3.2. Microstructure of As-Cast Haynes® 282®
The chemical composition of the as-cast Haynes
® 282
® given in
Table 1 reveals that the VIM IC process does not significantly change the chemical composition of the alloy. The observed marginal differences are the effect of segregation and evaporation of certain alloying elements, which is typical for casting as a process.
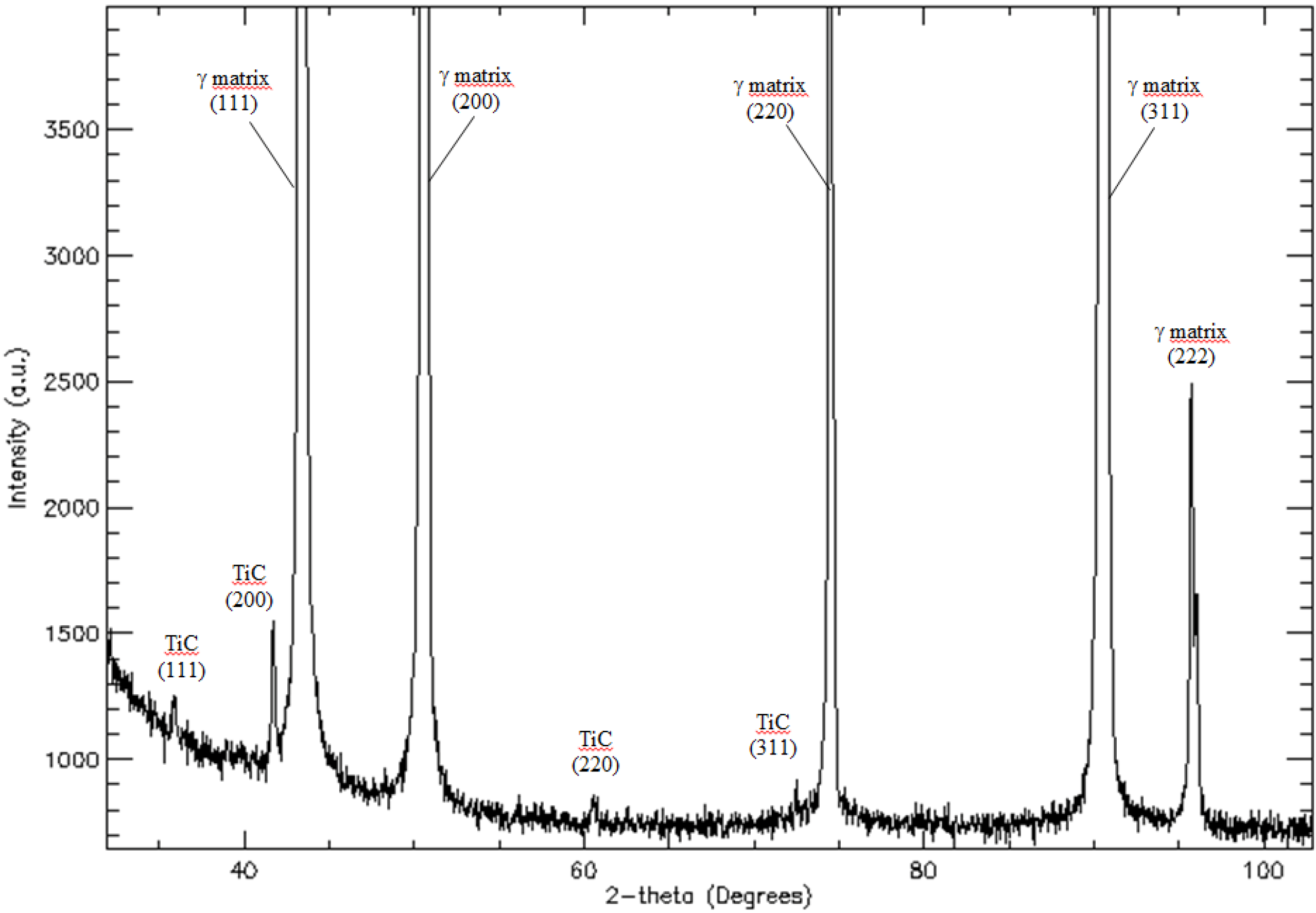
The XRD pattern for as-cast Haynes
® 282
® is shown in
Figure 2 and summarized in
Table 2. The pattern obtained, clearly reveals peaks of two phases: γ matrix and TiC phases. In
Table 2, the values of the
d(hkl) spacing and a
0 lattice parameter are given as measured on the XRD patterns. These values agree well with reference powder diffraction data both for TiC and γ(Ni, Fe) austenite.
Figure 2.
X-ray diffraction (XRD) patterns as obtained for the as-cast Haynes® 282®.
Figure 2.
X-ray diffraction (XRD) patterns as obtained for the as-cast Haynes® 282®.
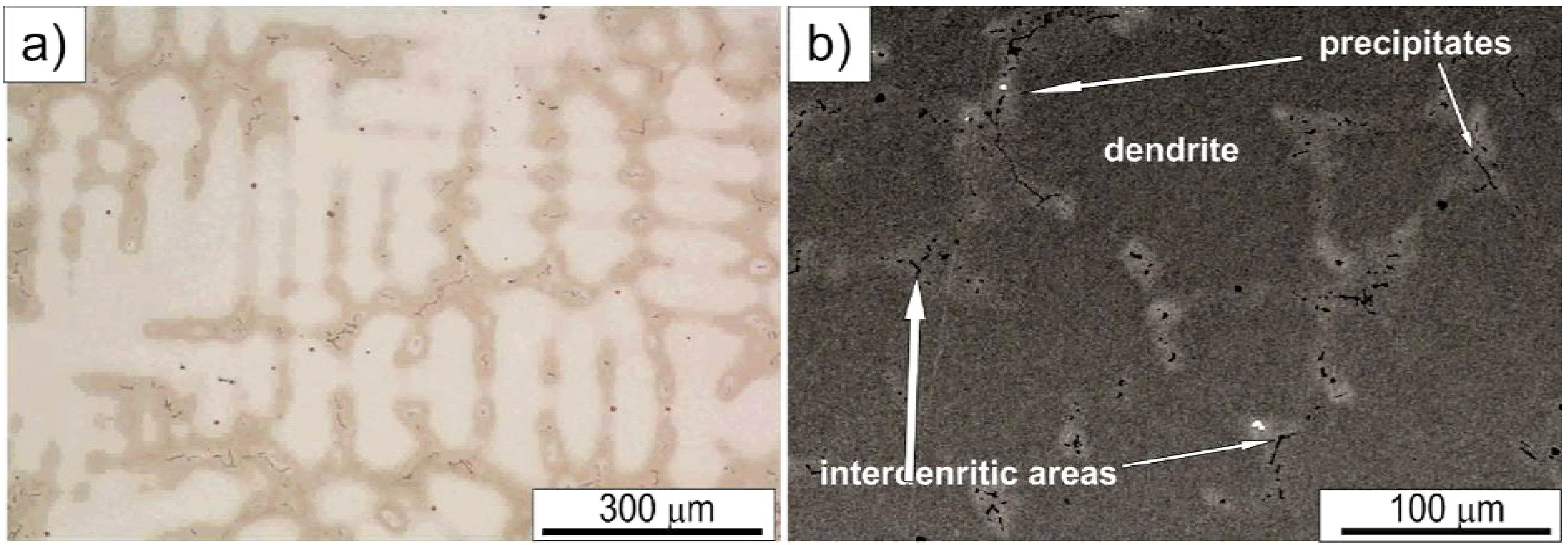
Microstructures representative of as-cast Haynes
® 282
® are shown in
Figure 3. As-cast samples reveal heterogeneous dendritic structure with various precipitates. A strong micro-segregation of alloying elements (Mo, Ti and Cr—confirmed by EDS analyses) is clearly seen on the etched samples due to a difference in contrast between dendrites and inter-dendritic areas. Quantitative image analysis of the as-cast microstructure revealed that the average secondary denrite arm spacing (SDAS) is in the range of 46–55 μm. The LM image reveals that large gray irregular carbides preferentially precipitated in inter-dendritic areas. On the other hand, nitrides are homogeneously dispersed in the as-cast microstructure (nitrides are easily identified due to their regular, angular shapes and orange-red color). SEM images, in
Figure 3b, reveal additional precipitates, apart from MC and MN, clustered in the inter-dendritic areas. Based on their mass contrast and chemical composition, these precipitates/particles were classified into four specific groups as described in more detail below.
Table 2.
