Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors
Abstract
:1. Introduction
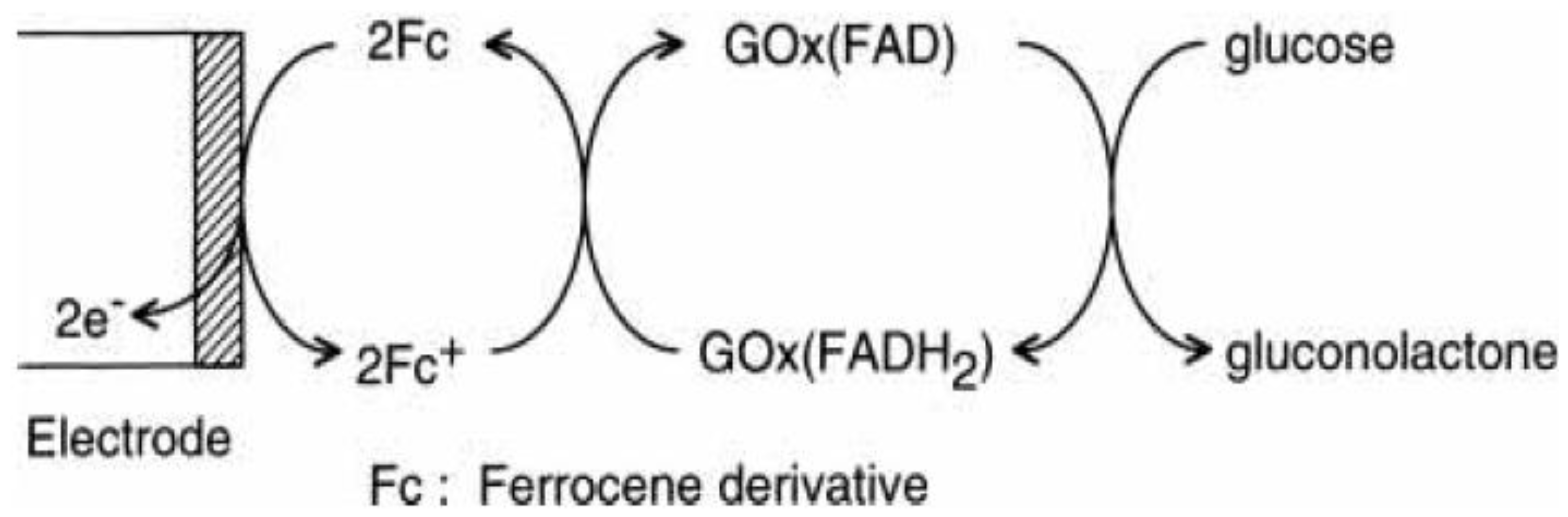
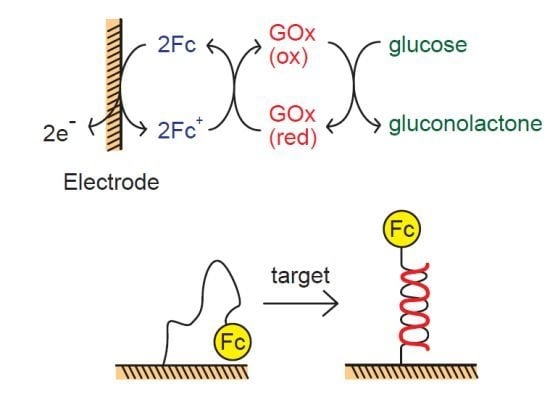
2. Fc-Containing Thin Films
2.1. In Situ Polymerized Fc Films
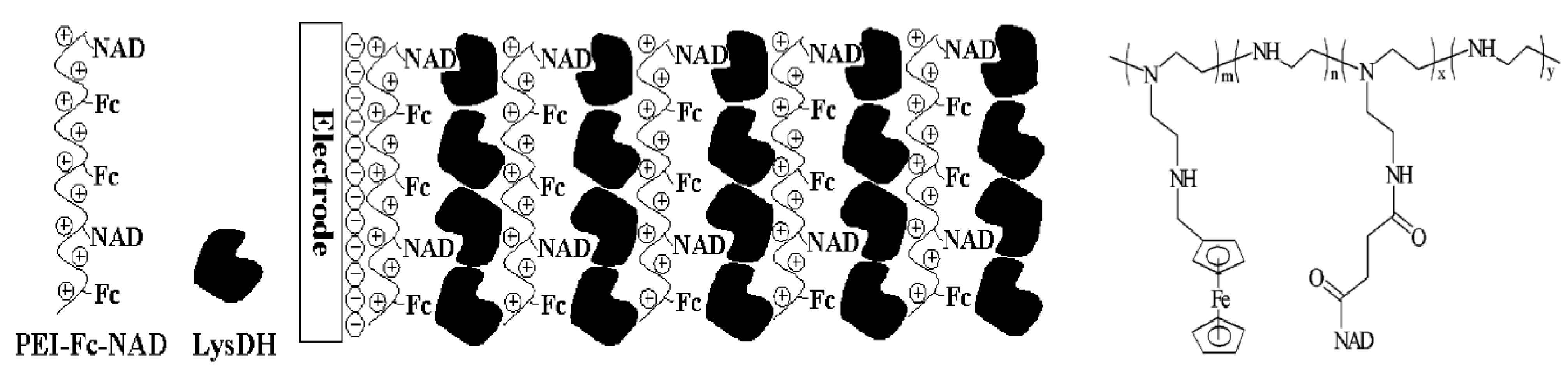
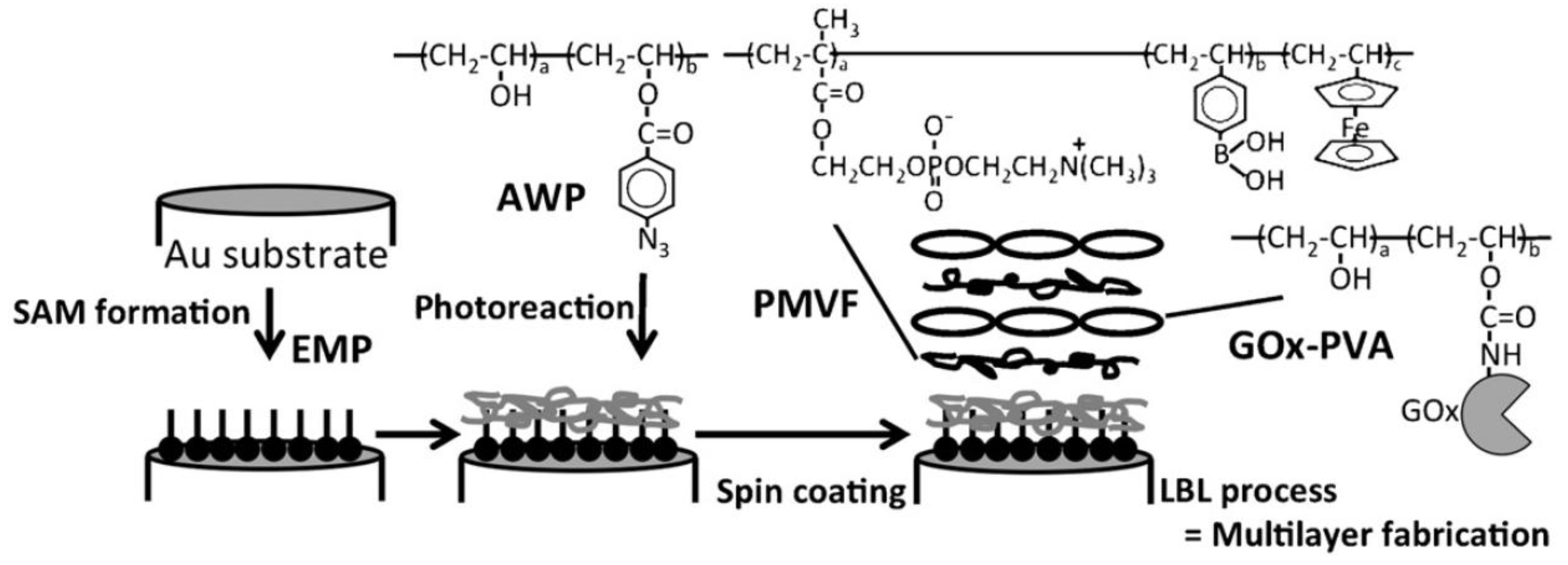
2.2. LbL-Deposited Fc Films
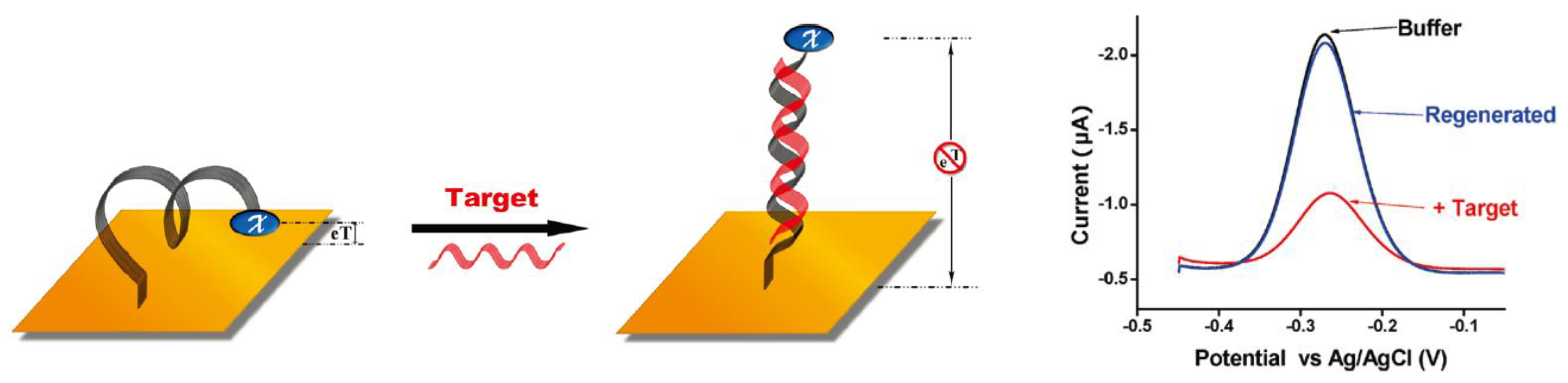
2.3. Host-Guest Complexation and Molecular Recognition

2.4. Miscellaneous
3. Fc-Modified Nanoparticles
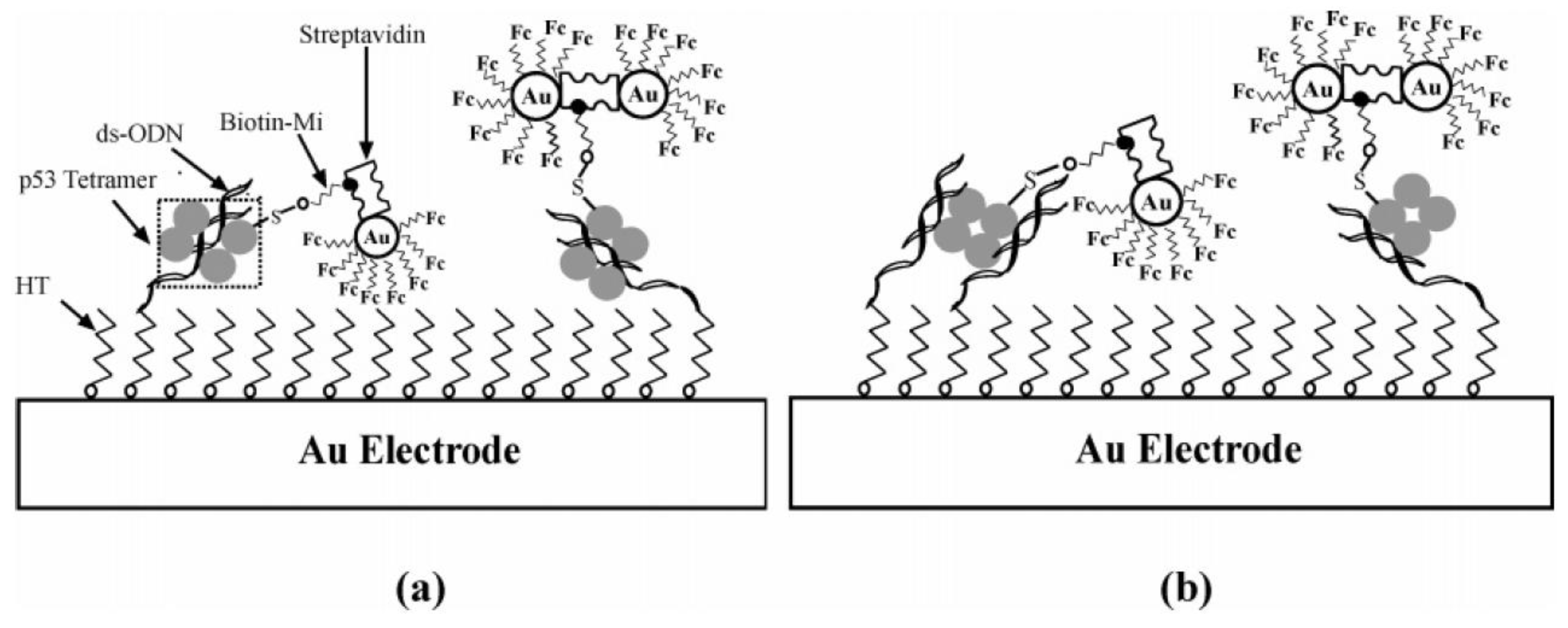
3.1. Fc-Modified Au Nanoparticles
3.2. Fc-Modified Inorganic Porous Materials
3.3. Fc-Modified Composite Nanoparticles

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ricci, F.; Palleschi, G. Sensors and biosensors preparation, optimization and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.K.; Vatsyayan, P.; Goswami, P.; Minteer, S.D. Recent advances in material science for developing enzyme electrodes. Biosens. Bioelectron. 2009, 24, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, K.; Weng, A.; Lei, Y.; Lin, L.; Chen, W.; Lin, X.; Chen, Y. Development of electrochemical DNA biosensors. Trend Anal. Chem. 2012, 37, 101–111. [Google Scholar] [CrossRef]
- Moyo, M.; Okonkwo, J.O.; Agyei, N.M. Recent advances in polymeric materials used as electron mediators and immobilizing materials in developing enzyme electrodes. Sensors 2012, 12, 923–953. [Google Scholar] [CrossRef] [PubMed]
- Kotanen, C.N.; Moussy, F.G.; Carrara, S.; Guiseppi-Elie, A. Implantable enzyme amperometric biosensors. Biosens. Bioelectron. 2012, 35, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Amer, W.A.; Wang, L.; Amin, A.M.; Ma, L.; Yu, H. Recent progress in the synthesis and applications of some ferrocene derivatives and ferrocene-based polymers. J. Inorg. Organomet. Polym. 2010, 20, 605–615. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hoshi, T.; Anzai, J. Glucose and lactate biosensors prepared by a layer-by-layer deposition of concanavalin A and mannose-labeled enzymes: Electrochemical response in the presence of electron mediators. Chem. Pharm. Bull. 2001, 49, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Zuo, X.; Yang, R.; Xia, F.; Plaxco, K.W.; White, R.J. Comparing the properties of electrochemical-based DNA sensors employing different redox tags. Anal. Chem. 2009, 81, 9109–9113. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-J.; Lee, S.-K.; Lee, J.-Y.; Kim, J.-H.; Lim, D.-S. Electrochemical sensor based on Au nanoparticles decorated boron-doped diamond electrode using ferrocene-tagged aptamer for proton detection. J. Electroanal. Chem. 2012, 677–680, 139–144. [Google Scholar] [CrossRef]
- Hiratsuka, A.; Kojima, K.; Muguruma, H.; Lee, K.; Suzuki, H.; Karube, I. Electron transfer mediator micro-biosensor fabrication by organic plasma process. Biosens. Bioelctron. 2005, 21, 957–964. [Google Scholar] [CrossRef]
- Muguruma, H.; Uehara, H. Electron transfer mediated biosensor with plasma-polymerized film containing redox site. IEICE Trans. Electron. 2006, E89-C, 1781–1785. [Google Scholar] [CrossRef]
- Bean, L.S.; Heng, L.Y.; Yamin, B.M.; Ahmad, M. Photocurable ferrocene-containing poly(2-hydroxyethyl methacrylate) films for mediated amperometric glucose biosensor. Thin Solid Films 2005, 477, 104–110. [Google Scholar] [CrossRef]
- Bean, L.S.; Heng, L.Y.; Yamin, B.M.; Ahmad, M. The electrochemical behavior of ferrocene in a photocurable poly(methyl methacrylate-co-2-hydroxyethyl methacrylate) film for a glucose biosensor. Bioelectrochemisty 2005, 65, 157–162. [Google Scholar] [CrossRef]
- Nagel, B.; Gajovic-Eichelmann, N.; Scheller, F.W.; Katterle, M. Ionic topochemical tuned biosensor interface. Langmuir 2010, 26, 9088–9093. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S. Biosensors based on electropolymerized films: New trends. Anal. Bioanal. Chem. 2003, 377, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, Y.; Chiba, S.; Ikezoe, H.; Anzai, J. Polypyrrole-supported graphite felt for acetylene coupling reaction in solid phase. Synlett 2004, 14, 2513–2516. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Chiba, S.; Anzai, J. Amperometric determination of optically active 1-phenylethenol using chiral nitroxyl radical-modified polypyrrole films prepared by electrochemical polymerization. J. Electroanal. Chem. 2004, 566, 257–262. [Google Scholar] [CrossRef]
