Magnetic Fe3O4-Based Sandwich-Type Biosensor Using Modified Gold Nanoparticles as Colorimetric Probes for the Detection of Dopamine
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of DTSSP-AuNPs
2.3. Synthesis of Magnetic Fe3O4 Particles
2.4. Detection of DA
3. Results and Discussion
3.1. Mechanism of DA Detection
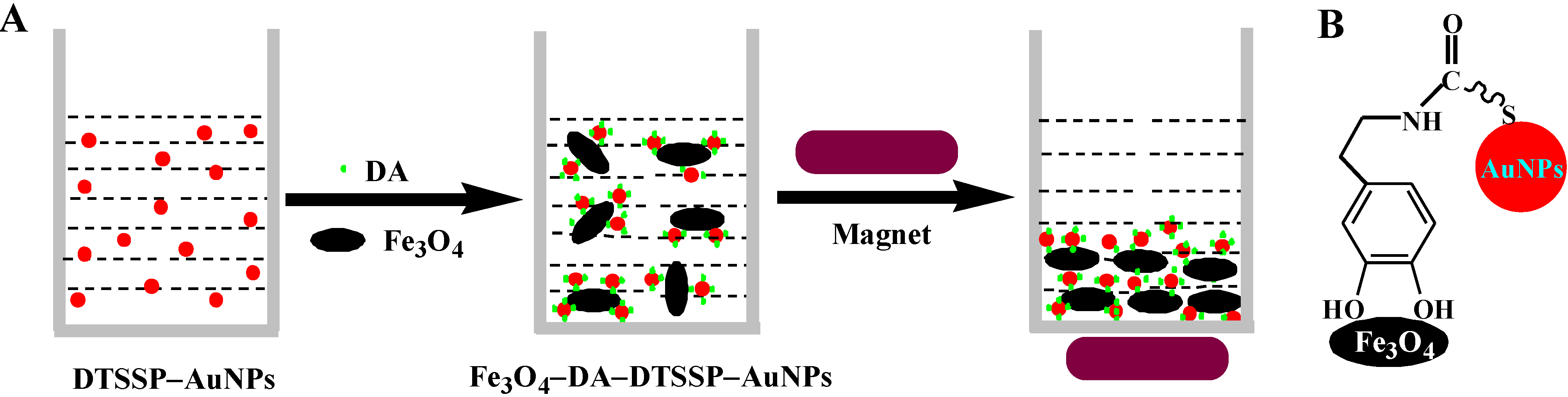
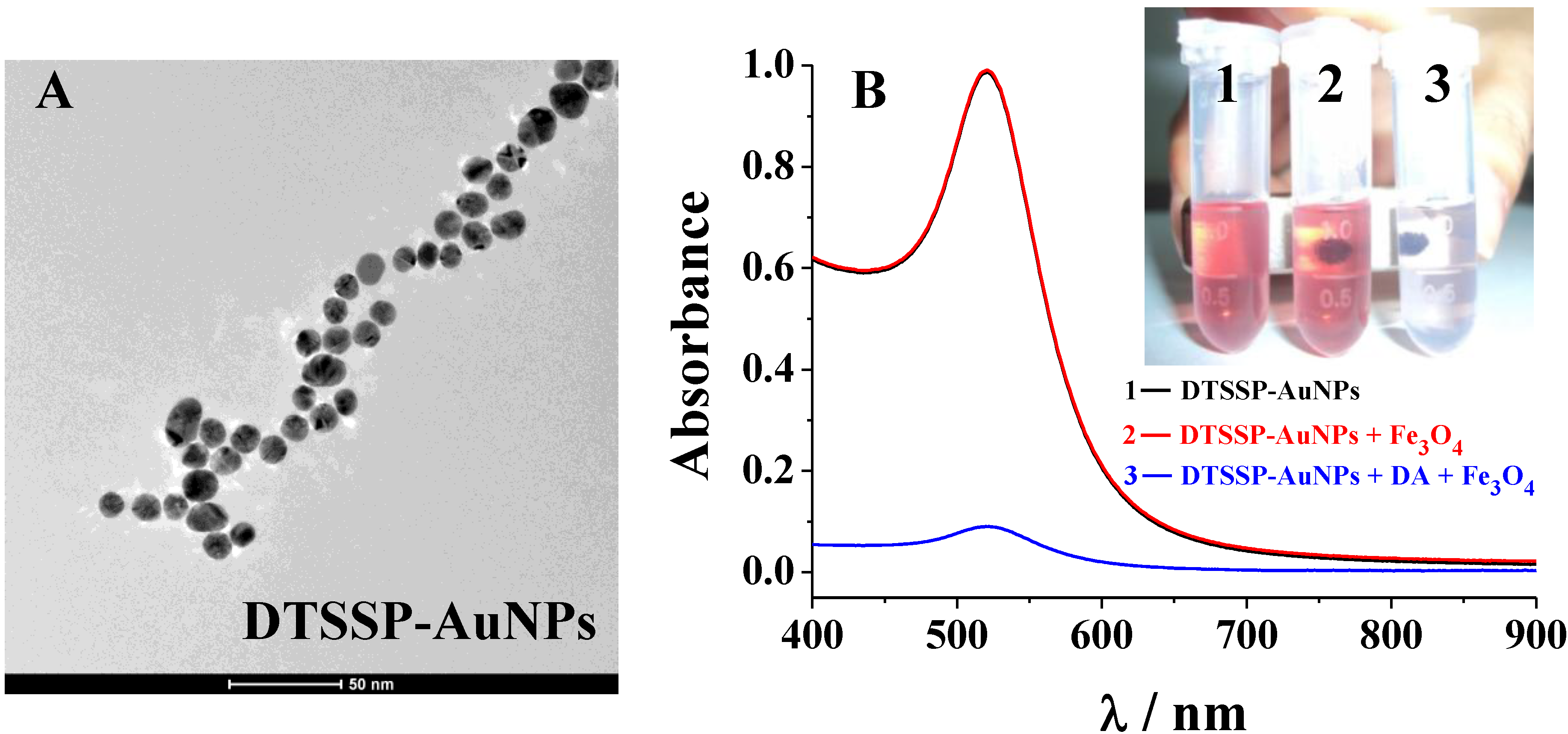
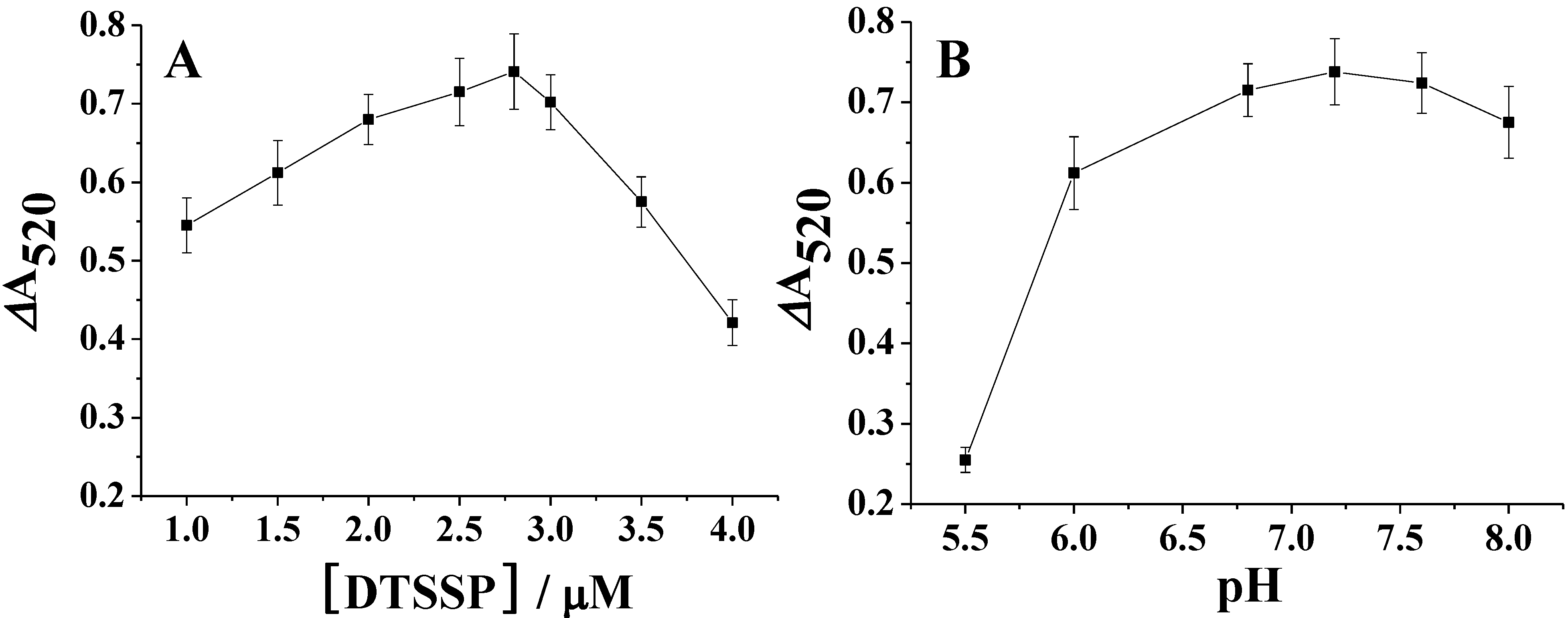
3.2. Colorimetric Assay for DA
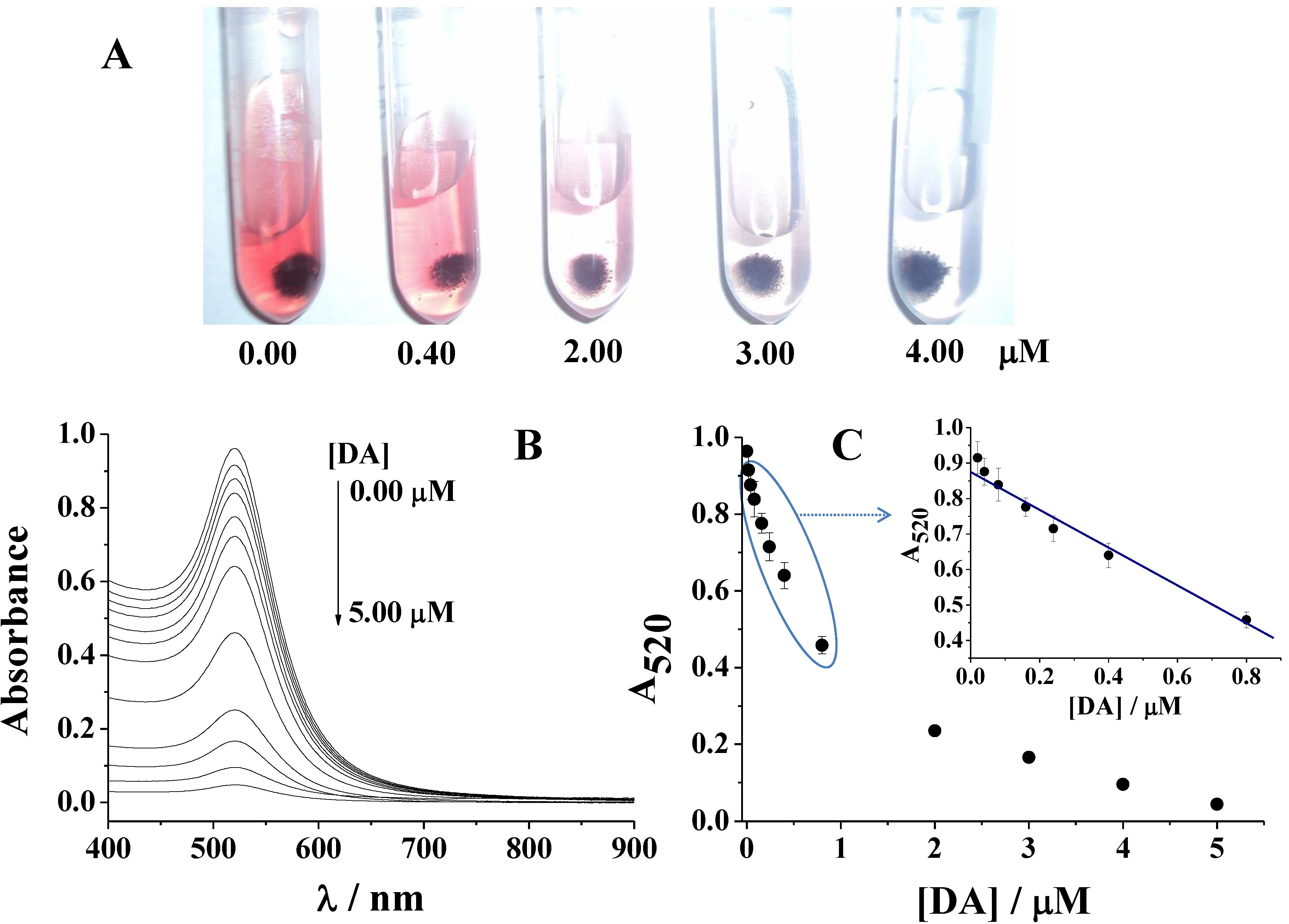
3.3. Sensitivity
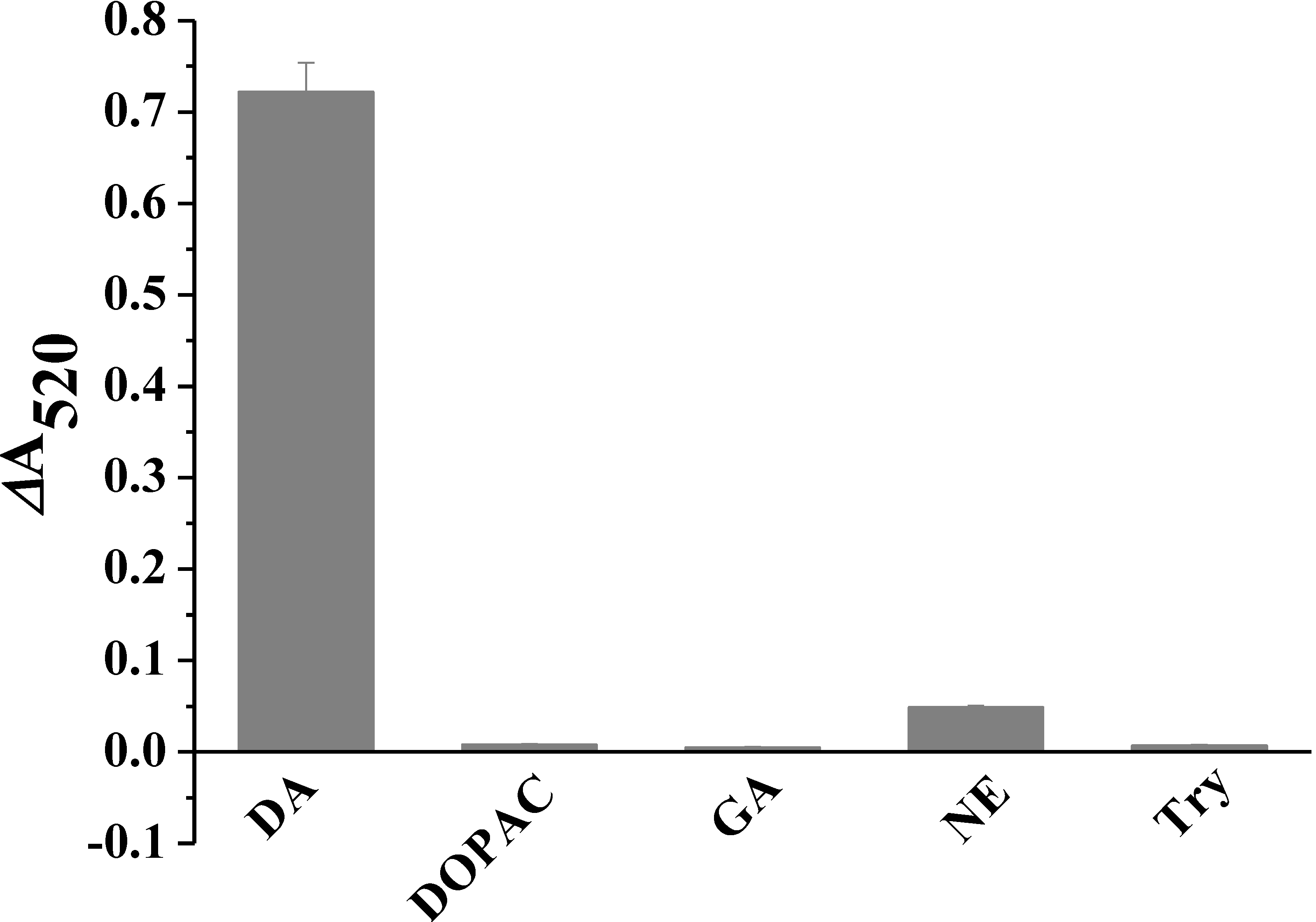
3.4. Selectivity
4. Conclusions/Outlook
Acknowledgments
Conflicts of Interest
References
- Yguerabide, J.; Yguerabide, E.E. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications—I. Theory. Anal. Biochem. 1998, 262, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, B.; Zhang, Y.; Zhang, Z. A simple and sensitive method for visual detection of heparin using positively-charged gold nanoparticles as colorimetric probes. Chem. Commun. 2011, 47, 12301–12303. [Google Scholar]
- Huang, C.-C.; Huang, Y.-F.; Cao, Z.; Tan, W.; Chang, H.-T. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal. Chem. 2005, 77, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zhu, A.; Luo, Y.; Tian, Y.; Yu, Y.; Shi, G. Sensitive and selective colorimetric visualization of cerebral dopamine based on double molecular recognition. Angew. Chem. Int. Ed. 2011, 50, 1837–1840. [Google Scholar] [CrossRef]
