Phosphorus Effects of Mesoporous Bioactive Glass on Occlude Exposed Dentin
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Setting Properties of Commercialized Product PerioGlas®
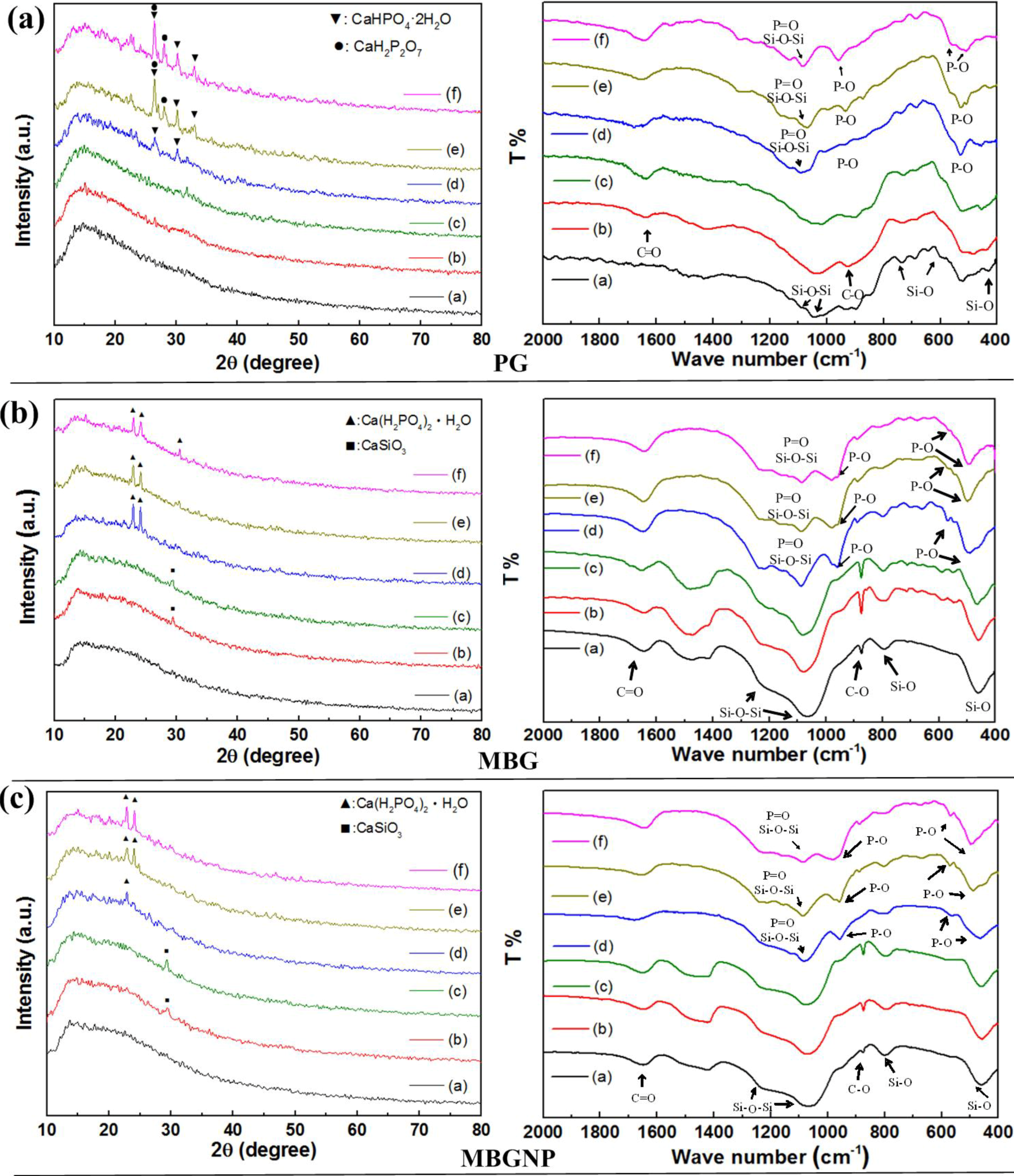
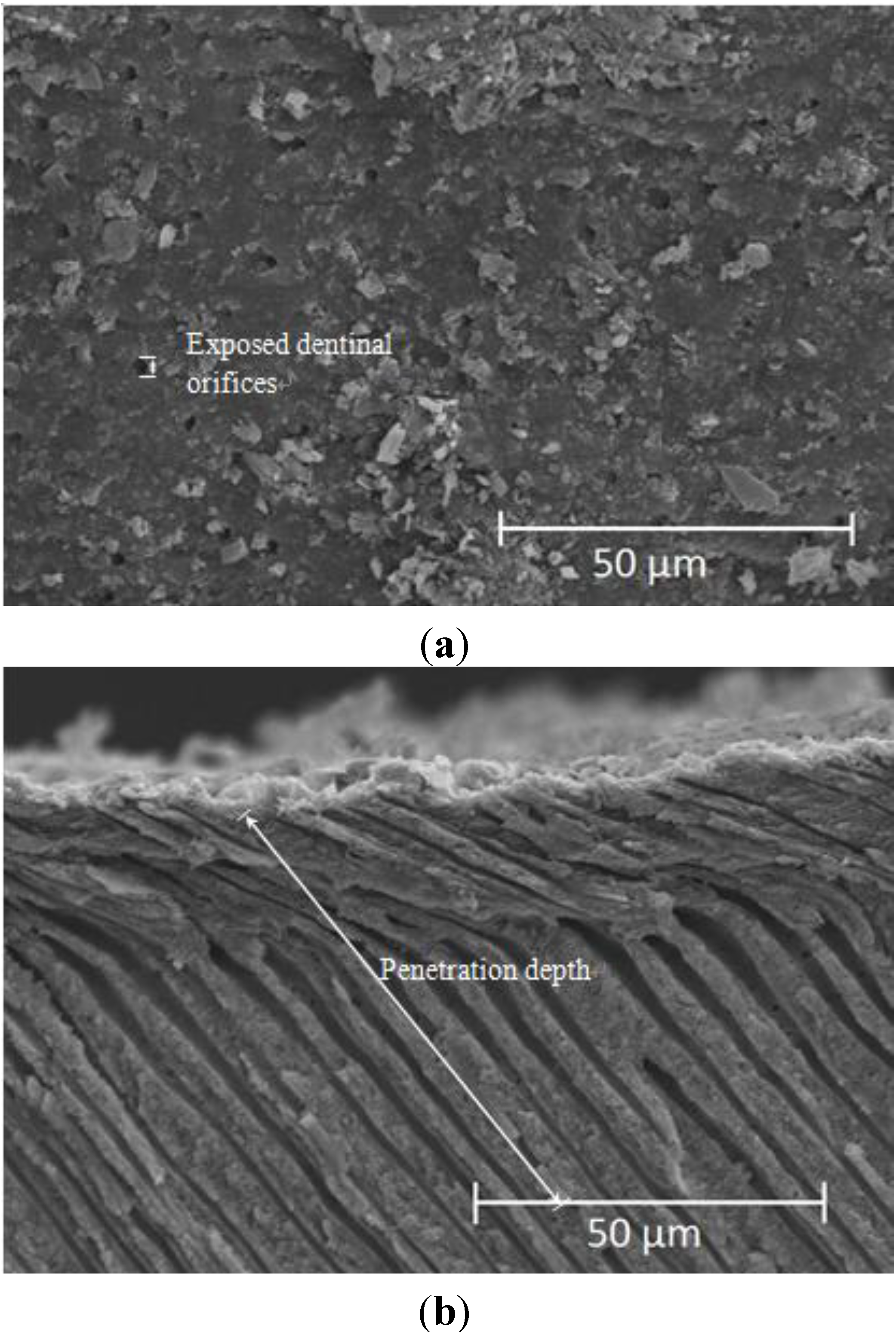
2.2. Effects of Commercialized PerioGlas® Brushed onto Dentin-Occlusion and Penetration in the Depths of Dentinal Tubules
| Occlusive agents mixed with varied hardening agents | PG | MBG | MBGNP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentrations of phosphate (wt %) | 20PA | 30PA | 40PA | 20PA | 30PA | 40PA | 20PA | 30PA | 40PA |
| Percentage of tubule occlusion (%) | 13% | 13% | 0 a | 50% | 68% | 65% | 0% | 0% | 25% |
| Average of penetration depth (μm) | 68.2 | 74.1 | 0 | 68.4 | 71.5 | 73.1 | 0 | 0 | 50.8 |
| Standard deviation of penetration depth (μm) | 2.0 | 2.1 | 0 | 9.2 | 11.1 | 9.4 | 0 | 0 | 7.2 |
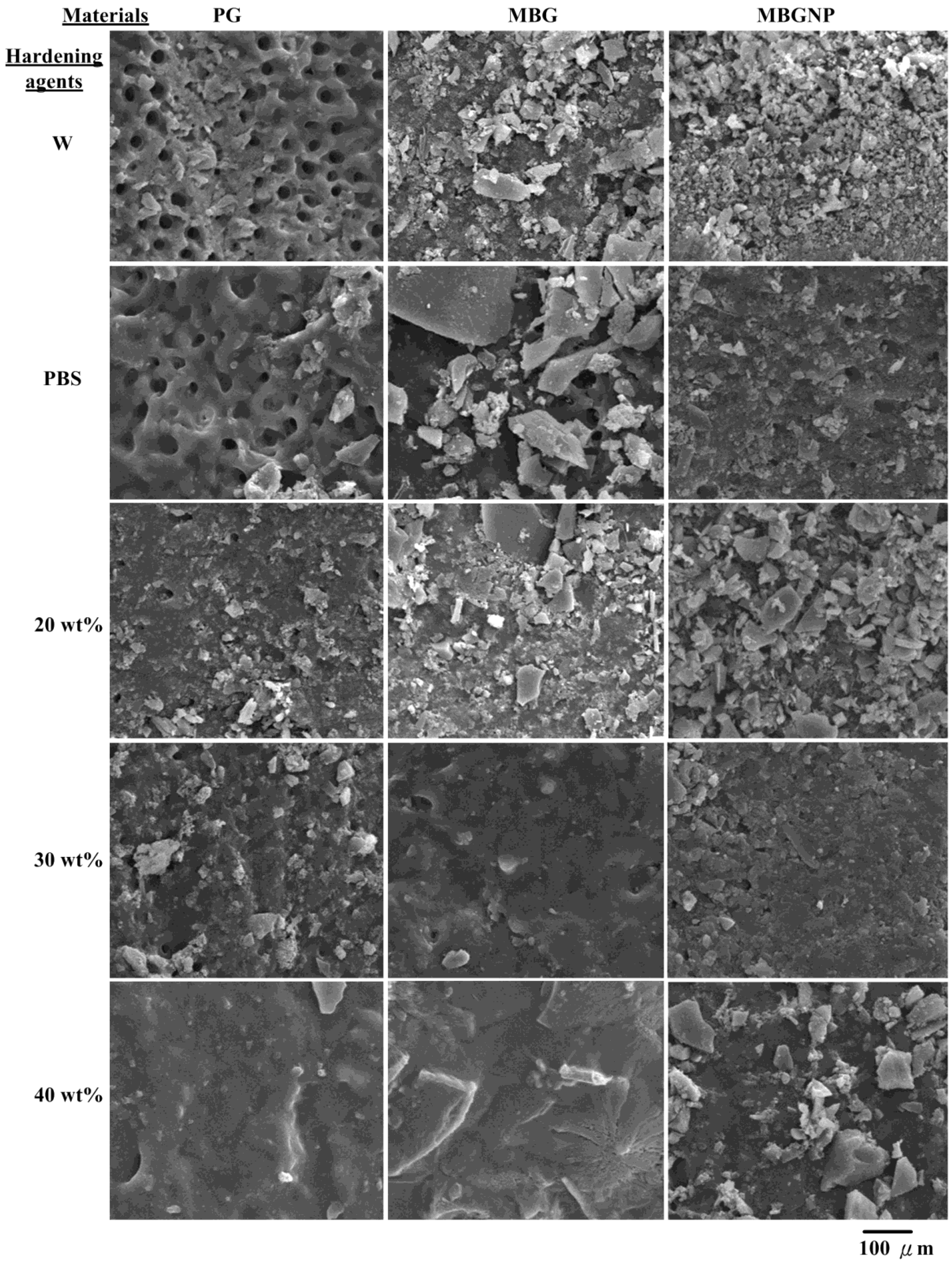
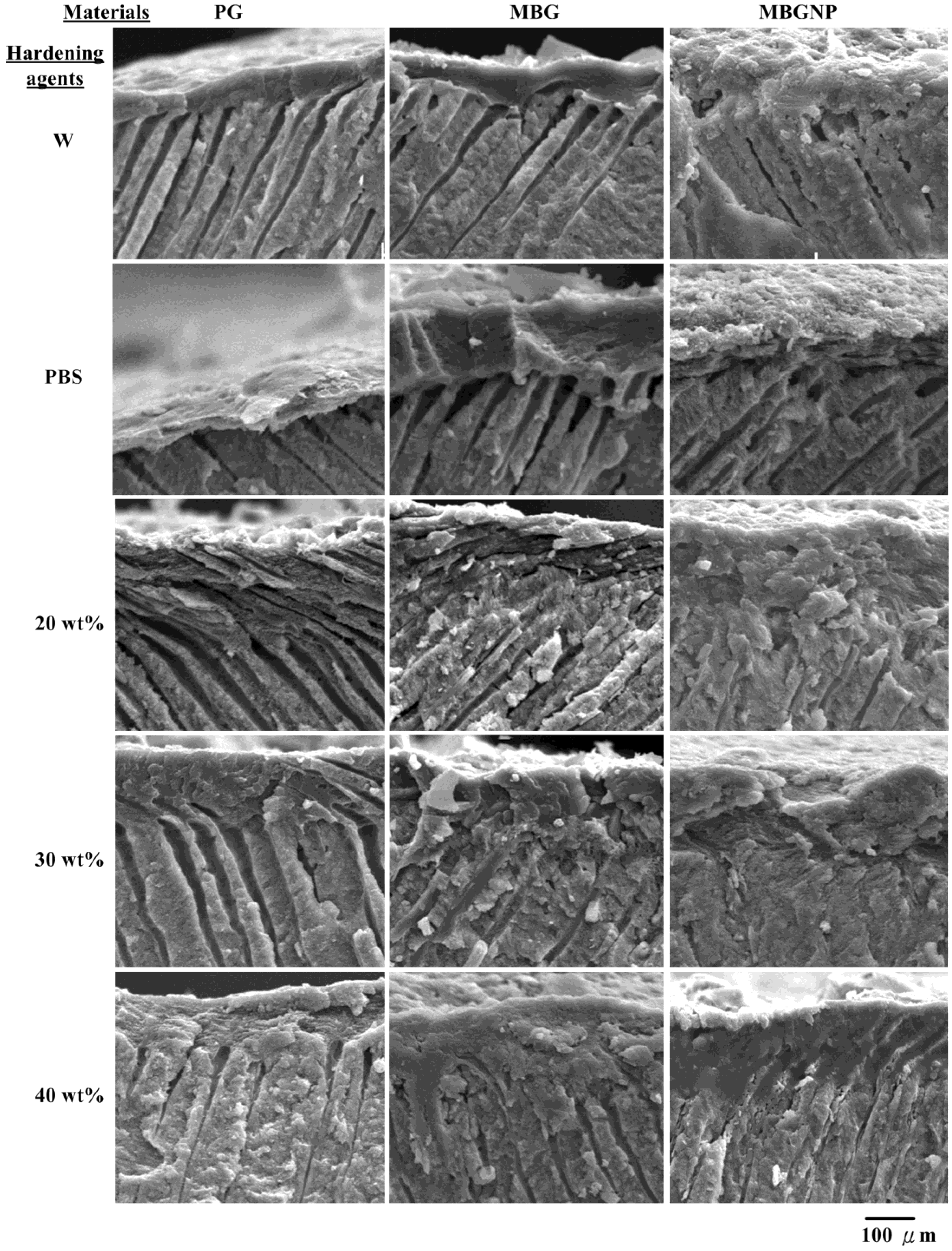
2.3. The Setting Properties of Meso-Porous Bioactive Glass (MBG) with Phosphate
2.4. Effects of MBG with Phosphorus on Penetration Depth of Dentinal Tubule
2.5. The Setting Properties of MBG without Phosphorus (MBGNP)
2.6. Effects of MBG without Phosphorus (MBGNP) on Penetrate in the Depth of Dentinal Tubule