- Geetha, S.; Rao, C.R.K.; Vijayan, M.; Trivedi, D.C. Biosensing and drug delivery by polypyrrole. Anal. Chim. Acta 2006, 568, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Rozlosnik, N. New directions in medical biosensors employing poly(3,4-ethylenedioxy thiophene) derivative-based electrodes. Anal. Bioanal. Chem. 2009, 395, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kathurouju, P.K.; Jampana, N. Polypyrrole based amperometric glucose biosensors. Sens. Actuators B 2009, 143, 430–443. [Google Scholar] [CrossRef]
- Higgins, M.J.; Molino, P.J.; Yue, Z.; Wallace, G.G. Organic conducting polymer-protein interactions. Chem. Mater. 2012, 24, 828–839. [Google Scholar] [CrossRef]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef]
- Chen, J.; Burrell, A.K.; Collis, G.E.; Officer, D.L.; Swiegers, G.F.; Ťoo, C.O.; Wallace, G.G. Preparation, characterisation and biosensor application of conducting polymers based on ferrocene substituted thiophene and terthiophene. Electrochim. Acta 2002, 47, 2715–2724. [Google Scholar] [CrossRef]
- Fang, B.; Jiao, S.; Li, M.; Qu, Y.; Jiang, X. Label-free electrochemical detection of DNA using ferrocene-containing cationic polythiophene and PNA probes on nanogold modified electrodes. Biosens. Bioelectron. 2008, 23, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Lepage, P.H.; Peytavi, R.; Bergeron, M.G.; Leclerc, M. Amplification strategy using aggregates of ferrocene-containing cationic polythiophene for sensitive and specific electrochemical detection of DNA. Anal. Chem. 2011, 83, 8086–8092. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Anzai, J. Use of polymeric indicator for electrochemical DNA sensors: Poly(4-vinylpyridine) derivative bearing [Os(5,6-dimethyl-1,10- phenanthroline)2Cl]2+. Anal. Chem. 2004, 76, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Şenel, M. Construction of reagentless glucose biosensor based on ferrocene conjugated polypyrrole. Synthe. Met. 2011, 161, 1861–1868. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Sukhishvili, S.A. Layer-by-layer hydrogen-bonded polymer films: From fundamentals to applications. Adv. Mater. 2009, 21, 3053–3065. [Google Scholar] [CrossRef]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Stuart, M.A.C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer construction of protein architectures through avidin-biotin and lectin-sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.K.; Ramani, K.P.; Singh, S.S.; Tekade, A.R.; Chatap, V.K.; Patil, G.B.; Bari, S.B. Stimuli-sensitive layer-by-layer (LbL) self-assembly systems: Targeting and biosensory applications. J. Control. Release 2013, 166, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sakr, O.S.; Borchard, G. Encapsulation of enzymes in layer-by-layer (LbL) structures: Latest advances and applications. Biomacromolecules 2013, 14, 2117–2135. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Akase, S.; Anzai, J. Preparation of multilayer thin films containing avidin through sugar-lectin interactions and their binding properties. Langmuir 2002, 18, 7024–7028. [Google Scholar] [CrossRef]
- Sato, K.; Kodama, D.; Naka, Y.; Anzai, J. Electrochemically induced disintegration of layer-by-layer-assembled thin films composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2006, 7, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suzuki, I.; Anzai, J. Preparation of polyelectrolyte-layered assemblies containing cyclodextrin and their binding properties. Langmuir 2003, 19, 7406–7412. [Google Scholar] [CrossRef]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayer. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Takita, R.; Yoshida, K.; Anzai, J. Redox properties of ferricyanide ion on layer-by-layer deposited poly(glutamic acid) film-coated electrodes and its use for electrocatalytic sensing of ascorbic acid. Sens. Actuators B 2007, 121, 54–60. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.-J.; Chen, H.-Y. Electrochemical biosensors based on layer-by-layer assemblies. Electroanalysis 2006, 18, 1737–1748. [Google Scholar] [CrossRef]