- Liu, C.-W.; Hsieh, Y.-T.; Huang, C.-C.; Lin, Z.-H.; Chang, H.-T. Detection of mercury(II) based on Hg(2+)-DNA complexes inducing the aggregation of gold nanoparticles. Chem. Commun. 2008, 2242–2244. [Google Scholar]
- Nam, J.-M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, B.; Li, J.; Wang, E.; Dong, S. Simple and sensitive aptamer-based colorimetric sensing of protein using unmodified gold nanoparticle probes. Chem. Commun. 2007, 3735–3737. [Google Scholar]
- Wilson, R. The use of gold nanoparticles in diagnostics and detection. Chem. Soc. Rev. 2008, 37, 2028–2045. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Addai-Mensah, J.; Losic, D. Magnetic-responsive delivery of drug carriers using titania nanotube arrays. J. Mater. Chem. 2012, 22, 6561–6563. [Google Scholar] [CrossRef]
- Perez, J.M. Iron oxide nanoparticles: Hidden talent. Nat. Nanotechnol. 2007, 2, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, S.I.; Huo, F.; Lee, J.-S.; Mirkin, C.A. Three-layer composite magnetic nanoparticle probes for DNA. J. Am. Chem. Soc. 2005, 127, 15362–15363. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Chen, W.; Liu, T.; Zhu, Y.; Jin, P.; Wang, L.; Liu, J.; Wei, Y.; Li, Y. Bifunctional Au-Fe3O4 nanoparticles for protein separation. ACS Nano 2007, 1, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.L.; Fleming, D.A.; Stone, M.B.; Schiffer, P.; Williams, M.E. Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett. 2004, 4, 719–723. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xie, J.; Ho, D.; Wang, C.; Kohler, N.; Walsh, E.G.; Morgan, J.R.; Chin, Y.E.; Sun, S. Au-Fe3O4 dumbbell nanoparticles as dual-functional probes. Angew. Chem. Int. Ed. 2008, 47, 173–176. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cai, Y.; Wang, T.; Shi, Y.; Jiang, G. Preparation of alkanethiolate-functionalized core/shell Fe3O4@Au nanoparticles and its interaction with several typical target molecules. Anal. Chem. 2008, 80, 9091–9096. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, G.K.; Wang, P.; Sreevatan, S.; Irudayaraj, J. Aptamer-mediated magnetic and gold-coated magnetic nanoparticles as detection assay for prion protein assessment. Biotechnol. Prog. 2007, 23, 1239–1244. [Google Scholar] [PubMed]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor. Anal. Chem. 2006, 78, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meyerhoff, M.E. Gold coated magnetic particles for solid phase immunoassays: Enhancing immobilized antibody binding efficiency and analytical performance. Anal. Chem. 2006, 78, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Jin, H.J.; Li, T.H.; Zheng, L. Fe3O4/Au magnetic nanoparticle amplification strategies for ultrasensitive electrochemical immunoassay of alfa-fetoprotein. Int. J. Nanomed. 2011, 6, 3259–3269. [Google Scholar] [CrossRef]