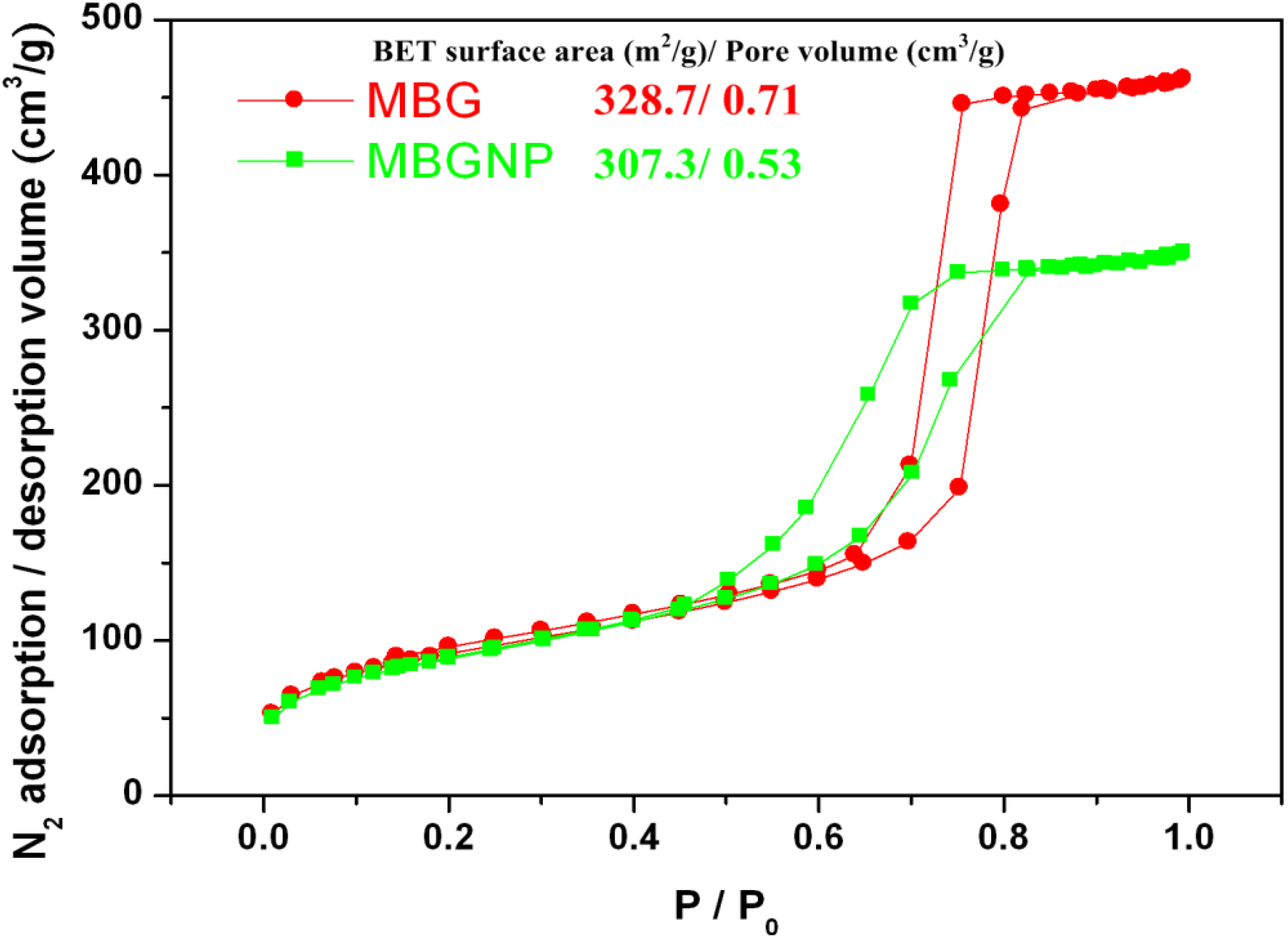
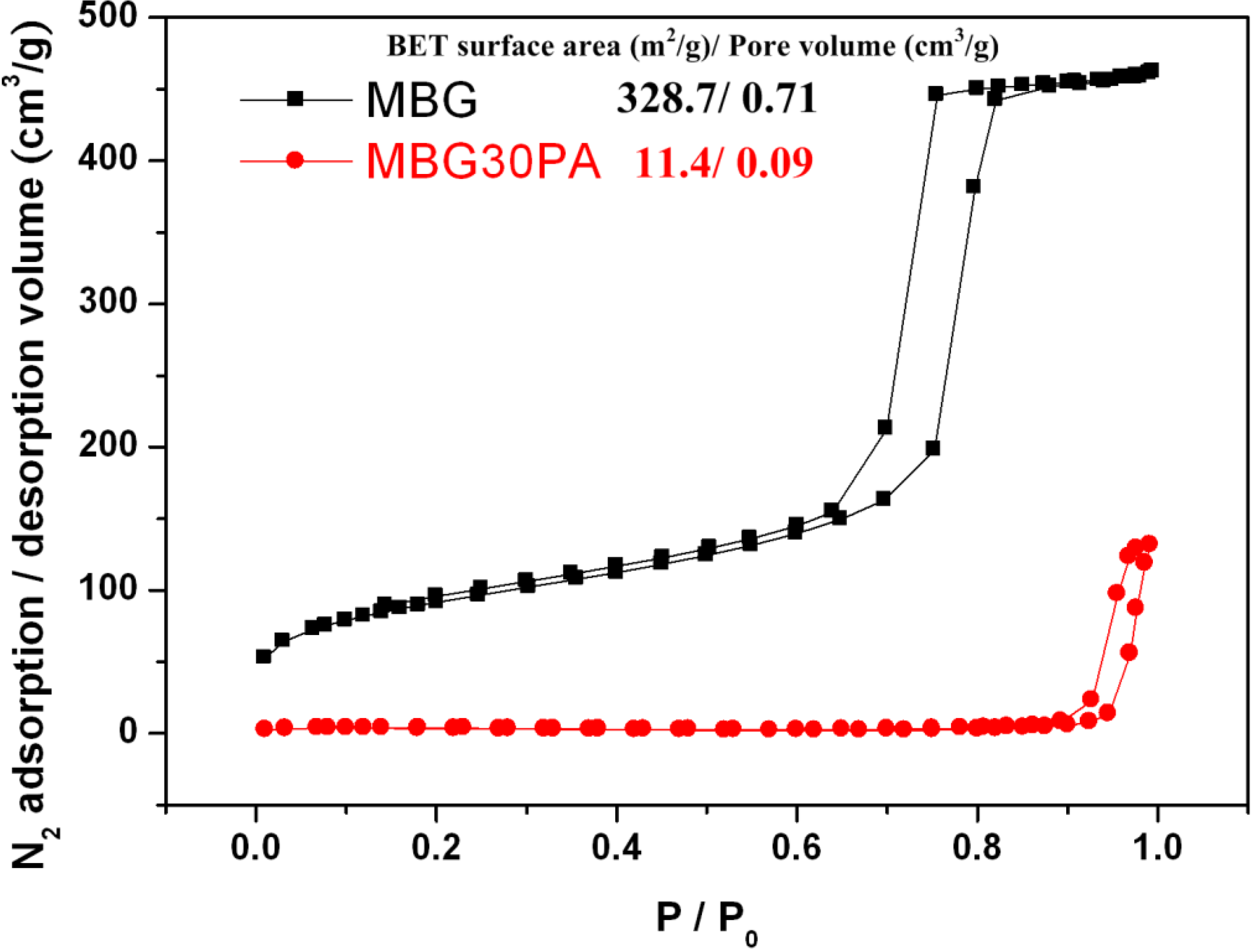
2.7. Nitrogen Adsorption-Desorption Texture Properties of MBG and MBGNP Mixed with 30 wt % Phosphoric Acid
3. Discussion
3.1. Commercialized PG and Synthesized MBG
3.2. Synthesized MBG with and without Element of Phosphorus
3.3. Textural Properties of MBG and MBGNP Reacted with 30 wt % Phosphoric acid
3.4. Summarized Discussions
3.5. Research Limitations
4. Experimental Section
4.1. Preparation of Materials
| Materials | formula | Company | Purity (%) |
|---|---|---|---|
| Pluronic F-127 | EO106–PO70–EO106 | BASF | – |
| Polyurethane foam | – | – | – |
| Calcium nitrate tetrahydrate | Ca(NO3)2·4H2O | SHOWA | 98.5 |
| Triethyl phosphate | C6H15O4P | HANAWA | 98.0 |
| Tetraethyl orthosilicate | C8H20O4Si | ACROS | 98.0 |
| Hydrochloric acid | HCl | Riedel-de Haen | 98.0 |
| Ethanol | C2H5OH | J. T. Baker | 99.9 |
| Phosphoric acid | H3PO4 | SHIMAKYU | 98.0 |
4.1.1. Compare groups of Commercialized Product
4.1.2. Powder Preparation of Meso-Pore Bioglass with Phosphate
4.1.3. Powder Preparation of Meso-Pore Bioglass without Phosphate
4.2. Remineralized Ability Evaluation to Penetrate into the Depth of Dentinal Tubule
4.3. Textural Characterization