- El-Hashani, A.; Toutianoush, A.; Tieke, B. Layer-by-layer assembled membranes of protonated 18-azacrown-6 and polyvinylsulfate and their application for highly efficient anion separation. J. Phys. Chem. B 2007, 111, 8582–8588. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Anzai, J. Stimuli-sensitive thin films prepared by a layer-by-layer deposition of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Langmuir 2005, 21, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hodak, J.; Etchenique, R.; Calvo, E.J. Layer-by-layer self-assembly of glucose oxidase with a poly(allylamine)ferrocene redox mediator. Langmuir 1997, 13, 2708–2716. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, W.; Niu, Y.; Sun, C. Multilayered construction of glucose oxidase and poly(allylamine)ferrocene on gold electrodes by means of layer-by-layer covalent attachment. Sens. Actuators B 2004, 101, 387–393. [Google Scholar] [CrossRef]
- Fushimi, T.; Oda, A.; Ohkita, H.; Ito, S. Fabrication and electrochemical properties of layer-by-layer deposited ultrathin polymer films bearing ferrocene moieties. Thin Solid Films 2005, 484, 318–323. [Google Scholar] [CrossRef]
- Liu, A.; Kashiwagi, Y.; Anzai, J. Polyelectrolyte multilayer films containing ferrocene: Effects of polyelectrolyte type and ferrocene contents in the film on the redox properties. Electroanalysis 2003, 15, 1139–1142. [Google Scholar] [CrossRef]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer film-coated electrodes: Electrocatalytic determination of ascorbic acid and use of inner blocking layers to improve the upper detection limit of the electrodes. Anal. Bioanal. Chem. 2004, 380, 98–103. [Google Scholar] [PubMed]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer films: Effects of electrochemically inactive surface layers on the redox properties. Langmuir 2003, 19, 4043–4046. [Google Scholar] [CrossRef]
- Sato, H.; Anzai, J. Preparation of layer-by-layer thin films composed of DNA and ferrocene-bearing poly(amine)s and their redox properties. Biomacromolecules 2006, 7, 2072–2076. [Google Scholar] [CrossRef] [PubMed]
- Suye, S.; Matsuura, T.; Kimura, T.; Zheng, H.; Hori, T.; Amano, Y.; Katayama, H. Amperometric DNA sensor using gold electrode modified with polymerized mediator by layer-by-layer adsorption. Microelectron. Eng. 2005, 81, 441–447. [Google Scholar] [CrossRef]
- Suye, S.; Zheng, H.; Okada, H.; Hori, T. Assembly of alternating polymerized mediator, polymerized coenzyme, and enzyme modified electrode by layer-by-layer adsorption technique. Sens. Actuators B 2005, 108, 671–675. [Google Scholar] [CrossRef]
- Zheng, H.; Hirose, Y.; Kimura, T.; Suye, S.; Hori, T.; Katayama, H.; Arai, J.; Kawakami, R.; Ohshima, T. L-proline sensor based on layer-by-layer immobilization of thermostable dye-linked L-proline dehydrogenase and polymerized mediator. Sci. Technol. Adv. Mater. 2006, 7, 243–248. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, J.; Okezaki, Y.; Suye, S. Construction of L-lysine sensor by layer-by-layer adsorption of L-lysine dehydrogenase and ferrocene-labeled high molecular weight coenzyme derivative on gold electrode. Electroanalysis 2008, 24, 2685–2691. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Han, S.; Shi, L.; Xu, G. Self-assembly of gold nanoparticles/electroactive polyelectrolyte multilayer films for tunable electrocatalysis. Electroanalysis 2010, 22, 963–968. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Yin, J.; Li, B.; Zhou, M.; Dong, S.; Wang, E. Solid-state probe based on electrochemical aptasensor for cocaine: A potentially convenient, sensitive, repeatable, and integrated sensing platform for drugs. Anal. Chem. 2010, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, C.; Yin, J.; Li, B.; Zhou, M.; Wang, E.; Dong, S. Layer-by-layer electrochemical biosensor with aptamer-appended active polyelectrolyte multilayer for sensitive protein determination. Biosens. Bioelectron. 2010, 25, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Konno, T.; Takai, M.; Ishihara, K. Fabrication of polymeric electron-transfer mediator/enzyme hydrogel multilayer on an Au electrode in a layer-by-layer process. Biosens. Bioelectron. 2012, 34, 191–196. [Google Scholar] [CrossRef]
