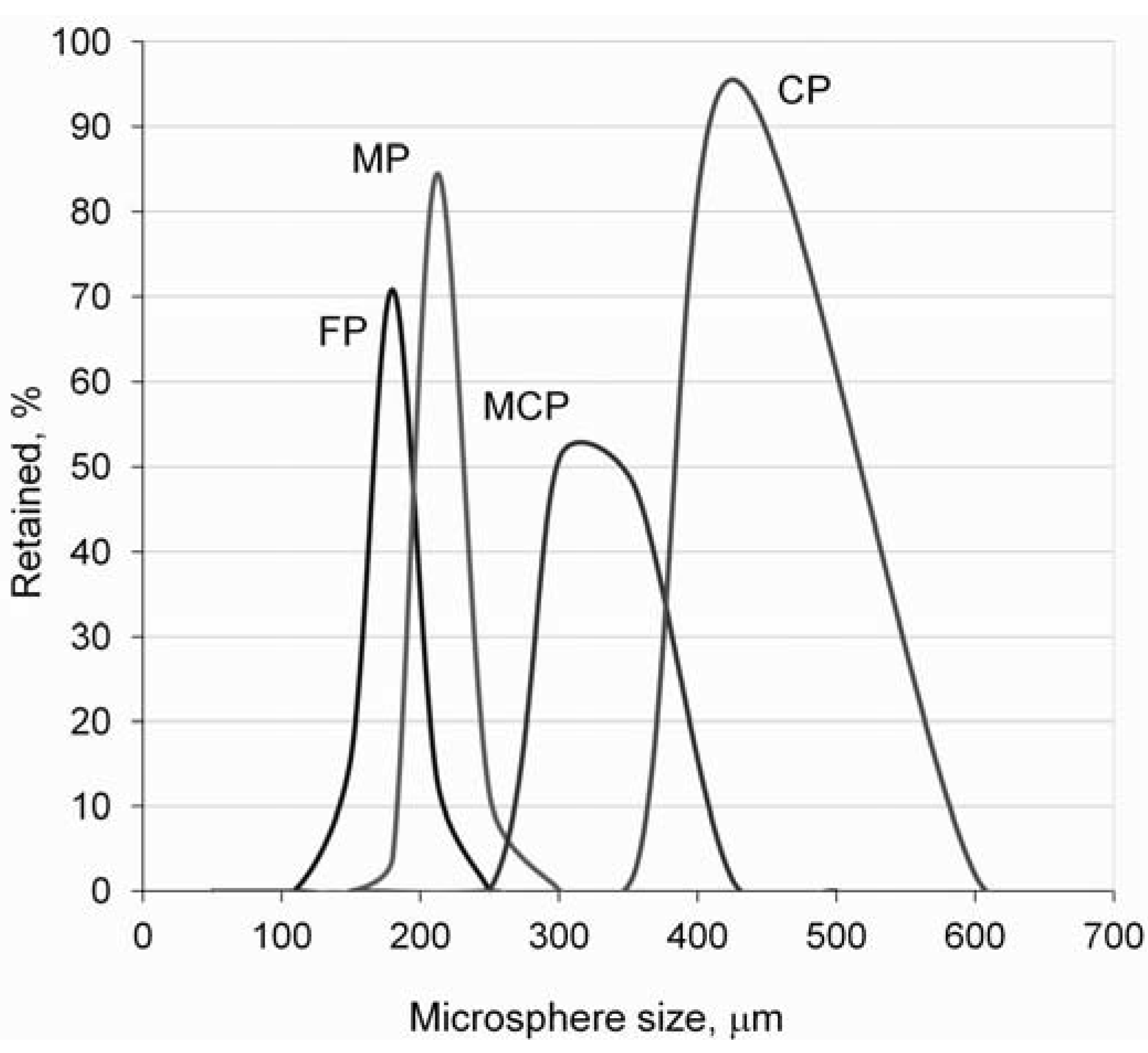
| Microsphere sizes | FP: −250/+180 | µm |
| MP: −300/+212 | ||
| MCP: −425/+300 | ||
| CP: −600/+425 |
2.3. Characterization of the Porous Samples
3. Results
3.1. Microstructure

3.2. Porosity
| Sample | Apparent density g/cm3 | Median diameter µm | d84 * vol % | Open porosity Vol % |
|---|---|---|---|---|
| FP | 2.95 | 54.6 | 72.7 | 32.1 |
| MP | 2.95 | 76.6 | 97.2 | 31.7 |
| MCP | 2.94 | 113.4 | 161.3 | 32.5 |
| CP | 2.95 | 140.1 | 175.9 | 31.2 |

3.3. Mechanical Properties
3.3.1. Bending Strength
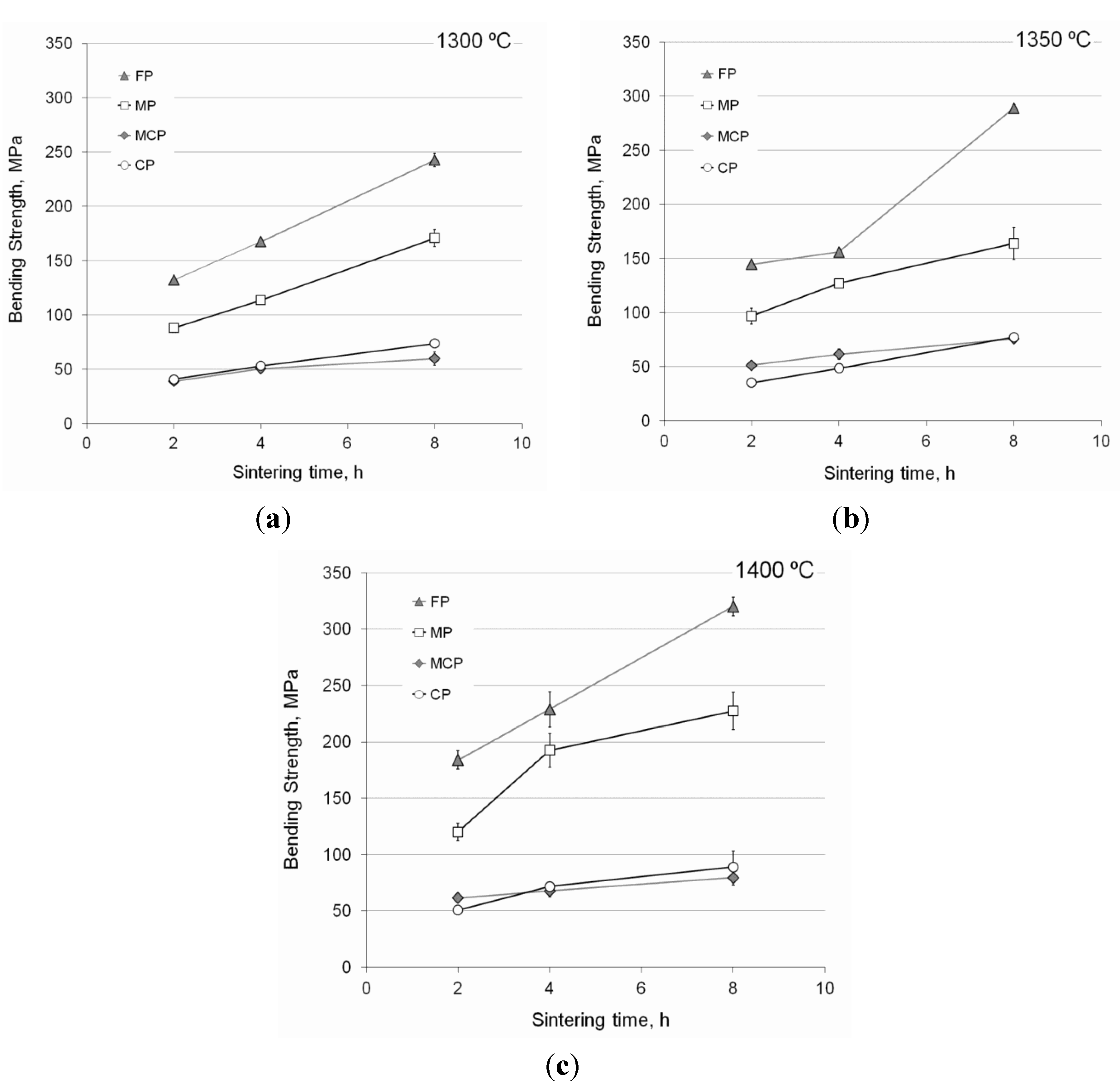
3.3.2. Compressive Strength
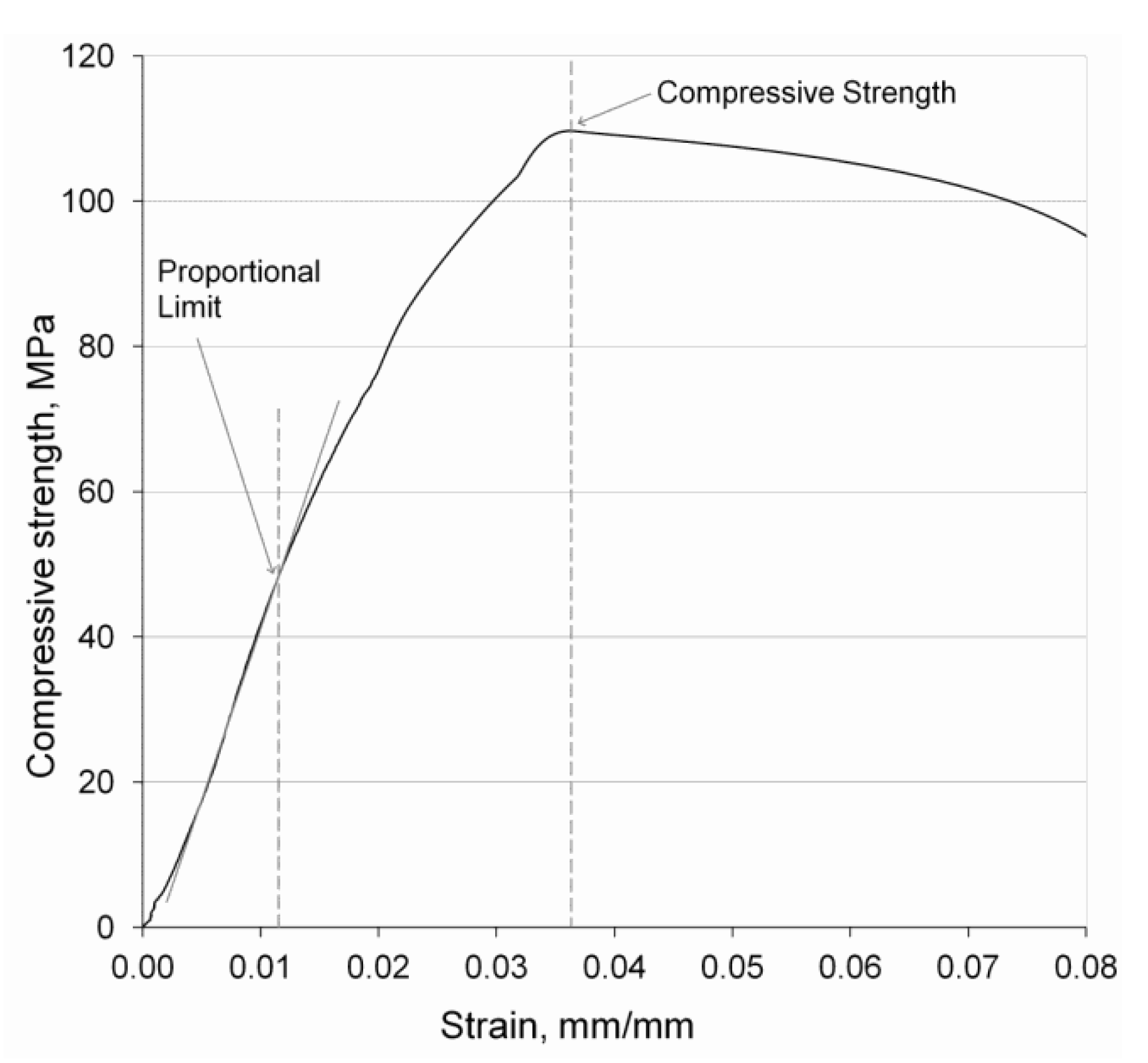
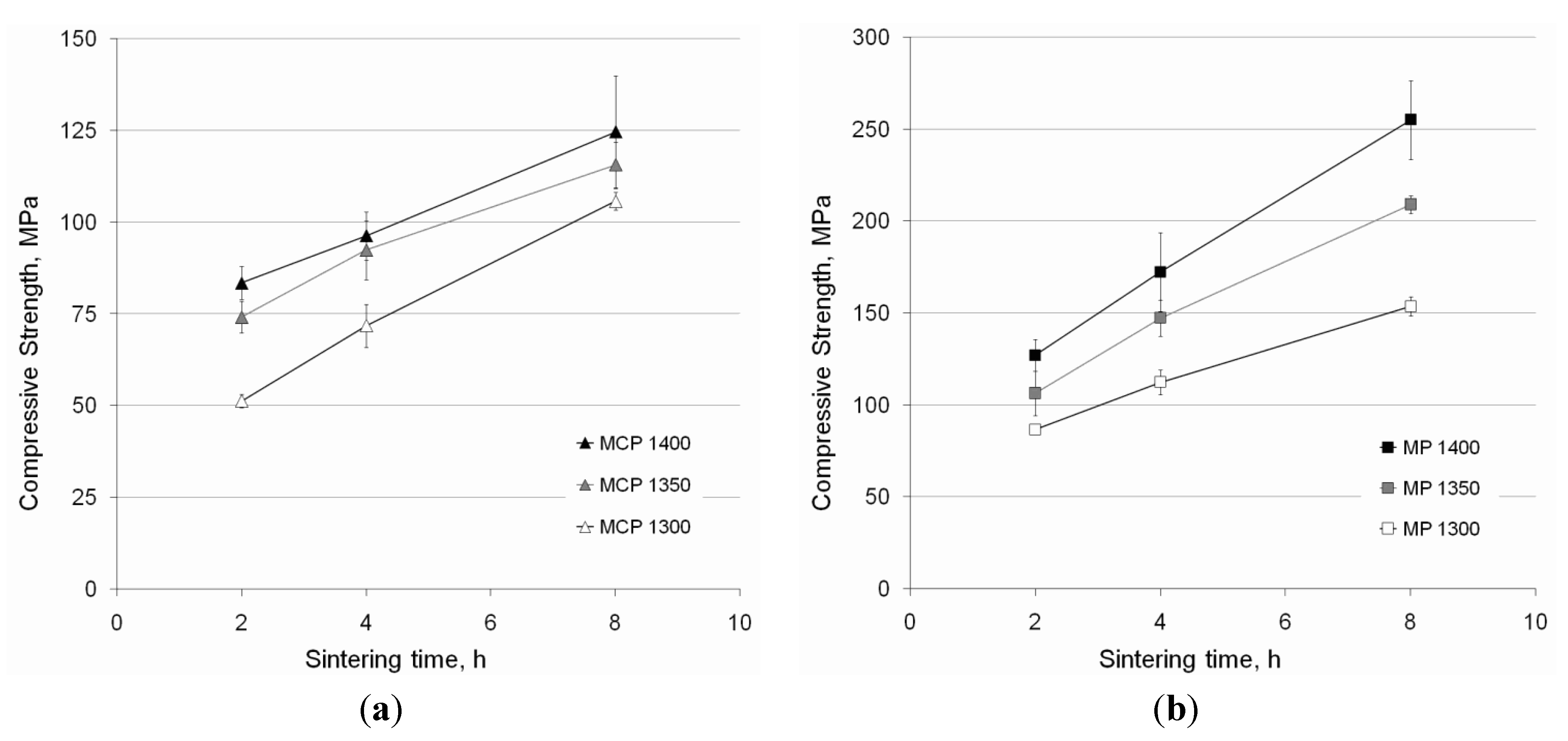
3.3.3. Stiffness
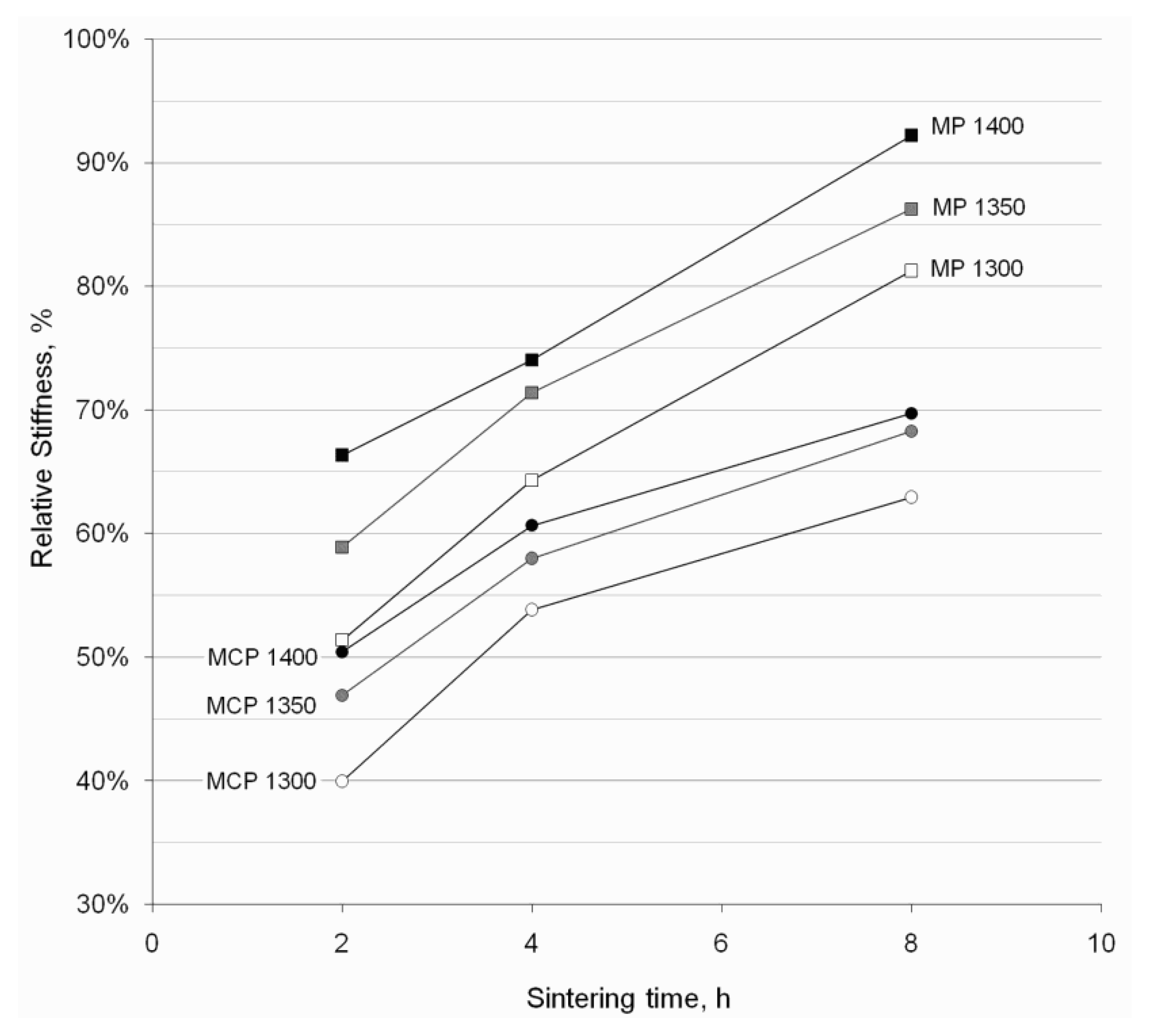
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Özcan, M.; Hämmerle, C. Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef] [Green Version]