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fischer, C.; Fischer, R.G.; Wennberg, A. Prevalence and distribution of cervical dentine hypersensitivity in a population in Rio de Janeiro, Brazil. J. Dent. 1992, 20, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Kanapka, J.A. Over-the counter dentifrices in the treatment of tooth hypersensitivity: Review of clinical studies. Dent. Clin. North. Am. 1990, 34, 545–560. [Google Scholar] [PubMed]
- Chabanski, M.B.; Gillam, D.G. Aetiology, prevalence and clinical features of cervical dentine sensitivity. J Oral Rehab. 1997, 24, 15–19. [Google Scholar] [CrossRef]
- Ritter, A.V.; Dias, W.L.; Miguez, P.; Caplan, D.J.; Swift, E.J., Jr. Treating cervical dentin hypersensitivity with fluoride varnish: A randomized clinical study. J. Am. Dent. Assoc. 2006, 137, 1013–1020. [Google Scholar] [CrossRef]
- Holland, G.R.; Narhi, M.N.; Addy, M.; Gangarosa, L.; Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J. Clin. Periodontol. 1997, 24, 808–813. [Google Scholar] [CrossRef]
- LeFleche, R.G.; Frank, R.M.; Steuer, P. The extent of the human odontoblast process as determined by transmission electron microscopy: The hypothesis of a retractable suspensor system. J. Biol. Buccale 1985, 13, 293–305. [Google Scholar] [PubMed]