- Hempenius, M.A.; Péter, M.; Robins, N.S.; Kooij, E.S.; Vancso, G.J. Water-soluble poly(ferrocenylsilanes) for supramolecualr assemblies by layer-by-layer deposition. Langmuir 2002, 18, 7629–7634. [Google Scholar] [CrossRef]
- Ma, Y.; Hempenius, M.A.; Vancso, G.J. Electrostatic assembly with poly(ferrocenylsilanes). J. Inorg. Organomet. Polym. Mater. 2007, 17, 3–18. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, W.; Hempenius, M.A.; Möhwald, H.; Vancso, G.J. Redox-controlled molecular permeability of composite-wall microcapsules. Nat. Mater. 2006, 5, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, W.; Kooij, E.S.; Hempenius, M.A.; Möhwald, H.; Vancso, G.J. Supramolecular assembly of water-soluble poly(ferrocenylsilanes): Multilayer structures on flat interfaces and permeability of microcapsules. Soft Matter 2007, 3, 889–895. [Google Scholar] [CrossRef]
- Song, J.; Janczewski, M.Y.; Hempenius, M.; Xu, J.; Vancso, G.J. Redox-controlled release of molecular payloads from multilayered organometallic polyelectrolyte films. J. Mater. Chem. B 2013, 1, 828–834. [Google Scholar] [CrossRef]
- Sui, X.; Feng, X.; Song, J.; Hempenius, M.A.; Vancso, G.J. Electrochemical sensing by surface-immobilized poly(ferrocenylsilane) grafts. J. Mater. Chem. 2012, 22, 11261–11267. [Google Scholar] [CrossRef]
- Ludden, M.J.W.; Peter, M.; Reinhoudt, D.N.; Huskens, J. Attachment of streptavidin to β-cyclodextrin molecular printboards via orthogonal host-guest and protein-ligand interactions. Small 2006, 2, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, R.; Cao, R.; Fragoso, A. Supramolecular chemistry of cyclodextrin in enzyme technology. Chem. Rev. 2007, 107, 3088–3116. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Torréns, M.; Alakulppi, N.; Strömbom, L.; Fragoso, A.; O’Sulivan, C.K. Amperometric supramolecular genosensor self-assembled on cyclodextrin-modified surfaces. Electrochem. Commun. 2011, 13, 578–581. [Google Scholar] [CrossRef]
- Fragoso, A.; Ortiz, M.; Sanroma, B.; O’Sulivan, C.K. Multilayered catalytic biosensor self-assembled on cyclodextrin-modified surfaces. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 355–360. [Google Scholar] [CrossRef]
- Scott, S.J.; Mortimer, R.J.; McKenzie, K.J.; Marken, F. Mesoporous TiO2 carboxymethyl-γ-cyclodextrin multi-layer host films: Effects on adsorption and electrochemistry of 1,1ʹ-ferrocenedimethanol. Analyst 2005, 130, 358–363. [Google Scholar] [CrossRef]
- Ortiz, M.; Torréns, M.; Canela, N.; Fragoso, A.; O’Sulivan, C.K. Supramolecular confinement of polymeric electron transfer mediator on gold surface for picomolar detection of DNA. Soft Matter 2011, 7, 10925–10930. [Google Scholar] [CrossRef]
- Suzuki, I.; Egawa, Y.; Mizukawa, Y.; Hoshi, T.; Anzai, J. Construction of positively-charged layered assemblies assisted by cyclodextrin complexation. Chem. Commun. 2002, 21, 164–165. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Gao, C. Stepwise assembly of the same polyelectrolytes using host-guest interaction to obtain microcapsules with multiresponsive properties. Chem. Mater. 2008, 20, 4194–4199. [Google Scholar] [CrossRef]
- Rao, S.V.; Anderson, K.W.; Bachas, L.G. Controlled layer-by-layer immobilization of horseradish peroxidase. Biotechnol. Bioeng. 1999, 65, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Sato, K.; Anzai, J. Disintegration of layer-by-layer assemblies composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2005, 6, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Anzai, J.; Kobayashi, Y. Construction of multilayer thin films of enzymes by means of sugar-lectin interactions. Langmuir 2000, 16, 2851–2856. [Google Scholar] [CrossRef]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, Z.; Jin, X.; Lin, X. HRP biosensor based on sugar-lectin biospecific interactions for the determination of phenolic compounds. Electrochim. Acta 2006, 52, 200–205. [Google Scholar] [CrossRef]