The measured values of d(hkl) spacing and a0 lattice parameters for γ, γʹ, metallic carbides (MC) and secondary carbides phases in the as-cast Haynes® 282®.
Table 2.
The measured values of d(hkl) spacing and a0 lattice parameters for γ, γʹ, metallic carbides (MC) and secondary carbides phases in the as-cast Haynes® 282®.
| (hkl) | d(hkl) [Å] | a0 [Å] | d(hkl) [Å] | a0 [Å] |
|---|
| Measurement: TEM *; XRD ** | Powder diffraction file |
|---|
| γ matrix FCC Fm3m (225) | PDF No. 47-1417 |
|---|
| (111) | 2.08 *; 2.08 ** | 3.61 *; 3.61 ** | 2.079 | 3.601 |
| (200) | 1.79 *; 1.80 ** | 3.58 *; 3.61 ** | 1.800 | 3.600 |
| (311) | 1.08 *; 1.09 ** | 3.57 *; 3.60 ** | 1.085 | 3.599 |
| (222) | 1.04 *; 1.04 ** | 3.60 *; 3.60 ** | 1.038 | 3.596 |
| (400) | 0.89 *; – ** | 3.56*; – ** | 0.900 | 3.600 |
| (331) | 0.83 *; – ** | 3.61*; – ** | 0.826 | 3.601 |
| γʹ FCC ordered L12 | PDF No. 09-0097 |
| (100) | 3.57 *; – ** | 3.57 *; – ** | 3.600 | 3.600 |
| (111) | 2.08 *; – ** | 3.61 *; – ** | 2.074 | 3.593 |
| (200) | 1.79 *; – ** | 3.58 *; – ** | 1.799 | 3.598 |
| (211) | 1.47 *; – ** | 3.60 *; – ** | 1.461 | 3.579 |
| (311) | 1.08 *; – ** | 3.57 *; – ** | 1.078 | 3.575 |
| (222) | 1.04 *; 1.02 ** | 3.60 *; 3.53 ** | 1.032 | 3.575 |
| (400) | 0.89 *; – ** | 3.56 *; – ** | 0.893 | 3.572 |
| (331) | 0.83 *; – ** | 3.61 *; – ** | 0.819 | 3.570 |
| MC (TiC) FCC Fm3m (225) | PDF No. 32-1383 |
| (111) | 2.46 *; 2.50 ** | 4.26 *; 4.33 ** | 2.499 | 4.328 |
| (200) | 2.14 *; 2.17 ** | 4.28 *; 4.17 ** | 2.164 | 4.164 |
| (220) | 1.49 *; 1.53 ** | 4.23 *; 4.32 ** | 1.530 | 4.328 |
| (311) | 1.29 *; 1.30 ** | 4.28 *; 4.32 ** | 1.305 | 4.327 |
| Secondary carbides: M23C6 FCC Fm3m (225)/M6C FCC Fd3m (227) | PDF No. 85-1281/PDF No. 47-1192 |
| (111) | 6.19 *; – ** | 10.72 *; – ** | 6.154 /6.429 | 10.659 /11.130 |
| (200) | 5.40 *; – ** | 10.79 *; – ** | 5.330 /5.569 | 10.659 /11.130 |
| (220) | 3.79 *; – ** | 10.71 *; – ** | 3.769 /3.939 | 10.660 /11.130 |
| (311) | 3.30 *; – ** | 10.96 *; – ** | 3.214 /3.359 | 10.660 /11.130 |
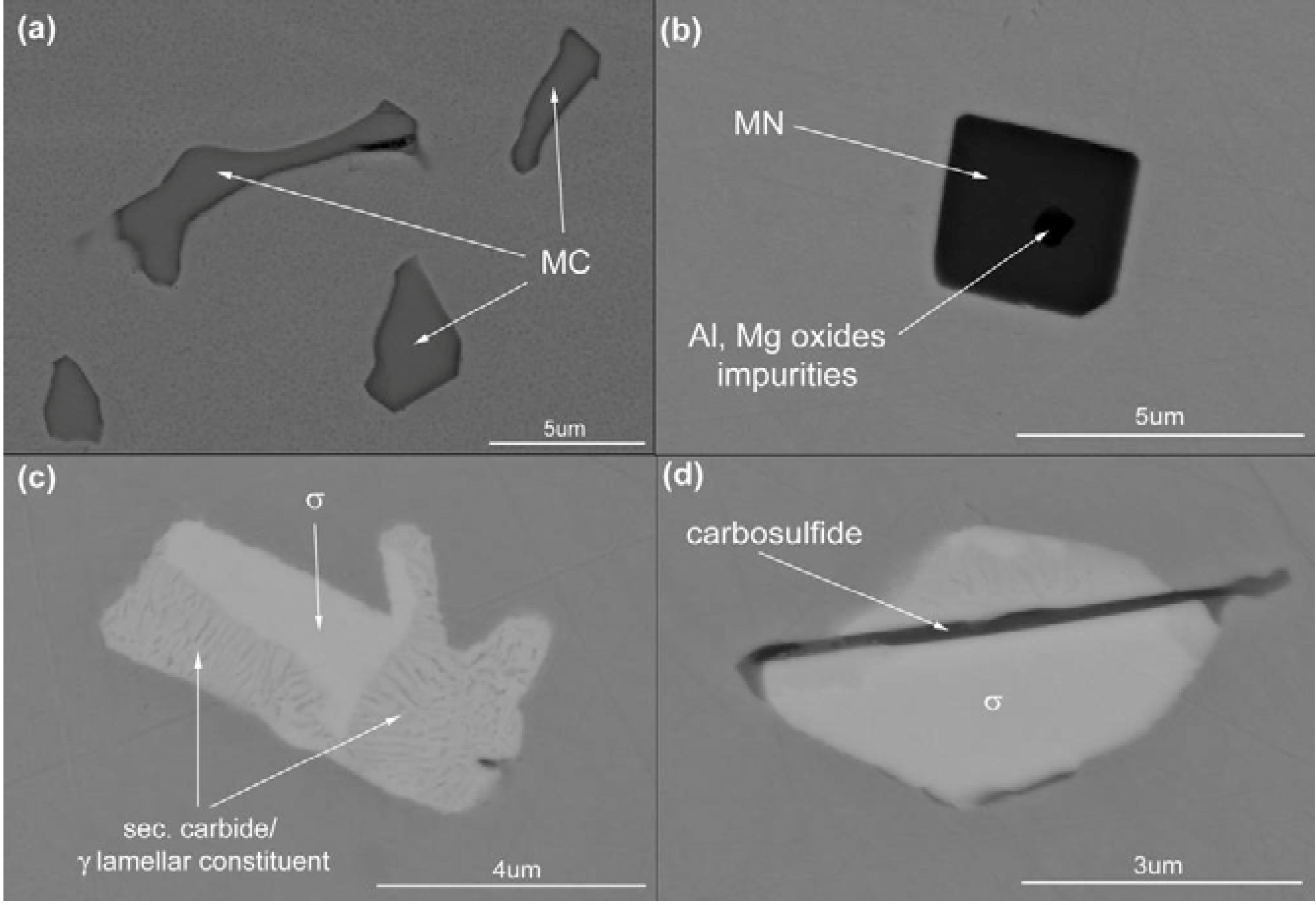
MC precipitates, appear in large number (see
Table 3) as “gray” particles. These precipitates are concentrated in the interdendritic areas. Representative MC particles are shown at high magnification in
Figure 4a (Please note that the Z-contrast is the same for all these precipitates). Semi-quantitative EDS analyses of MC precipitates revealed approximately 50 at % of C, 30 at % of Ti, 14 at % of Mo and 2 at % of Cr (see
Table 4). The last three elements, Ti, Mo and Cr, are carbide forming elements [
25,
26,
27,
28].
Figure 3.
Dendritic microstructure of the as-cast Haynes® 282®: (a) Light Microscopy (LM) image; (b) Scaning Electron Microscopy in backscattered electrons mode (SEM-BSE) image.
Figure 3.
Dendritic microstructure of the as-cast Haynes® 282®: (a) Light Microscopy (LM) image; (b) Scaning Electron Microscopy in backscattered electrons mode (SEM-BSE) image.
Table 3.
Size and distribution frequency of secondary phases in the as-cast Haynes® 282® alloy (metallic carbides (MC), metallic nitrides (MN)).
Table 3.