- Asaoka, K.; Kon, M. Sintered porous titanium and titanium alloys as advanced biomaterials. Mater. Sci. Forum 2003, 426–432, 3079–3084. [Google Scholar]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Reig, L.; Amigó, V.; Busquets, D.J.; Calero, J.A. Development of porous Ti6Al4V samples by microsphere sintering. J. Mater. Process. Technol. 2012, 212, 3–7. [Google Scholar] [CrossRef]
- Sumitomo, N.; Noritake, K.; Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, M. Experiment study on fracture fixation with low rigidity titanium alloy. J. Mater. Sci. Mater. Med. 2008, 19, 1581–1586. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Brown, S.A.; Lemons, J.E. Medical Applications of Titanium and its Alloys; ASTM International: West Conshohocken, PA, USA, 1996; pp. 76–87. [Google Scholar]
- Zhou, Y.L.; Niinomi, M. Ti–25Ta alloy with the best mechanical compatibility in Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. 2009, 29, 1061–1065. [Google Scholar] [CrossRef]
- Máleka, J.; Hnilicaa, F.; Veselýa, J.; Smolac, B.; Bartákovád, S.; Vaněkd, J. Microstructure and mechanical properties of Ti–35Nb–6Ta alloy after thermomechanical treatment. Mater. Charact. 2012, 66, 75–82. [Google Scholar] [CrossRef]
- Afonso, C.R.M.; Aleixo, G.T.; Ramirez, A.J.; Caram, R. Influence of cooling rate on microstructure of Ti–Nb alloy for orthopedic implants. Mater. Sci. Eng. 2007, 27, 908–913. [Google Scholar] [CrossRef]
- Nugroho, A.W.; Leadbeater, G.; Davies, I.J. Processing of a porous titanium alloy from elemental powders using a solid state isothermal foaming technique. J. Mater. Sci. Mater. Med. 2010, 21, 3103–3107. [Google Scholar] [CrossRef]
- Barbas, A.; Bonnet, A.S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous titanium bone substitutes. J. Mech. Behav. Biomed. 2012, 9, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Wieding, J.; Jonitz, A.; Bader, R. The effect of structural design on mechanical properties and cellular response of additive manufactured titanium scaffolds. Materials 2012, 5, 1336–1347. [Google Scholar] [CrossRef]