- Padeste, C.; Steiger, B.; Grubelnik, A.; Tiefenauer, L. Redox labeled avidin for enzyme sensor architectures. Biosens. Bioelectron. 2003, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Padeste, C.; Steiger, B.; Grubelnik, A.; Tiefenauer, L. Molecular assembly of redox-conductive ferrocene-streptavidin conjugates—Towards bio-electrochemical devices. Biosens. Bioelectron. 2004, 20, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Kwaon, S.J.; Park, N.; Kwon, D.; Kwak, J. Electrochemical detection of biomolecule with mixed self-assembled monolayer of ferrocene-undecanethiol. J. Nanosci. Nanotechnol. 2011, 11, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Casas-Solvas, J.M.; Ortiz-Salmerón, E.; García-Fuentes, L.; Vargas-Berenguel, A. Ferrocene-mannose conjugates as electrochemical molecular sensors for concanavalin A lectin. Org. Biomol. Chem. 2008, 6, 4230–4235. [Google Scholar] [CrossRef] [PubMed]
- Martos-Maldonado, M.C.; Casas-Solvas, J.M.; Quesada-Soriano, I.; García-Fuentes, L.; Vargas-Berenguel, A. Poly(amido amine)-based mannose-glycodendrimers as multielectron redox probes for improving lectin sensing. Langmuir 2013, 29, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Martos-Maldonado, M.C.; Thygesen, M.B.; Jensen, K.J.; Vargas-Berenguel, A. Gold-ferrocene glycol-nanoparticles for high-sensitivity electrochemical detection of carbohydrate-lectin interactions. Eur. J. Org. Chem. 2013, 2793–2801. [Google Scholar]
- Sato, K.; Kodama, D.; Anzai, J. Electrochemical determination of sugars by use of multilayer thin films of ferrocene-appended glycogen and concanavalin A. Anal. Bioanal. Chem. 2006, 386, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, P.; He, P.; Fang, Y. A solid-state electrochemiluminescence sensing platform for detection of adenosine based on ferrocene-labeled structure-switching signaling aptamer. Anal. Chim. Acta 2010, 658, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zheng, J.; Zhang, J.; Sheng, Q. Carbon nanotube-enhanced electrochemical aptasensor for the detection of thrombin. Talanta 2010, 81, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Gao, S.; Xie, L.; Chen, H.; Liu, Q.; Lin, Z.; Qiu, B.; Chen, G. An ultra-sensitive electrochemical sensor for ascorbic acid based on click chemistry. Analyst 2011, 136, 3962–3966. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, C. Click chemistry-based functionalization on non-oxidized silicon substrates. Chem. Asian J. 2011, 6, 2592–2605. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gill, R.; Freeman, R.; Willner, I. Probing of enzyme reactions by the biocatalyst-induced association or dissociation of redox labels liked to monolayer-functionalized electrodes. Chem. Commun. 2006, 5027–5029. [Google Scholar]
- Halámek, J.; Wollenberger, U.; Stöcklein, W.; Scheller, F.W. Development od a biosensor for glycated hemoglobin. Electrochim. Acta 2007, 53, 1127–1133. [Google Scholar] [CrossRef]
- Chien, H.-C.; Chou, T.-C. A nonenzymatic amperometric method for fructosy-valin sensing using ferroceneboronic acid. Electroanalysis 2011, 23, 402–408. [Google Scholar] [CrossRef]
- Takahashi, S.; Abiko, N.; Haraguchi, N.; Fujita, H.; Seki, E.; Ono, T.; Yoshida, K.; Anzai, J. Voltammetric response of ferroceneboronic acid to diol and phenolic compounds as possible pollutants. J. Environ. Sci. 2011, 23, 1027–1032. [Google Scholar] [CrossRef]
- Takahashi, S.; Haraguchi, N.; Abiko, N.; Ono, T.; Yoshida, K.; Anzai, J. Voltammetric determination of salicylic acid and derivatives based on ferroceneboronic acid. Sens. Lett. 2011, 9, 1845–1848. [Google Scholar] [CrossRef]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar] [CrossRef]
- Wang, Z.; Etienne, M.; Quilès, F.; Kohring, G.W.; Walcarius, A. Durable cofactor immobilization in sol-gel bio-composite thin films for reagentless biosensors and bioreactors using dehydrogenases. Biosens. Bioelectron. 2012, 32, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.A.; Tran, T.O.; Meredith, M.T.; Cline, T.C.; Glatzhofer, D.T.; Schmidtke, D.W. High-sensitive amperometric biosensors based on ferrocene-modified linear poly(ethyleneimine). Langmuir 2009, 25, 7736–7742. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.T.; Hickey, D.P.; Redemann, J.P.; Schmidtke, D.W.; Glatzhofer, D.T. Effects of ferrocene methylation on ferrocene-modified linear poly(ethyleneimine) bioanodes. Electrochim. Acta 2013, 92, 226–235. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Lee, J.M.; Shin, W. Electrochemical properties of ferrocene modified polysiloxane/chitosan nanocomposite and its application to glucose sensor. Electrochim. Acta 2009, 54, 6508–6514. [Google Scholar] [CrossRef]