- Eldarrat, A.H.; High, A.S.; Kale, G.M. In vitro analysis of “smear layer” on human dentine using ac-impedance spectroscopy. J. Dent. 2004, 32, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Berman, L.H. Dentinal sensation and hypersensitivity. A review of mechanisms and treatment alternatives. J. Periodontol. 1985, 56, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Scherman, A.; Jacobsen, P.L. Managing dentin hypersensitivity: What treatment to recommend to patients. J. Am. Dent. Assoc. 1992, 123, 57–61. [Google Scholar] [PubMed]
- Camilotti, V.; Zilly, J.; Busato, P.M.R.; Nassar, C.A.; Nassar, P.O. Desensitizing treatments for dentin hypersensitivity: A randomized, split-mouth clinical trial. Braz. Oral Res. 2012, 26, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kems, D.G.; Scheidt, M.J.; Pashley, D.H.; Horner, J.A.; Strong, S.L.; van Dyke, T.E. Dentinal tubule occlusion & root hypersensitivity. J. Periodontol. 1991, 62, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Vargas, M.A.; Denehy, G.E.; Boyer, D.B. Dentin desensitizing agents: SEM & X-ray microanalysis assessment. Am. J. Dent. 1997, 1, 21–26. [Google Scholar]
- Pereira, J.C.; Segala, A.D.; Gillam, D.G. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments-an in vitro study. Dent. Mater. 2005, 21, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Tsai, H.Y.; Tsai, Y.L.; Lan, W.H.; Lin, C.P. In vitro study of DP-bioglass paste for treatment of dentin hypersensitivity. Dent. Mater. J. 2005, 24, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Kang, S.H.; Wang, Y.L.; Lin, F.H.; Lin, C.P. In vitro study of dentinal tubule occlusion with sol-gel DP-bioglass for treatment of dentin hypersensitivity. Dent. Mater. J. 2007, 26, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.C.; Lee, B.S.; Kang, S.H.; Lin, F.H.; Lin, C.P. Cytotoxicity of DP-bioglass paste used for treatment of dentin hypersensitivity. J. Endod. 2007, 33, 451–454. [Google Scholar] [CrossRef]
- Stanley, H.R.; Hall, M.B.; Clark, A.E.; King, C.J.; Hench, L.L.; Berte, J.J. Using 45S5bioglass cones as endosseous ridge maintenance implants to prevent alveolarridge resorption: A 5-year evaluation. Int. J. Oral Maxill. Implants 1997, 12, 95–105. [Google Scholar]
- Wilson, J.; Clark, A.E.; Hall, M.; Hench, L.L. Tissue response to bioglass endosseousridge maintenance implants. J. Oral Implantol. 1993, 19, 295–302. [Google Scholar] [PubMed]