- Zhou, H.K.; Gan, N.; Li, T.H.; Cao, Y.T.; Zeng, S.L.; Zheng, L.; Guo, Z.Y. The sandwich-type electrochemiluminescence immunosensor for alpha-fetoprotein based on enrichment by Fe3O4-Au magnetic nano probes and signal amplification by CdS-Au composite nanoparticles labeled anti-AFP. Anal. Chim. Acta 2012, 746, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, J.; Li, S.; Yuan, B.; Han, H.; Jing, M.; Xia, N. Amplified voltammetric detection of dopamine using ferrocene-capped gold nanoparticle/streptavidin conjugates. Biosens. Bioelectron. 2013, 41, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, K.; Lee, H.-Y.; Xu, C.; Hsu, A.R.; Peng, S.; Chen, X.; Sun, S. Ultrasmall c(RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin αvβ3-rich tumor cells. J. Am. Chem. Soc. 2008, 130, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.W.; Yang, Z.M.; Gao, J.H.; Chang, C.K.; Xu, B. Heterodimers of nanoparticles: Formation at a liquid-liquid interface and particle-specific surface modification by functional molecules. J. Am. Chem. Soc. 2005, 127, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, Q.; He, J.; Li, T.; Zhao, Y.; Cai, Y.; Du, B.; Qian, Z.; Yang, M. Electrochemical immunosensors for cancer biomarker with signal amplification based on ferrocene functionalized iron oxide nanoparticles. Biosens. Bioelectron. 