Size and distribution frequency of secondary phases in the as-cast Haynes® 282® alloy (metallic carbides (MC), metallic nitrides (MN)).
| Precipitation | MN | MC | σ and lamellar constituent | M2SC | γʹ dendrite | γʹ interdendritic |
|---|
| d [μm] | 4.04 | 2.48 | 2.87 | 0.76 | 0.074 | 0.113 |
| NA [L/mm2] | 55 | 550 | 19 | 6 | – | – |
| NV [L/mm3] | 14 × 103 | 222 × 103 | 7 × 103 | 8 × 103 | – | – |
| VV [%] | – | – | – | – | 9.6 | 8.5 |
Figure 4.
Representative precipitates in the as-cast Haynes® 282®: (a) metallic carbides, MC; (b) metallic nitrides, MN, and oxide impurities; (c) σ phase and secondary carbide/ γ lamellar constituent; (d) M2SC and σ phase.
Figure 4.
Representative precipitates in the as-cast Haynes® 282®: (a) metallic carbides, MC; (b) metallic nitrides, MN, and oxide impurities; (c) σ phase and secondary carbide/ γ lamellar constituent; (d) M2SC and σ phase.
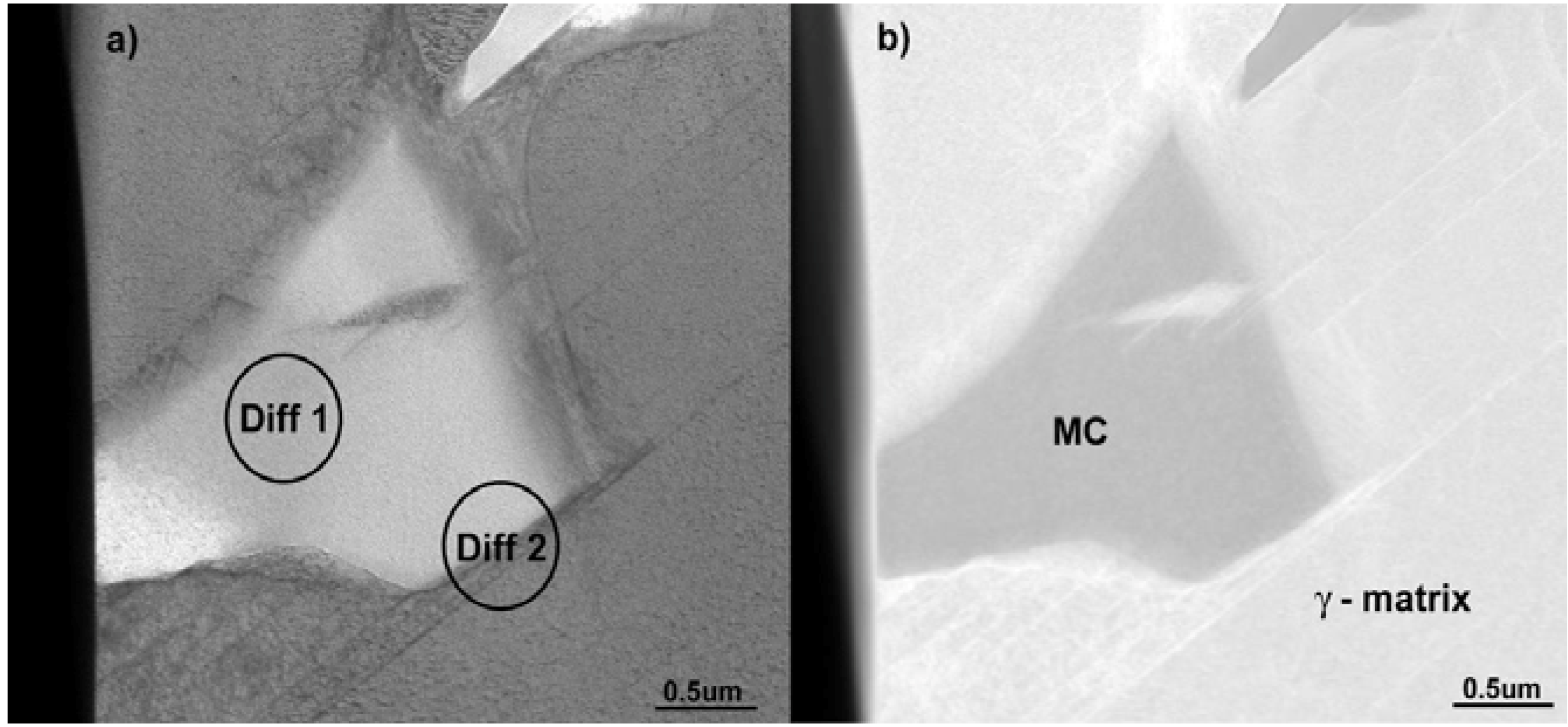
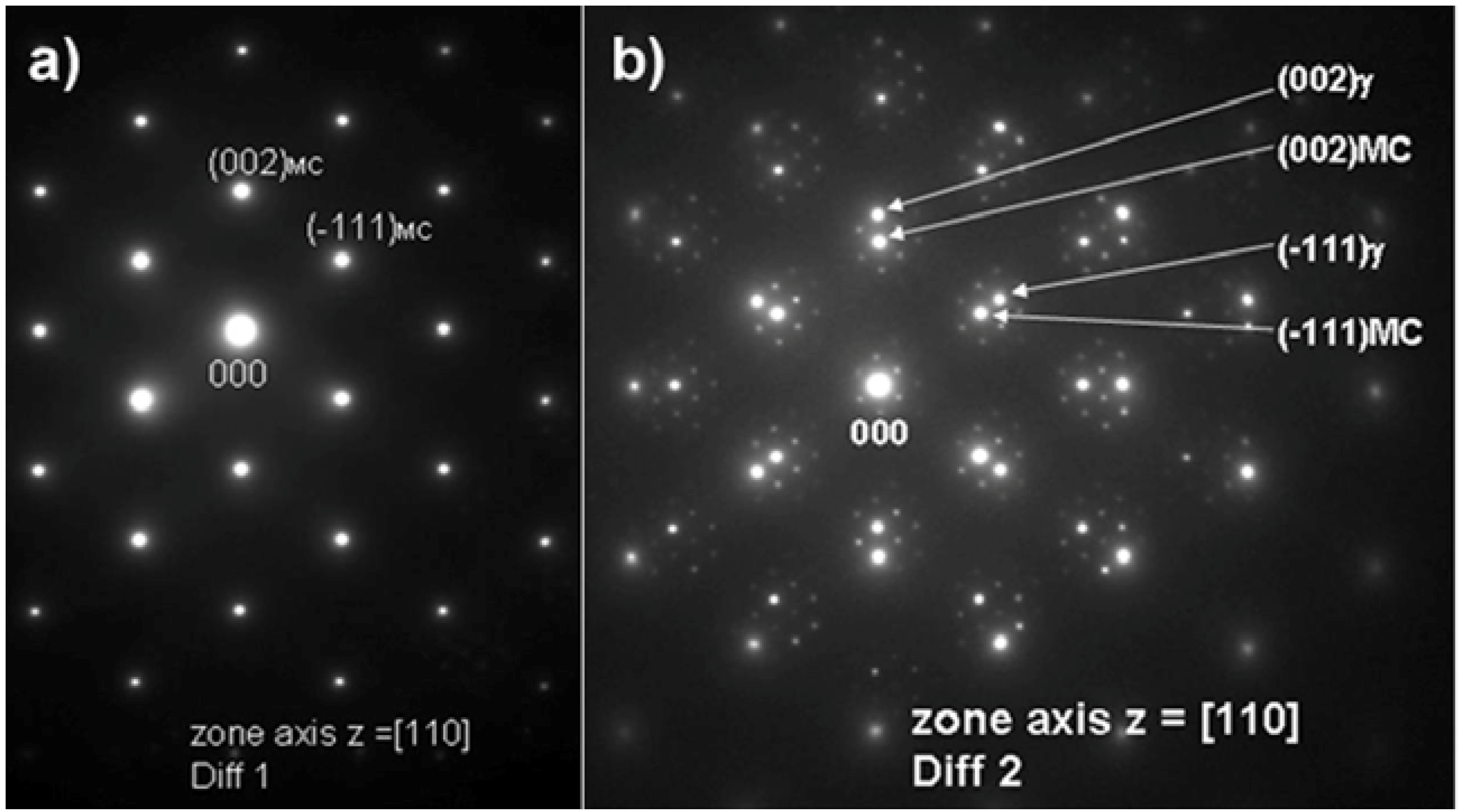
The MC particles were also analyzed using TEM. Bright field (BF) and high angle annular dark field (HAADF) images of these particles are shown with corresponding electron diffraction patterns in
Figure 5 and
Figure 6, respectively. It should be noted that the pattern shown in
Figure 6a clearly reveals reflections from a FCC crystal structure. The
d(hkl)-spacing and a
0 lattice parameters measured on the basis of electron diffraction patterns, listed in
Table 2, are in good agreement with the theoretical values.