- Dezfuli, S.N.; Sadrnezhaad, S.K.; Shokrgozar, M.A.; Bonakdar, S. Fabrication of biocompatible titanium scaffolds using space holder technique. J. Mater. Sci. Mater. Med. 2012, 23, 2483–2488. [Google Scholar] [CrossRef]
- Reig, L.; Amigó, V.; Busquets, D.; Calero, J.A. Stiffness variation of porous titanium developed using space holder method. Powder Metall. 2011, 54, 389–392. [Google Scholar] [CrossRef]
- Amigó, V.; Reig, L.; Busquets, D.J.; Ortiz, J.L.; Calero, J.A. Analysis of bending strength of porous titanium processed by space holder method. Powder Metall. 2011, 54, 67–70. [Google Scholar] [CrossRef]
- Amigó, V.; Salvador, M.D.; Romero, F.; Solves, C.; Moreno, J.F. Microstructural evolution of Ti–6Al–4V during the sintering of microspheres of Ti for orthopedic implants. J. Mater. Process. Technol. 2003, 141, 117–122. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Sargeant, T.D.; Stupp, S.I.; Dunand, D.C. Porous NiTi for bone implants: A review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef]
- Bram, M.; Bogdanski, S.H.; Koller, M.; Buchkremer, H.P.; Stover, D. Evaluation of Mechanical and Biological Properties of Highly Porous Titanium Parts. In Proceedings of the Euro PM 2005 Congress & Exhibition, Praga, Czech Republic, 2–5 October 2005; pp. 517–522.
- Leyens, C.; Peters, M. Titanium and Titanium Alloys. Fundamentals and Applications; Wiley Vch Gmbh & Co.: Köln, Germany, 2003; p. 423. [Google Scholar]
- German, R.M. Powder Metallurgy and Particulate Materials Processing: The Processes, Materials, Products, Properties and Applications; Metal Powder Industries Federation: Princeton, NJ, USA, 2005. [Google Scholar]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Kujala, S.; Ryhanen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Comín, M.; Peris, J.L.; Prat, J.M.; Decoz, J.R.; Vera, P.M.; Hoyos, J.V. Biomecánica de la Fractura ósea y Técnicas de Reparación; (in Spanish). Instituto de Biomecánica de Valencia: Valencia, Spain, 1999. [Google Scholar]
- Heimann, R.B. Materials science of crystaline bioceramics: A review of basic properties and applications. CMU J. 2002, 1, 23–46. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reig, L.; Tojal, C.; Busquets, D.J.; Amigó, V. Microstructure and Mechanical Behavior of Porous Ti–6Al–4V Processed by Spherical Powder Sintering. Materials 2013, 6, 4868-4878. https://doi.org/10.3390/ma6104868
Reig L, Tojal C, Busquets DJ, Amigó V. Microstructure and Mechanical Behavior of Porous Ti–6Al–4V Processed by Spherical Powder Sintering. Materials. 2013; 6(10):4868-4878. https://doi.org/10.3390/ma6104868
Chicago/Turabian StyleReig, Lucía, Concepción Tojal, David J. Busquets, and Vicente Amigó. 2013. "Microstructure and Mechanical Behavior of Porous Ti–6Al–4V Processed by Spherical Powder Sintering" Materials 6, no. 10: 4868-4878. https://doi.org/10.3390/ma6104868