- Schepers, E.J.G.; Ducheyne, P. Bioactive glass particles of narrow size range forthe treatment of oral bone defects: A 1–24 month experiment with severalmaterials and particle sizes and size range. J. Oral Rehab. 1997, 24, 171–181. [Google Scholar]
- Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J. R. Soc. Interface 2009, 6, 349–360. [Google Scholar] [CrossRef]
- Chen, W.C.; Kung, J.C.; Chen, C.H.; Hsiao, Y.C.; Shih, C.J.; Chien, C.S. Effects of bioactive glass with and without mesoporous structures on desensitization in dentinal tubule occlusion. Appl. Surf. Sci. 2013, 283, 833–842. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Telle, E. On a theory of the van der waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chen, H.J.; Liu, H.C.; Kang, S.H.; Lee, B.S.; Lin, F.H.; Lin, H.P.; Lin, C.P. A novel mesoporous biomaterial for treating dentin hypersentitivity. J. Dent. Res. 2010, 89, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H. Smear layer: Overview of structure and function. Proceed. Finn. Dent. Soc. 1992, 88, 215–224. [Google Scholar]
- Hoppenbrouwers, P.M.M.; Driessens, F.C.M.; Borggreven, J.M.P.M. The vulnerability of unexposed human dentinal roots to demineralization. J. Dent. Res. 1986, 65, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.J.; Närhi, M.V.O.; Hakumäki, M.O.K. The excitability of dog pulp nerves in relation to the condition of the dentin surface. J. Endod. 