Table 4.
Chemical composition in at % of MC, MN, σ and secondary carbide/γ lamellar constituent as obtained through X-ray spectroscopy (EDS) analyses.
Table 4.
Chemical composition in at % of MC, MN, σ and secondary carbide/γ lamellar constituent as obtained through X-ray spectroscopy (EDS) analyses.
| Phase | Chemical composition in at % |
|---|
| MC | MN | σ | secondary carbide/γ lamellar constituent |
|---|
| N | – | 56.7 ± 0.5 | – | – |
| C | 52.7 ± 2.4 | – | – | 20.7 ± 7.9 |
| Si | – | – | 1.0 ± 0.4 | 0.7 ± 0.1 |
| Ti | 30.7 ± 1.8 | 42.5 ± 0.9 | 1.0 ± 0.3 | – |
| Cr | 1.7 ± 0.5 | 0.3 ± 0.1 | 29.0 ± 0.7 | 22.4 ± 3.4 |
| Co | – | – | 9.5 ± 0.7 | 8.0 ± 1.3 |
| Ni | 1.4 ± 0.7 | 0.7 ± 0.1 | 27.8 ± 0.8 | 23.7 ± 6.0 |
| Mo | 13.6 ± 0.8 | – | 31.0 ± 0.6 | 24.1 ± 0.7 |
| Al | MC | MN | 0.5 ± 0.1 | – |
Based on the electron diffraction patterns (
Figure 6b) the following, typical cube-cube orientation relationships between the MC and γ-matrix were confirmed:
It should be noted that MC carbides are important constituents in Ni based superalloys. Formation of primary carbides begins in the melt due to segregation of carbon, which reacts with elements such as Ti, Mo, Cr and Nb. Fine MC precipitates at grain boundaries or in the matrix which strengthens the alloy and also ties-up some elements that would otherwise promote phase instability during heat treatment and service. These carbides may transform into M
23C
6 and/or M
6C at 760–980 °C and 815–980 °C, respectively, which are rich in Cr, Mo and/or W [
7,
26]. The most common transformation can be described as:
The homogenously distributed precipitates exemplified in
Figure 4b are characterized as TiN. These particles have significantly lower number density in comparison with MC—see
Table 3. SEM images of these precipitates (
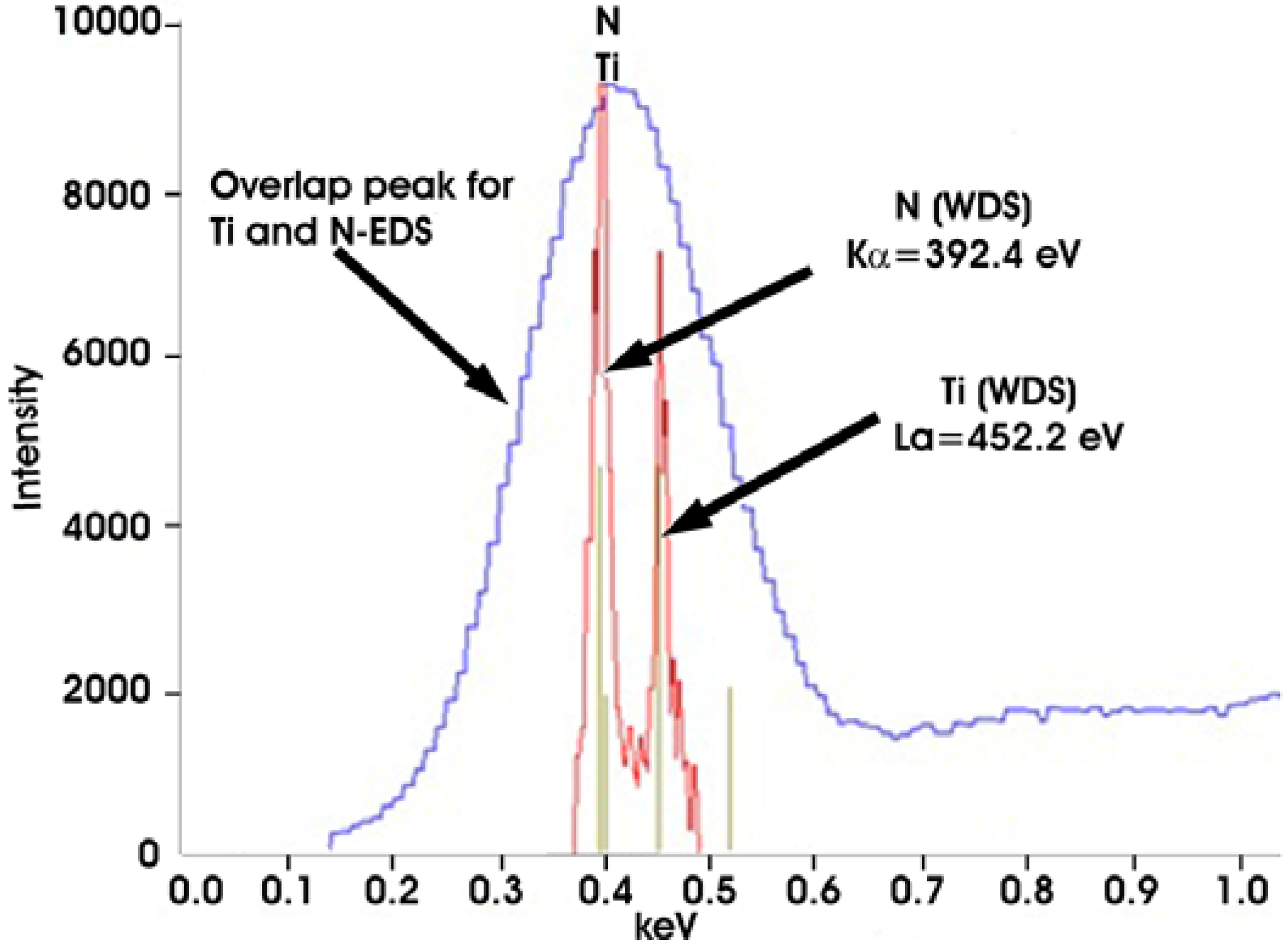
Figure 4b) exhibit homogenous Z-contrast with a dark nucleus centre of regular shape. Results from the measurement of their chemical analyses are given in
Table 4. Since the spectra show a strong peak in the region of overlapping lines of Ti and N, additional WDS analyses were conducted (
Figure 7) with the conclusion that these are TiN precipitates which confirms the findings using LM (orange-red color) [
26].
The EDS chemical analyses also revealed strong signals of Mg, Al and O at the centre of the TiN precipitates. This suggests that nitrides nucleate around non-metallic oxide impurities. It should be noted that TiN particles are not influenced by the potential heat treatments of the casting and are insoluble up to the melting point. At their standard concentration in superalloys, they generally have little influence on the mechanical properties [
26].
Figure 5.
Microstructure of a MC: (a) Scanning Transmission Electron Microscpy in Bright Field (BF-STEM); and (b) Scanning Transmission Electron Microscpy in High-Angle Annular Dark Field (HAADF-STEM).
Figure 5.
Microstructure of a MC: (a) Scanning Transmission Electron Microscpy in Bright Field (BF-STEM); and (b) Scanning Transmission Electron Microscpy in High-Angle Annular Dark Field (HAADF-STEM).
Figure 6.
Selected area electron diffraction patterns for the MC phase constituent shown in (a) together with superimposed Moiré electron diffraction pattern for MC and γ phase in (b).
Figure 6.
Selected area electron diffraction patterns for the MC phase constituent shown in (a) together with superimposed Moiré electron diffraction pattern for MC and γ phase in (b).
Figure 7.
EDS and wave length dispersive X-ray spectroscopy (WDS) patterns for MN.
Figure 7.
EDS and wave length dispersive X-ray spectroscopy (WDS) patterns for MN.