2011, 26, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D.; Reveles, J.U.; Khanna, S.N.; Carpenter, E.E. Reactive nature of dopamine as a surface functionalization agent in iron oxide nanoparticles. J. Am. Chem. Soc. 2007, 129, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.D.; Xu, C.J.; Xie, J.; Yang, Z.Y.; Sun, S.H. pH controlled release of chromone from chromone-Fe3O4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 14436–14437. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar] [CrossRef] [PubMed]
- Durocher, S.; Rezaee, A.; Hamm, C.; Rangan, C.; Mittler, S.; Mutus, B. Disulfide-linked, gold nanoparticle based reagent for detecting small molecular weight thiols. J. Am. Chem. Soc. 2009, 131, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Deng, D.; Zhang, L.; Yuan, B.; Jing, M.; Du, J.; Liu, L. Sandwich-type electrochemical biosensor for glycoproteins detection based on dual-amplification of boronic acid-gold nanoparticles and dopamine-gold nanoparticles. Biosens. Bioelectron. 2013, 43, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, C.Z.; Li, Y.F.; Ling, J.; Liu, Y.L.; Fei, L.R.; Xie, J.P. Magnetic particle-based sandwich sensor with DNA-modified carbon nanotubes as recognition elements for detection of DNA hybridization. Anal. Chem. 2008, 80, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.J.; Hu, P.P.; Wu, X.D.; Zou, Y.L.; Chen, L.Q.; Peng, L.; Ling, J.; Zhen, S.J.; Zhan, L.; Li, Y.F.; et al. Sensitive discrimination and detection of prion disease-associated isoform with a dual-aptamer strategy by developing a sandwich structure of magnetic microparticles and quantum dots. Anal. Chem. 2010, 82, 9736–9742. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, D.; Xing, Y.; Li, S.; Yuan, B.; Chen, J.; Xia, N. Activity analysis of the carbodiimide-mediated amine coupling reaction on self-assembled monolayers by cyclic voltammetry. Electrochim. Acta 2013, 89, 616–622. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.; Bai, Y.; Wei, W.; Xia, N.; Du, Y. Magnetic Fe3O4-Based Sandwich-Type Biosensor Using Modified Gold Nanoparticles as Colorimetric Probes for the Detection of Dopamine. Materials 2013, 6, 5690-5699. https://doi.org/10.3390/ma6125690
Wang Z, Bai Y, Wei W, Xia N, Du Y. Magnetic Fe3O4-Based Sandwich-Type Biosensor Using Modified Gold Nanoparticles as Colorimetric Probes for the Detection of Dopamine. Materials. 2013; 6(12):5690-5699. https://doi.org/10.3390/ma6125690
Chicago/Turabian StyleWang, Zhiyong, Yanyan Bai, Wenchao Wei, Ning Xia, and Yuhui Du. 2013. "Magnetic Fe3O4-Based Sandwich-Type Biosensor Using Modified Gold Nanoparticles as Colorimetric Probes for the Detection of Dopamine" Materials 6, no. 12: 5690-5699. https://doi.org/10.3390/ma6125690