1984, 10, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Dippel, M.; Borggreven, J.M.P.M.; Hoppenbrouwers, P.M.M. Morphology and and permeability of the dentinal smear layer. J. Prosthet. Dent. 1984, 52, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Gusman, H.; Gomes, B.P.; Simão, R.A. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J. Endod. 2011, 37, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H. The effects of acid etching on the pulpodentin complex. Oper. Dent. 1992, 17, 229–242. [Google Scholar] [PubMed]
- Retief, D.H.; Austin, J.C.; Fatti, L.P. Pulpal response to phosphoric acid. J. Oral Pathol. Med. 1974, 3, 114–122. [Google Scholar] [CrossRef]
- Stanley, H.R.; Going, R.E.; Chauncey, H.H. Human pulp response to acid pretreatment of dentin and to composite restoration. J. Am. Dent. Assoc. 1975, 21, 817–825. [Google Scholar]
- Cox, C.F.; Keall, C.L.; Keall, H.J.; Ostro, E.; Bergenholtz, G. Biocompatibility of surface-sealed dental materials against exposed pulps. J. Prosthet. Dent. 1987, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fuks, A.B.; Funnell, B.; Cleaton-Jones, P. Pulp response to a composite resin inserted in deep cavities with and without a surface seal. J. Prosthet. Dent. 1990, 63, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Dowell, P.; Addy, M. Dentine hypersensitivity—A review. Aetiology, symptoms and theories of pain production. J. Clin. Periodontol. 1983, 10, 341–350. [Google Scholar] [PubMed]
- Shih, C.J.; Chen, H.T.; Huang, L.F.; Lu, P.S. Synthesis and in vitro bioactivity of mesoporous bioactive glass scaffolds. Mater. Sci. Eng. C 2011, 30, 657–663. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, W.-C.; Chen, C.-H.; Kung, J.-C.; Hsiao, Y.-C.; Shih, C.-J.; Chien, C.-S. Phosphorus Effects of Mesoporous Bioactive Glass on Occlude Exposed Dentin. Materials 2013, 6, 5335-5351. https://doi.org/10.3390/ma6115335
Chen W-C, Chen C-H, Kung J-C, Hsiao Y-C, Shih C-J, Chien C-S. Phosphorus Effects of Mesoporous Bioactive Glass on Occlude Exposed Dentin. Materials. 2013; 6(11):5335-5351. https://doi.org/10.3390/ma6115335
Chicago/Turabian StyleChen, Wen-Cheng, Cheng-Hwei Chen, Jung-Chang Kung, Yu-Cheng Hsiao, Chi-Jen Shih, and Chi-Sheng Chien. 2013. "Phosphorus Effects of Mesoporous Bioactive Glass on Occlude Exposed Dentin" Materials 6, no. 11: 5335-5351. https://doi.org/10.3390/ma6115335