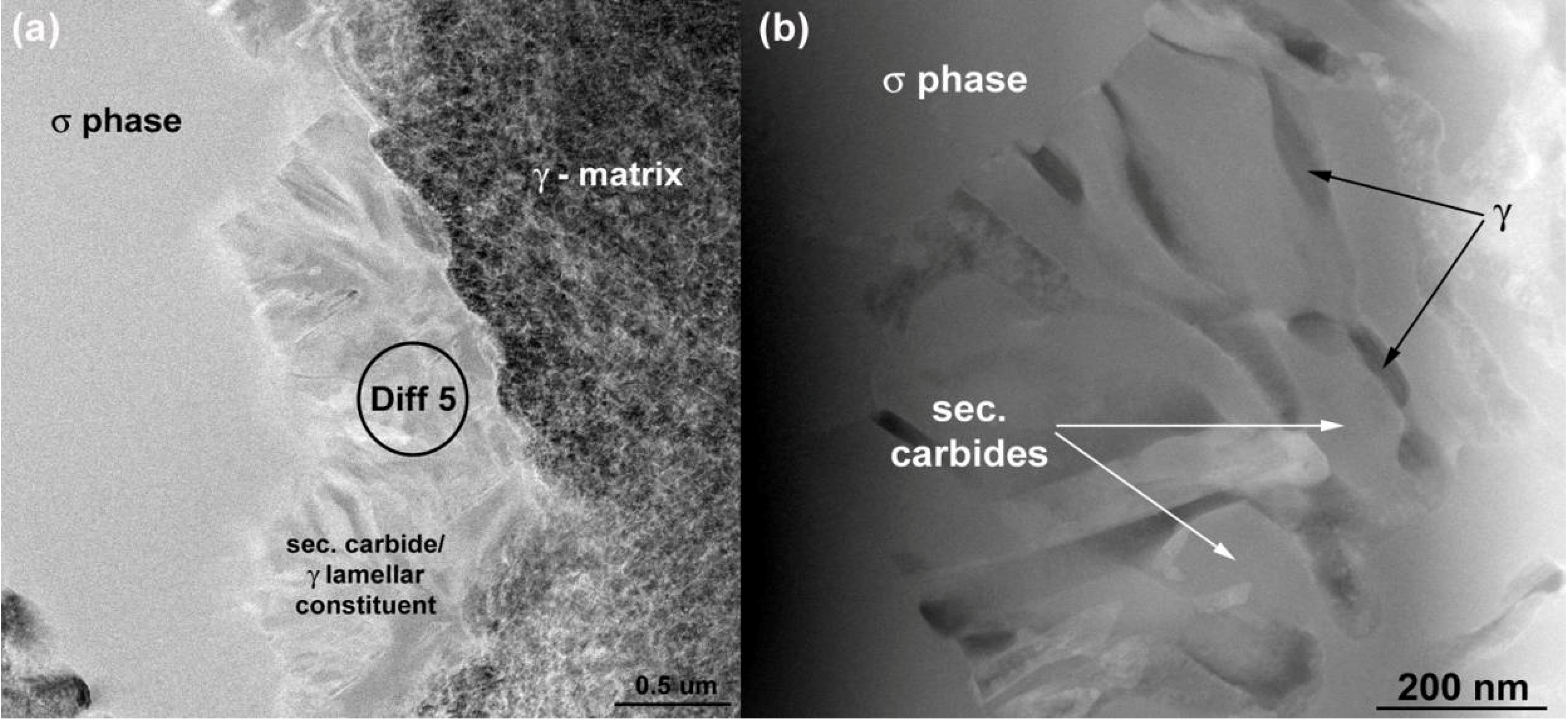
The “white phase” particles are present in the interdendritic areas. The number density of these particles is much lower than the MC carbides, see
Table 3. Detailed SEM examinations (
Figure 4c) have shown that they often constitute: (a) compact-precipitation with homogenous Z-contrast and (b) lamellar appearance. The results of the EDS analyses for both regions are listed in
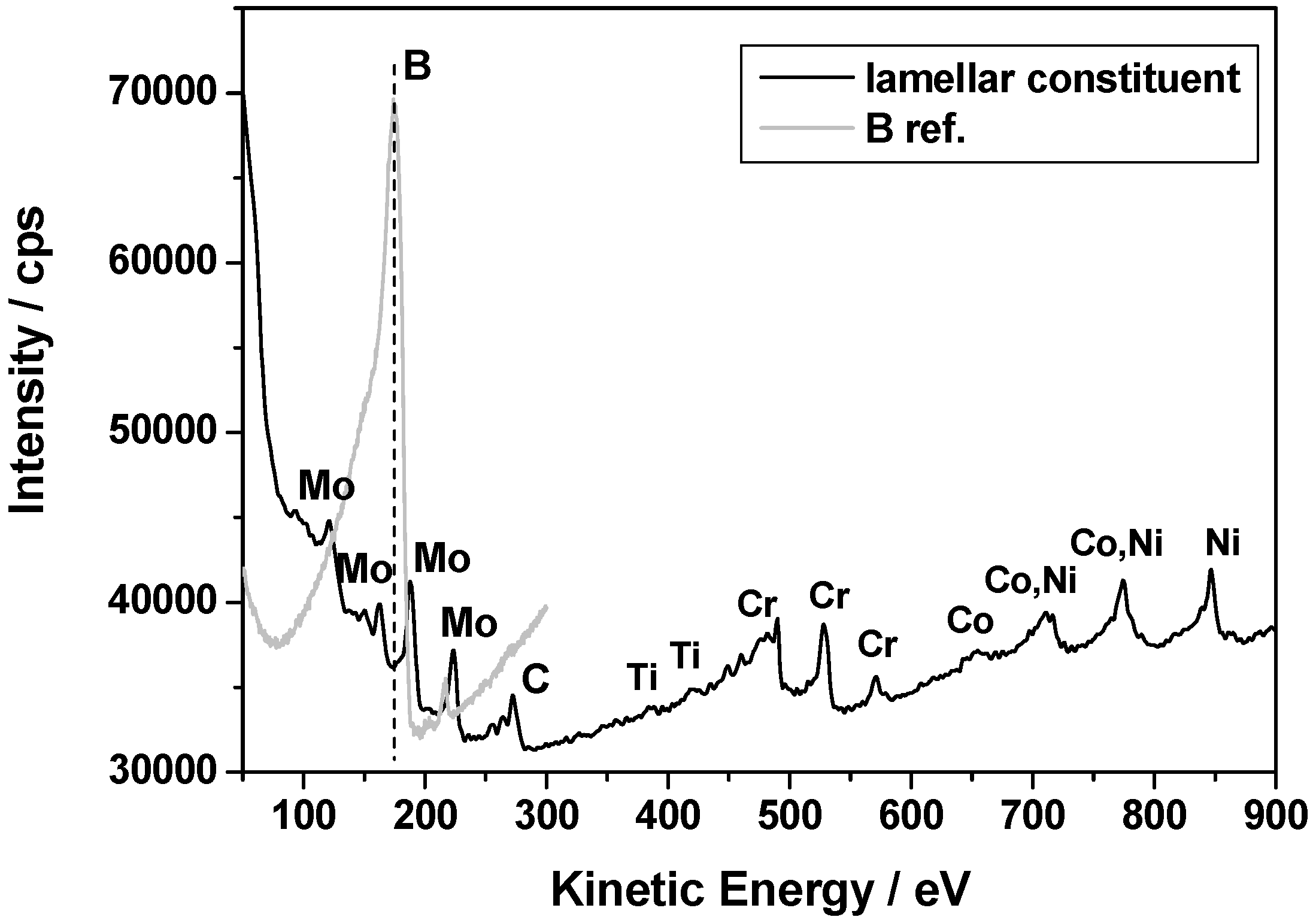
Table 4 above. The compact phase contains equal amounts (30 at %) of Cr, Ni and Mo and 10 at % of Co. The chemical composition of the lamellar constituent areas are similar to that of the compact phase, however, with a significant concentration of C. As the spatial resolution of the EDS method is relatively low, FIB samples were cut-out from the compact precipitation and lamellar constituent regions and were investigated by AES and TEM analysis methods.