- Frasconi, M.; Deriu, D.; D’Annibale, A.; Mazzei, F. Nanostructured materials based on the integration of ferrocenyl-tethered dendrimer and redox proteins on self-assembled monolayers: An efficient biosensor interface. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Anzai, J. Dendrimers in layer-by-layer assemblies: Synthesis and applications. Molecules 2013, 18, 8440–8460. [Google Scholar] [CrossRef] [PubMed]
- Merechant, S.A.; Glatzhofer, D.T.; Schmidtke, D.W. Effects of electrolyte and pH on the behavior of cross-liked films of ferrocene-modified poly(ethyleneimine). Langmuir 2007, 23, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Bunte, C.; Rühe, J. Photochemical generation of ferrocene-based redox-polymer networks. Macromol. Rapid Commun. 2009, 30, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Chakrabarty, T.; Singh, A.K.; Shahi, V.K. Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosens. Bioelectron. 2013, 41, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Siangproh, W.; Dungchai, W.; Rattanarat, P.; Chailapakul, O. Nanoparticle-based electrochemical detection in conventional and miniaturized systems and their bioanalytical applications: A review. Anal. Chim. Acta 2011, 690, 10–25. [Google Scholar]
- Fang, Y.; Zhang, D.; Qin, X.; Miao, Z.; Takahashi, S.; Anzai, J.; Chen, Q. A non-enzymatic hydrogen peroxide sensor based on poly(vinyl alcohol)-multiwalled carbon nanotubes-platinum nanoparticles hybrids modified glassy carbon electrode. Electrochim. Acta 2012, 70, 266–271. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singhg, S.P. Recent advances in ZnO nanoparticles and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, Y.; Huang, J.; Zhao, Z.; Xu, X.; Anzai, J.; Osa, T.; Chen, Q. Amperometric choline biosensors prepared by layer-by-layer deposition of choline oxidase on the Prussian blue-modified platinum electrode. Talanta 2006, 70, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Anzai, J. Phenylboronic acid monolayer-modified electrodes sensitive to sugars. Langmuir 2005, 21, 5102–5107. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Chen, J.; Wu, X.; Fang, K.; Jia, A.; Liu, J. Immobilization of Prussian blue nanoparticles onto thiol SAM modified Au electrodes for electroanalytical or biosensor applications. J. Nanosci. Nanotechnol. 2007, 7, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Baca, A.J.; Hu, J.; Zhou, F.; Yan, W.; Pang, D.-W. Amplified voltammetric determination of DNA hybridization via oxidation of ferrocene caps on gold nanoparticle/streptavidin conjugates. Anal. Chem. 2003, 75, 3941–3945. [Google Scholar] [CrossRef] [PubMed]
- Baca, A.J.; Zhou, F.; Wang, J.; Hu, J.; Li, J.; Wang, J.; Chikneyan, Z.S. Attachment of ferrocene-capped gold nanoparticle/streptavidin conjugates onto electrode surfaces covered with biotinylated biomolecules for enhanced voltammetric analysis. Electroanalysis 2004, 16, 73–80. [Google Scholar] [CrossRef]
- Jimenez, O.A.; Chikneyan, S.; Baca, A.J.; Wang, J.; Zhou, F. Sensitive detection of sulfhydryl groups in surface-confined metallothioneins and related species via ferrocene-capped gold nanoparticle/streptavidin conjugates. Environ. Sci. Technol. 2005, 39, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Tu, Q.; Guo, Q.; Zarui, C.S.; Momand, J.; Sun, X.Z.; Zhou, F. Capture of p53 by electrodes modified with consensus DNA duplexes and amplified voltammetric detection using ferrocene-capped gold nanoparticle/streptavidin conjugates. Anal. Chem. 2008, 80, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kiu, L.; Du, J.; Li, S.; Yuan, B.; Han, H.; Jing, M.; Xia, N. Amplified voltammetric detection of dopamine using ferrocene-capped gold nanoparticle/streptavidin conjugates. Biosens. Bioelectron. 2013, 41, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, W.; Hu, J.; Yao, X.; Li, J. Site-specific DNA cleavage of EcoR1 endonuclease probed by electrochemical analysis using ferrocene capped gold nanoparticles as reporter. Electrochem. Commun. 2007, 9, 1086–1090. [Google Scholar] [CrossRef]