Since elements like Mo and Cr are generally known to have a high affinity for boron/carbon and the solid solubility of boron/carbon in the γ phase is very low, it is likely that borides (like M
3B
2 and/or M
5B
3) and/or secondary carbides (like M
23C
6 and/or M
6C) easily form. This is commonly observed in many Ni based superalloys [
29,
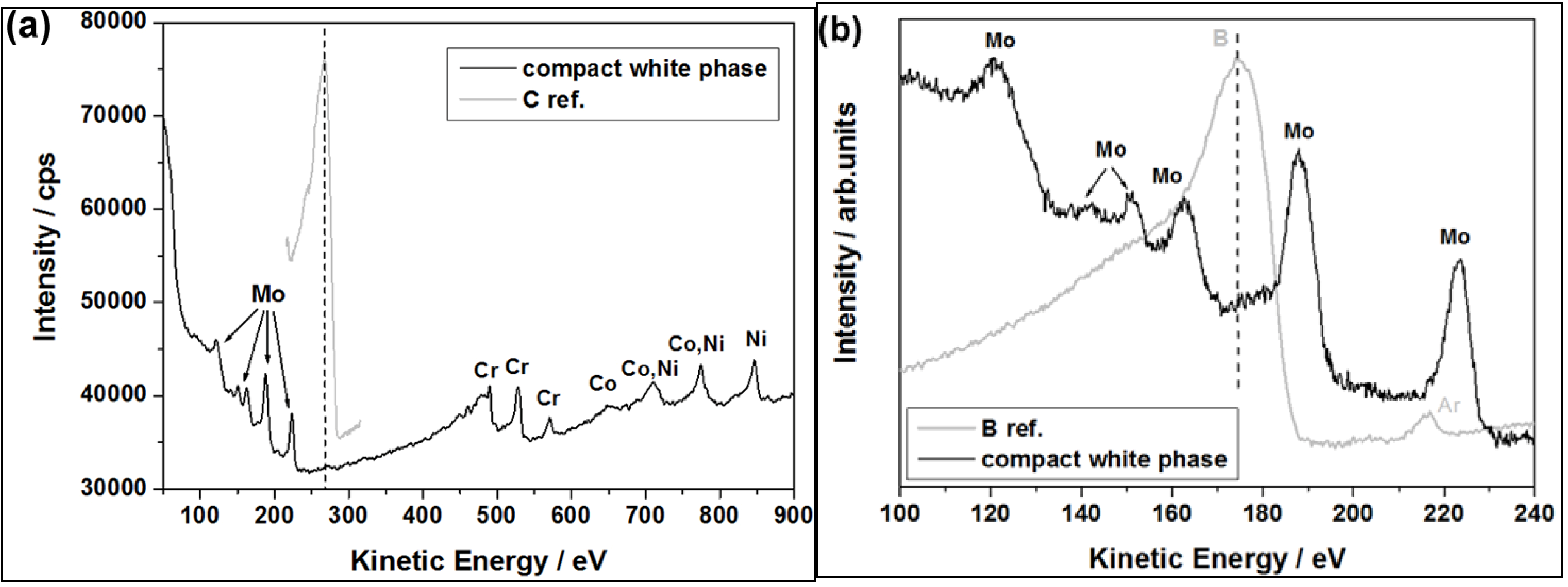
30]. Thus, AES was used to analyze light elements (C and B) in the “white phase”. A representative local auger spectrum for the compact “white phase” is shown in
Figure 8. The obtained results confirm that the phase is rich in Mo, however boron as well as carbon was not detected. It suggests that the compact “white phase” should be one of the TCP phases, and not a boride or carbide.
Figure 8.
Local auger electron spectroscopy (AES) spectra for the compact “white phase” (a) with reference carbon peak; and (b) with reference boron peak.
Figure 8.
Local auger electron spectroscopy (AES) spectra for the compact “white phase” (a) with reference carbon peak; and (b) with reference boron peak.
A TEM micrograph of a TCP particle is shown in
Figure 9 with corresponding electron diffraction pattern in
Figure 10. This pattern clearly reveal reflections from a tetragonal, P4
2/mnm crystal structure with a = 8.7 Å and c = 4.7 Å. The measured values of the d
(hkl) spacing, a
0 and c
0 lattice parameters, are listed in
Table 5 together with powder diffraction data for σ phase of various chemical compositions. A good agreement between the reference and experimental data obtained in this study can be noted. Furthermore, the current data reveal an orientation relationship in between the TCP phase precipitates and the γ matrix which has been already reported in [
16,
22]:
The intermetallic σ phase is hard and brittle. It is detrimental to the mechanical properties of superalloys if present in the form of elongated particles or as grain boundary films. On the other hand, precipitates that are small and spherical in shape may improve creep resistance. Nevertheless, the formation of the σ phase is always a concern as it depletes refractory metals in the γ matrix, causing reduction in strength [
15,
16,
17,
18,
19,
20,
21,
22,
26].
Figure 9.
Microstructure of σ phase particles: (a) HAADF-STEM and (b) BF-STEM.
Figure 9.
Microstructure of σ phase particles: (a) HAADF-STEM and (b) BF-STEM.
Figure 10.
Diffraction pattern of the sigma phase particle shown in
Figure 9a.
Figure 10.
Diffraction pattern of the sigma phase particle shown in
Figure 9a.
Table 5.
The measured values of d(hkl)-spacing and lattice parameters for σ phase in the as-cast Haynes® 282® superalloy.
Table 5.
The measured values of d(hkl)-spacing and lattice parameters for σ phase in the as-cast Haynes® 282® superalloy.
| (hkl) | d(hkl) [Å] | a0 [Å] | c0 [Å] | a0 [Å] | c0 [Å] |
|---|
| Measurement | Powder diffraction data |
|---|
| σ phase-Tetragonal, P42/mnm (136) |
|---|
| (110) | 6.14 | – | – | – | – |
| (001) | 4.66 | – | – | – | – |
| (111) | 3.73 | – | – | 9.170 * | 4.741 * |
| (221) | 2.57 | – | – | 8.810 ** | 4.560 ** |
| (112) | 2.20 | 8.68 | 4.66 | 8.790 *** | 4.544 *** |
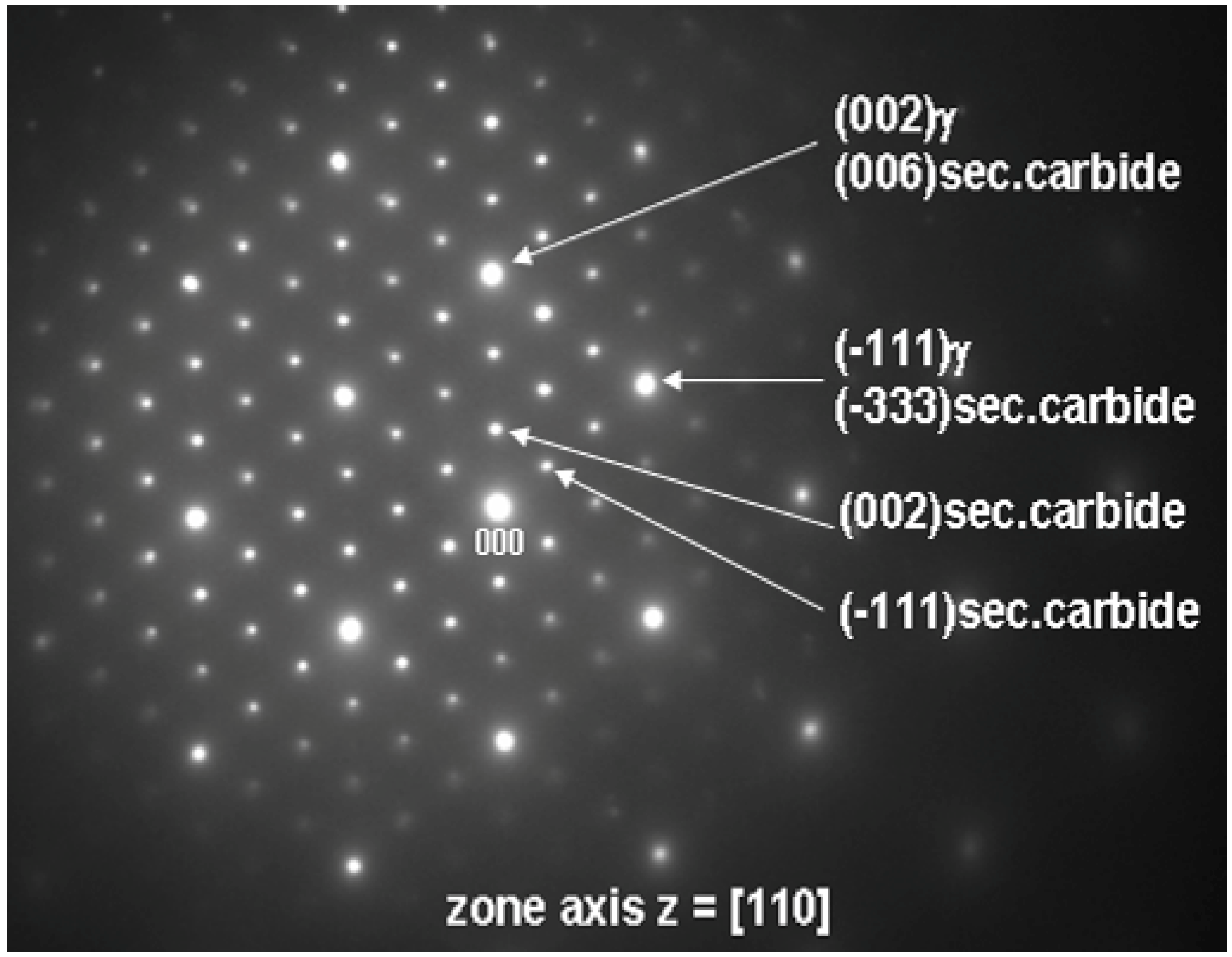
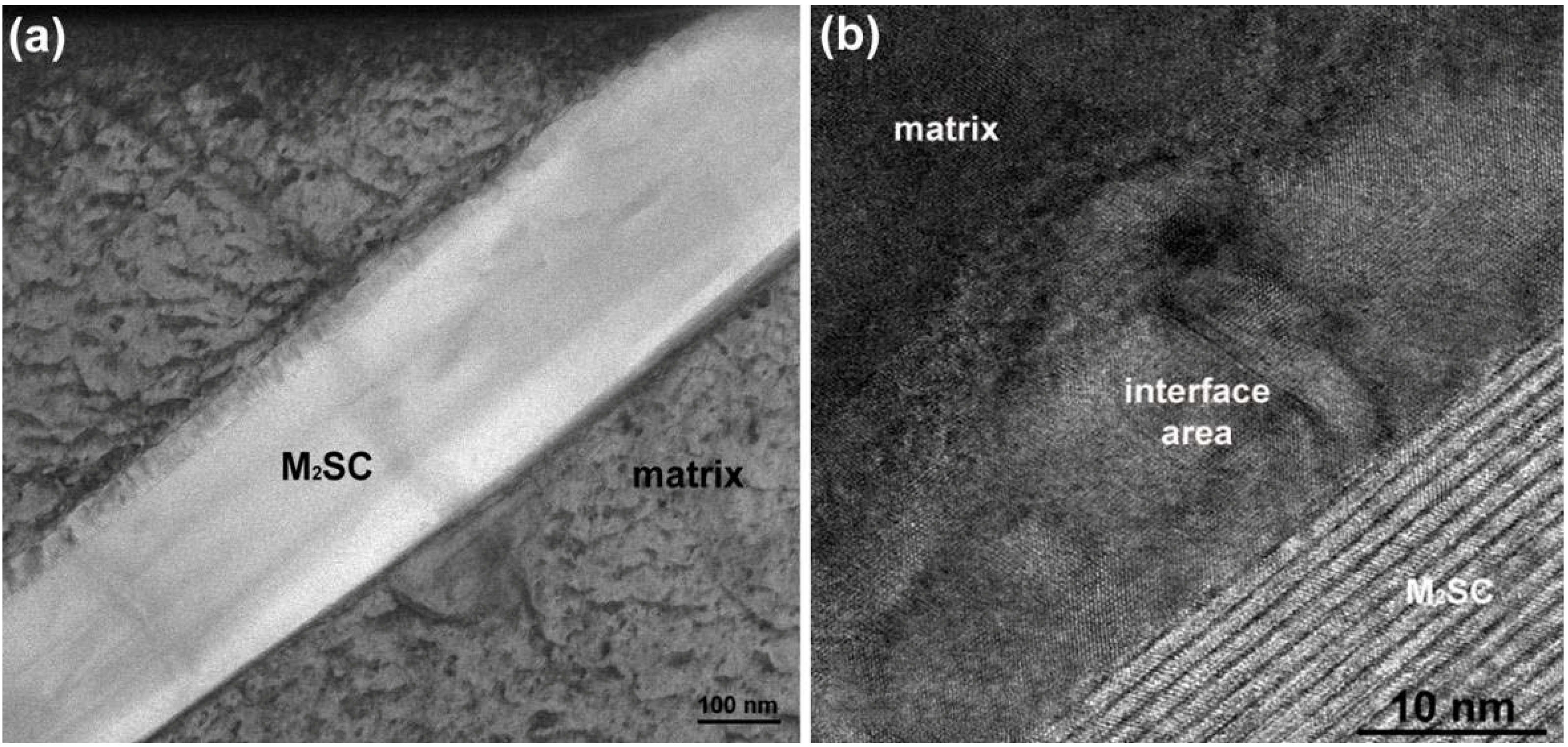
TEM was also used to investigate the lamellar areas, exemplified in
Figure 11. The corresponding electron diffraction pattern (
Figure 12) demonstrates that both phases possess FCC crystal structure. Reflections from the “bright phase” are present at every third gray phase dot and the following, typical cube-cube orientation relationships were confirmed:
Figure 11.
Microstructure of lamellar secondary carbide/γ constituent (HAADF-STEM).
Figure 11.
Microstructure of lamellar secondary carbide/γ constituent (HAADF-STEM).
Such relationships are characteristic of secondary (M
23C
6 and/or M
6C) carbides and γ matrix in cast superalloys [
18,
25]. The values of
d(hkl) spacing and a
0 lattice parameters estimated from the diffraction patterns are listed in
Table 5 together with reference powder diffraction data for Cr
23C
6 and Mo
3Fe
3C carbides. Best fit was achieved for M
23C
6 carbide, however M
6C is also probable. EDS measurements (
Table 4) indicate that the “bright phase” located within the lamellar constituent is rich in Mo, however significant solubilities for Cr, Ni, Co and C have been also observed. Local AES spectra, as shown in
Figure 13, confirm the results obtained by the EDS technique. It should be noticed that boron was not detected. It is reasonable to believe that the boron content in the alloy is too low to form any borides in the as-cast state which suggests that B is soluble in the matrix. Boron may reduce solubility of carbon in the γ matrix, which promote precipitation of carbides [
26]. From this part of the study it can therefore be concluded that the “white phase” constitutes a TCP σ phase together with a lamellar constituent consisting of γ and secondary carbide. To the authors’ knowledge, these type of objects have not previously been reported for in the Haynes
® 282
® superalloy.
The “black needles/platelets” constituents, which have been analyzed in this study, are observed adjacent to σ phase precipitates (see
Figure 4d). Their density is relatively low, see
Table 3. HR-STEM micrographs of a “needle” shown in
Figure 14 reveals so called “nano-laminates” within the microstructure being typical for MAX phases (M
n+1AX
n, where
n = 1, 2 or 3, M is an early transition metal, A is an A-group element, and X is either C or N) [
31].
Figure 12.
Selected area electron diffraction patterns for lamellar secondary carbide/γ obtained from regions shown in
Figure 11a.
Figure 12.
Selected area electron diffraction patterns for lamellar secondary carbide/γ obtained from regions shown in
Figure 11a.
Figure 13.
Local AES spectra for “bright phase” in lamellar constituent with reference boron peak.
Figure 13.
Local AES spectra for “bright phase” in lamellar constituent with reference boron peak.
Figure 14.
Microstructure of a M2SC carbosulphide: (a) BF-STEM; and (b) high resolution image.
Figure 14.
Microstructure of a M2SC carbosulphide: (a) BF-STEM; and (b) high resolution image.