- Chen, M.; Diao, G. Electrochemical study of mono-6-thio-b-cyclodextron/ferrocene capped on gold nanoparticles: Characterization and application to the design of glucose amperometric biosensor. Talanta 2009, 80, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xu, Y.; Wang, Y.; Song, W. Assembly of ferrocenylhexanethiol functionalized gold nanoparticles for ascorbic acid determination. Microchim. Acta 2010, 171, 81–89. [Google Scholar] [CrossRef]
- Escorcia, A.; Dhirani, A. Electrochemical properties of ferrocenylalkane dithiol-gold nanoparticle films prepared by layer-by-layer self-assembly. J. Electroanal. Chem. 2007, 601, 260–268. [Google Scholar] [CrossRef]
- Song, Z.; Yuan, R.; Chai, Y.; Zhou, Y.; Jiang, W.; Su, H.; Che, X.; Li, J. Horseradish peroxidase-functionalized Pt hollow nanospheres and multiple redox probes as trace labels for a sensitive simultaneous multianalyte electrochemical immunoassay. Chem. Commun. 2010, 46, 6750–6752. [Google Scholar] [CrossRef]
- Cai, W.; Xu, Q.; Zhao, X.; Zhu, J.; Chen, H. Porous gold-nanoparticle-CaCO3 hybrid material: Preparation, characterization, and application for horseradish peroxidase assembly and direct electrochemistry. Chem. Mater. 2006, 18, 279–284. [Google Scholar] [CrossRef]
- Kirdeciler, S.K.; Soy, E.; Öztürk, S.; Kucherenko, I.; Soldatkin, O.; Dzyadevych, S.; Akata, B. A novel urea conductometric biosensor based on zeolite immobilized urease. Talanta 2011, 85, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhou, Q.; Gu, Z.; Gu, X.; Zhao, L.; Li, Y.; Zheng, J. Hydrogen peroxide biosensor based on microperosidase-11 immobilized in a silica cavity array electrode. Talanta 2013, 107, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, Z.; Zhang, L.; Qi, X.; Li, G.; Wu, J.; Wang, M. Application of Fe3O4 mesoporous sphere modified carbon ionic liquid electrode as electrochemical hemoglobin biosensor. Colloid. Surf. B 2013, 101, 177–182. [Google Scholar] [CrossRef]
- Li, T.; Yang, M. Electrochemical sensor utilizing ferrocene loaded porous polyelectrolyte nanoparticles as label for the detection of protein biomarker IL-6. Sens. Actuators B 2011, 158, 361–365. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Zhao, H.; Xu, J.; Sun, Y. Reagentless amperometric glucose biosensor based on the immobilization of glucose oxidase on a ferrocene@NaY zeolite composite. Microchim. Acta 2011, 174, 281–288. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Q.; Wang, X.; Sun, Z.; Zhu, Z.; Xian, Y.; Jin, L.; Yamamoto, K. Amperometric sensor based on ferrocene-doped silica nanoparticles as an electron transfer mediator for the determination of glucose in rat brain coupled to in vivo microdialysis. J. Electroanal. Chem. 2004, 571, 133–138. [Google Scholar] [CrossRef]
- Li, H.; Wei, Q.; He, J.; Li, T.; Zhao, Y.; Cai, Y.; Du, B.; Qian, Z.; Yang, M. Electrochemical immunosensors for cancer biomarker with signal amplification based on ferrocene functionalized iron oxide nanoparticles. Biosens. Bioelectron. 2011, 26, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Wang, X.; Sun, B.; Cheng, Z.; Ai, S. β-cyclodextrin-ferrocene host-guest complex multifunctional labeling triple amplification strategy for electrochemical immunoassay of subgroup J of avian leukosis viruses. Biosens. Bioelectron. 2013, 45, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, C.; Xie, J.; Yang, Z.; Sun, S. pH controlled release of chromone from chromone-Fe3O4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 14436–14437. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.A.; Hrapovic, S.; Luong, J.H.T. Picomolar detection of protease using peptide/single walled carbon nanotube/gold nanoparticle-modified electrode. ACS Nano 2008, 2, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.A.; Luong, J.H.T. Impedance method for detecting HIV-1 protease and screening for its inhibitors using ferrocene-peptide conjugate/Au nanoparticle/single-walled carbon nanotube modified electrode. Anal. Chem. 2008, 80, 7056–7062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chai, Y.; Yuan, R.; Mao, L.; Yuan, Y.; Han, J. Glucose oxidase and ferrocene labels immobilized at Au/TiO2 nanocomposites with high load amount and activity for sensitive immunoelectrochemical measurement of proGRP biomarker. Biosens. Bioelectron. 2011, 26, 3838–3844. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.D.; Yang, Y.; Shi, H.B.; Song, Z.; Zhao, Z.X.; Anzai, J.; Osa, T.; Chen, Q. Multi-walled carbon nanotubes-based glucose biosensor prepared by a layer-by-layer technique. Mater. Sci. Eng. C 2006, 26, 113–117. [Google Scholar] [CrossRef]
- Huang, J.D.; Song, Z.; Li, J.; Yang, Y.; Shi, H.B.; Wu, B.; Anzai, J.; Osa, T.; Chen, Q. A highly-sensitive L-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Mater. Sci. Eng. C 2007, 27, 29–34. [Google Scholar] [CrossRef]
- Pereira, A.C.; Kisner, A.; Duran, N.; Kubota, L.T. The effects of dimensionality on electrochemical sensors based on carbon nanotubes and metallic nanowires. J. Nanosci. Nanotechnol. 2010, 10, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, B.; He, X.; Li, F.; Ding, Y.; Fei, J. Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim. Acta 2013, 180, 935–956. [Google Scholar] [CrossRef]
- Goff, A.L.; Moggia, F.; Debou, N.; Jegou, P.; Artero, V.; Fontecave, M.; Jousselme, B.; Palacin, S. Facile and tunable functionalization of carbon nanotube electrodes with ferrocene by covalent coupling and p-stacking interactions and their relevance to glucose bio-sensing. J. Electroanal. Chem. 2010, 641, 57–63. [Google Scholar] [CrossRef]
- Moore, K.E.; Flavel, B.S.; Yu, J.; Abell, A.D.; Shapter, J.G. Increased redox-active peptide loading on carbon nanotube electrodes. Electrochim. Acta 2013, 89, 206–211. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, W.; Guo, J.; Wang, R.; Liang, R. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Griveau, S.; Bedioui, F.; Nyokong, T. Layer-by-layer electrode functionalization using carbon nanotubes, electrochemical grafting of azide-alkyne functions and click chemistry. Electroanalysis 2012, 24, 1833–1838. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, J.; Li, D.; Xu, L.; Liu, Y.; Wang, E. Biocompatible conductive architecture with surface-confined probe for non-invasive electrochemical cytosensing. Electrochem. Commun. 2012, 18, 81–84. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Li, D.; Wang, T.; Liu, Y.; Wang, J.; Wang, E. Highly sensitive and selective detection of cancer cell with a label-free electrochemical cytosensor. Biosens. Bioelectron. 2013, 41, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Miao, Y.; Wang, L.; Gan, T.; Yu, M.; Wang, L. Direct electrochemistry of hemoglobin based on chitosan-ionic liquid-ferrocene/graphene composite film. Process Biochem. 2012, 47, 1171–1177. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Q.; Wang, K.; Li, F.; Niu, L. Ferrocene functionalized grapheme: Preparation, characterization and efficient electron transfer toward sensors of H2O2. J. Mater. Chem. 2012, 22, 6165–6170. [Google Scholar] [CrossRef]
- Song, Y.; Kang, X.; Zuckermann, N.B.; Phebus, B.; Konopelski, J.P.; Chen, S. Ferrocene-functionalized carbon nanoparticles. Nanoscale 2013, 3, 1984–1989. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Ho, K. Synthesis of redox polymer nanobeads and nanocomposites for glucose biosensors. ACS Appl. Mater. Interfaces 2013, 5, 7852–7861. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takahashi, S.; Anzai, J.-i. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742-5762. https://doi.org/10.3390/ma6125742
Takahashi S, Anzai J-i. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials. 2013; 6(12):5742-5762. https://doi.org/10.3390/ma6125742
Chicago/Turabian StyleTakahashi, Shigehiro, and Jun-ichi Anzai. 2013. "Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors" Materials 6, no. 12: 5742-5762. https://doi.org/10.3390/ma6125742