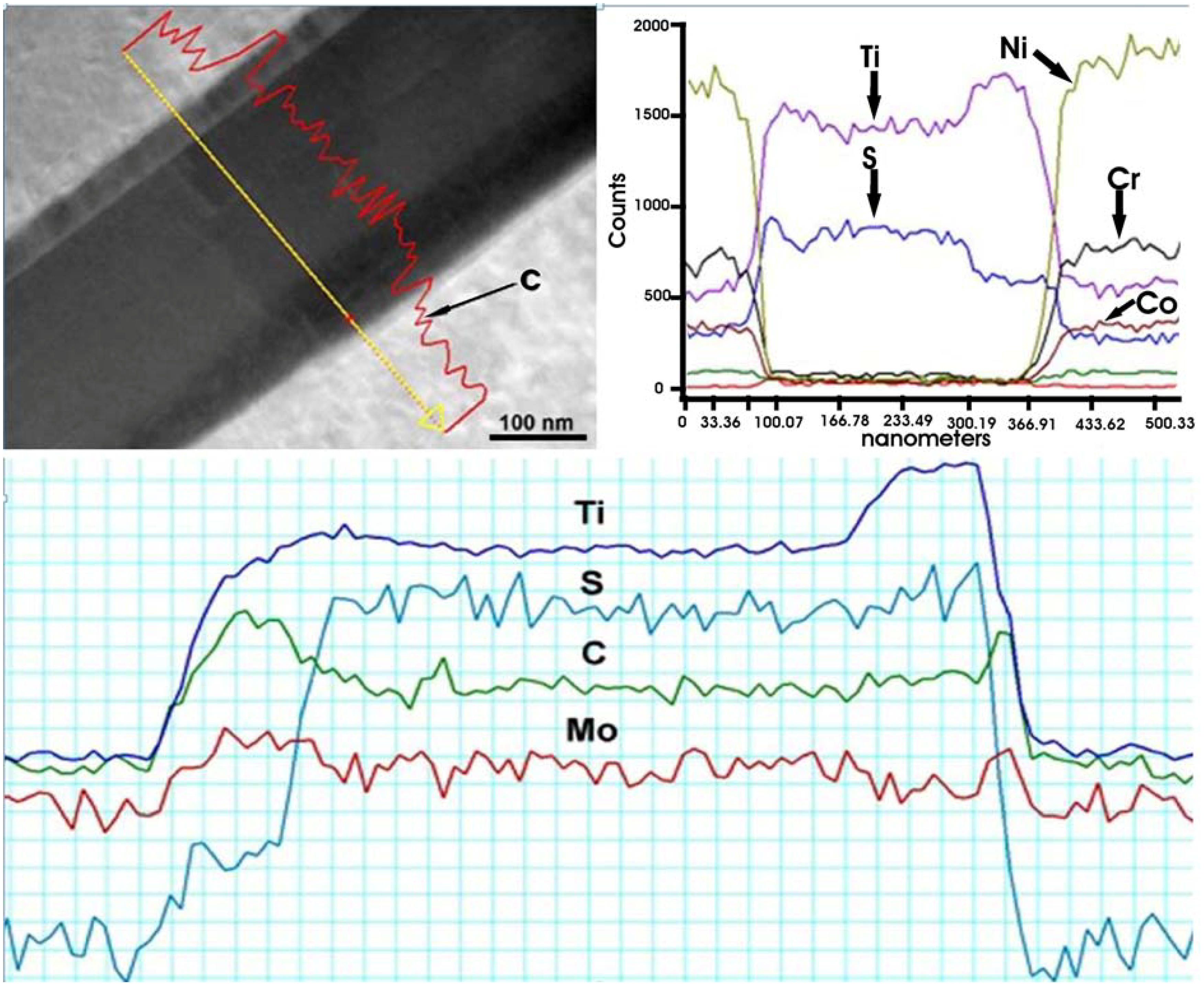
The EDS and EELS spectra (
Figure 15) reveal that the “needles” contain S and Ti. Some concentration of C (especially on the interface) and Mo were also detected. Sulfur is known to be a harmful element in superalloys. It may segregate to the grain boundaries [
26,
27,
28,
30] increasing the susceptibility towards embrittlement (intergranular fracture). Sulfur also reduces ductility through the formation of sulfide/carbosulfide particles with needle/plate morphology (mostly with titanium) [
26] of M
2SC stoichiometry (where M = Ti, Mo, Nb, Cr, and Fe). The distance in between the “nano-laminates” measured in this study is 11.17 Å, which is in a good agreement with the theoretical value of c
0 lattice constant for Ti
2SC. It should be noted that Ti
2SC has a hexagonal unit cell (P6
3/mmc space group) with lattice constants of
a0 = 3.22 Å and
c0 = 11.22 Å [
32].
Figure 15.
EDS and electron energy-loss spectrometry (EELS) line-scans of elements in M2SC carbosulfide.
Figure 15.
EDS and electron energy-loss spectrometry (EELS) line-scans of elements in M2SC carbosulfide.
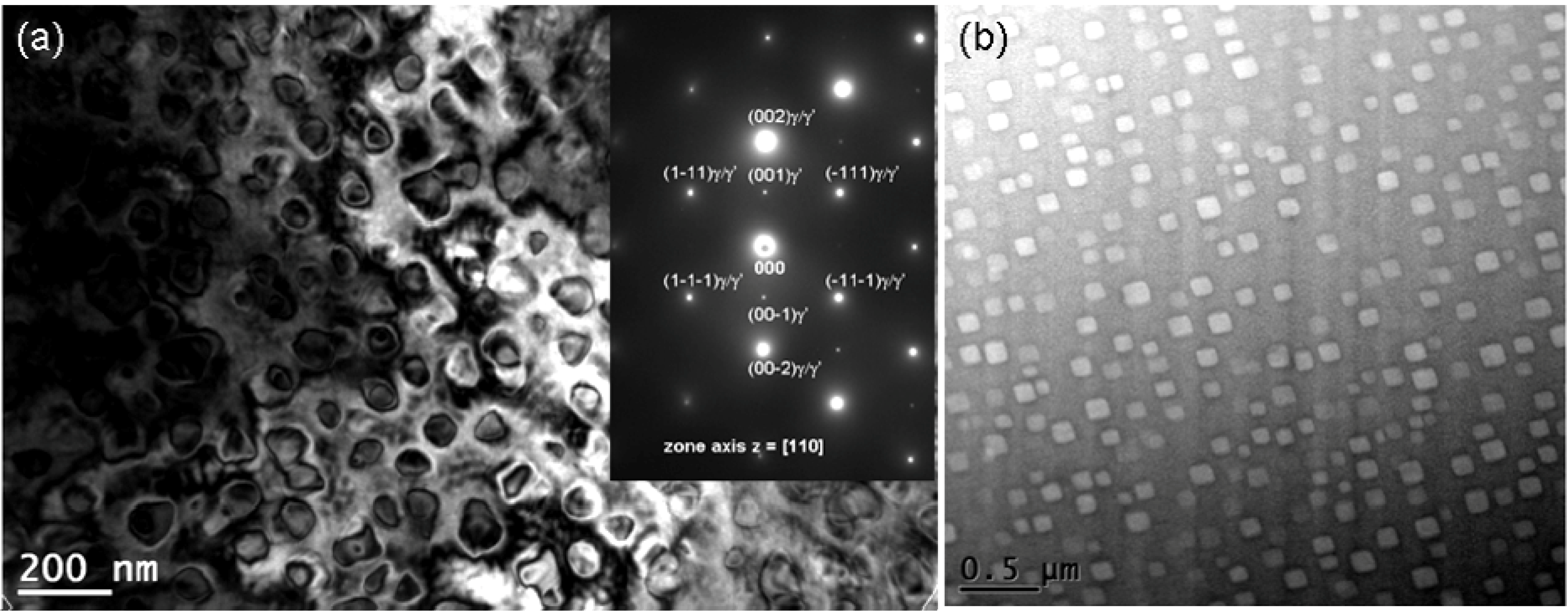
A bright-field TEM image of the matrix nanostructure of the as-cast Haynes
® 282
® alloy is shown in
Figure 16 with the corresponding electron diffraction pattern. Within the dendrites, the nano particles appears mostly in the form of spherical, fine precipitates (74 nm), while coarser (113 nm), cubic precipitates are present in the interdendritic areas. The volume content of the precipitates within the dendrites and interdendritic areas is 9.6% and 8.5%, respectively. The electron diffraction pattern in
Figure 16 reveals reflections from two phases; FCC ordered L1
2 and FCC A1. The estimates of
d(hkl) spacing and a
0 lattice parameters are listed in
Table 2 together with the powder diffraction reference data for γʹ-Ni
3Al and γ-(Ni,Fe) austenite. The agreement between these data suggests that these precipitates are nickel aluminides. Such a conclusion is confirmed by EDS analyses for the γʹ phase, which primarily revealed Ni, Al and Ti. It is thus concluded that the FCC A1 matrix (γ phase Ni-Cr-Co-Mo solid solution) of as-cast Haynes
® 282
® alloy is precipitation strengthened by the FCC ordered L1
2, coherent Ni
3(Al,Ti) γʹ phase. These phases remain in the following crystallographic orientation relationship:
In the as-cast state of Ni-based superalloys which contain a high number and concentration of alloying elements, variation in the nature (size, shape and volume content) of γʹ precipitates is commonly observed due to the effects of dendritic microsegregation and local cooling rates during cast solidification. This results in the formation of finer γʹ precipitates in the dendrites and coarser γʹ precipitates in the interdendritic regions [
33].
Figure 16.
A BF TEM image and selected area electron diffraction pattern for γ/γʹ type microstructure in the as-cast Haynes® 282® superalloy: (a) dendrite; and (b) interdendritic area.
Figure 16.
A BF TEM image and selected area electron diffraction pattern for γ/γʹ type microstructure in the as-cast Haynes® 282® superalloy: (a) dendrite; and (b) interdendritic area.