Effect of Thermal Treatment of Veneer on Formaldehyde Emission of Poplar Plywood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Measurement of Formaldehyde Emission
2.3. Strength Test of Heated Veneer
2.4. Adsorption Properties of Heated Veneer
3. Results and Discussion
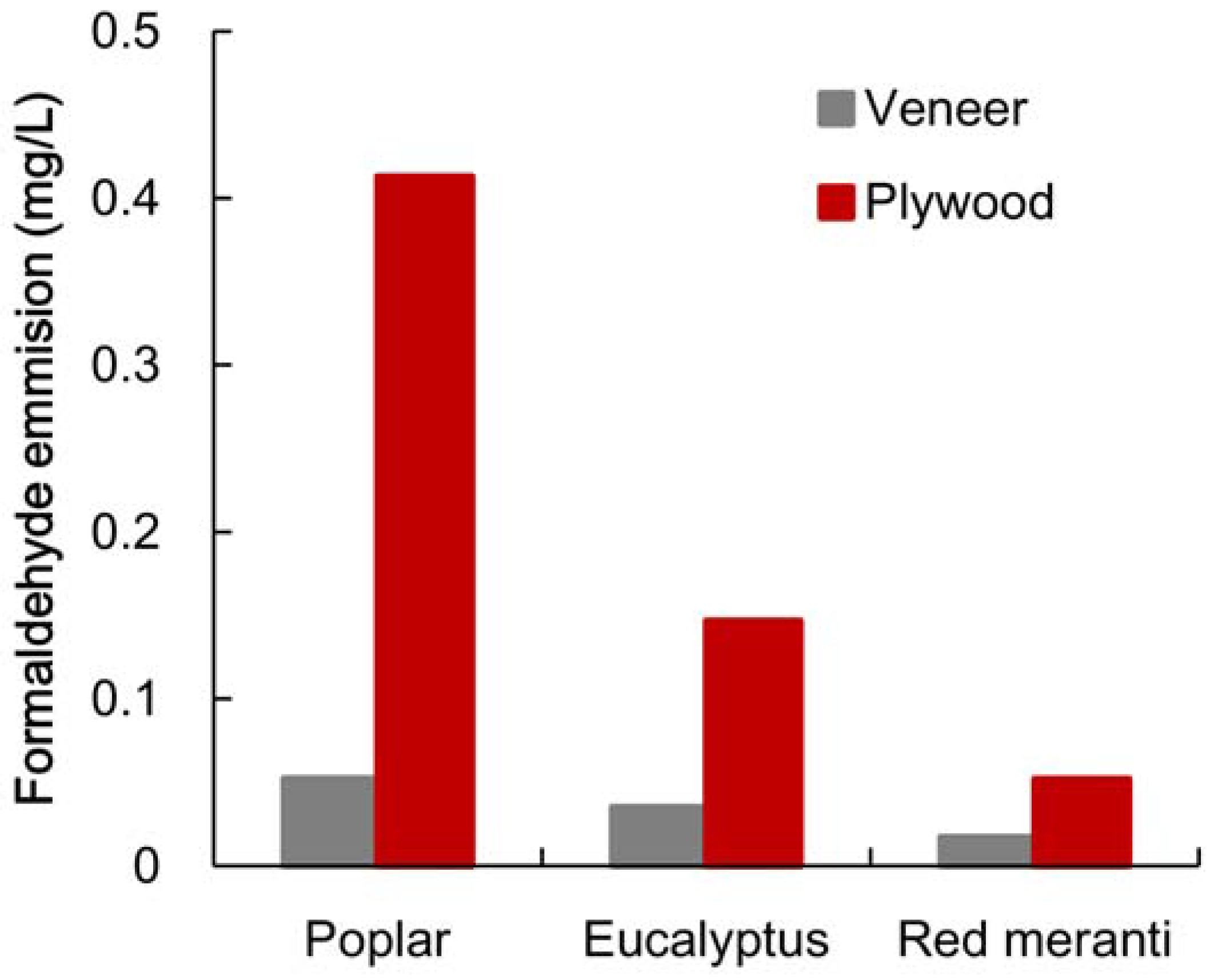
3.1. Formaldehyde Emission
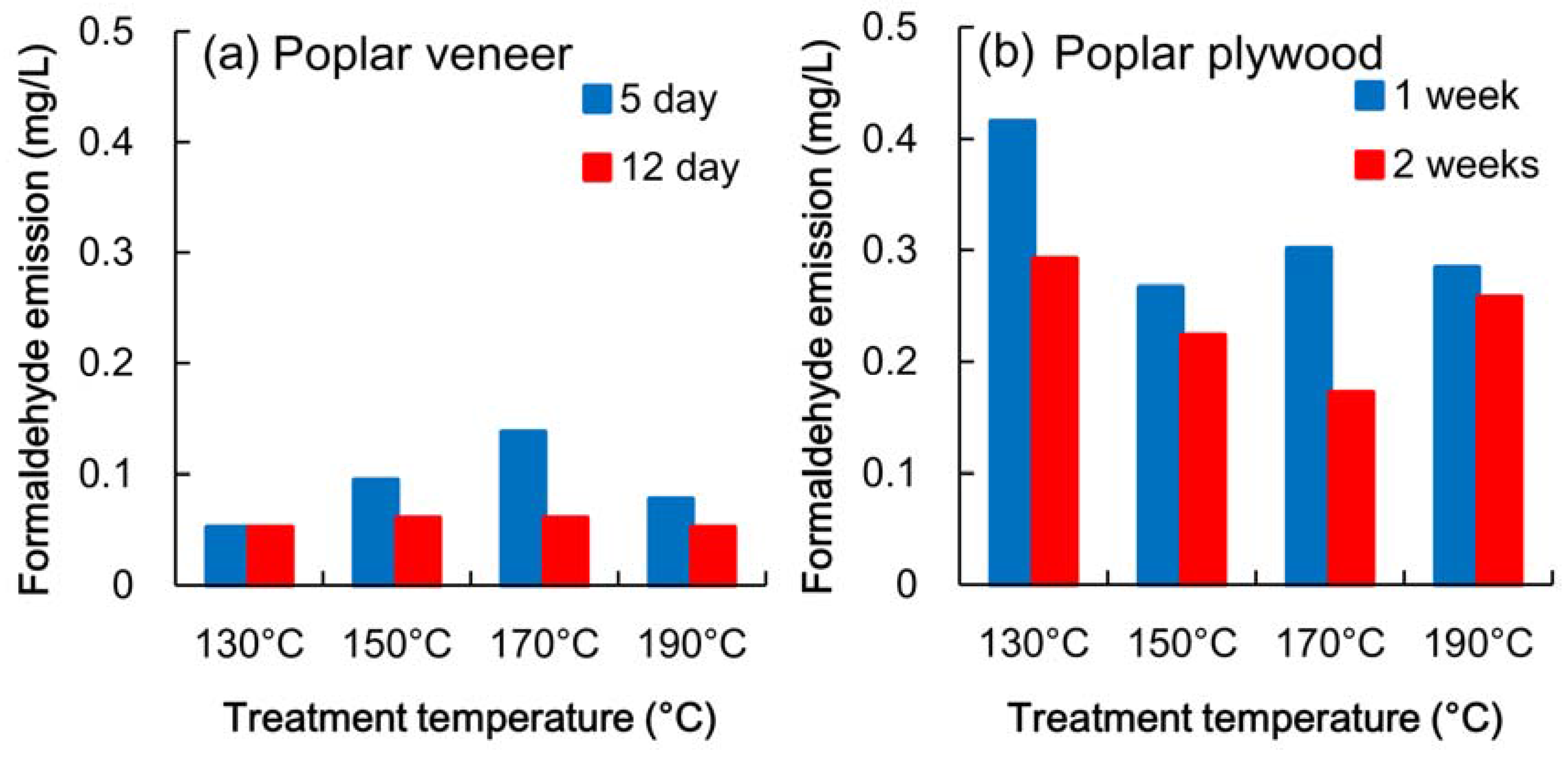
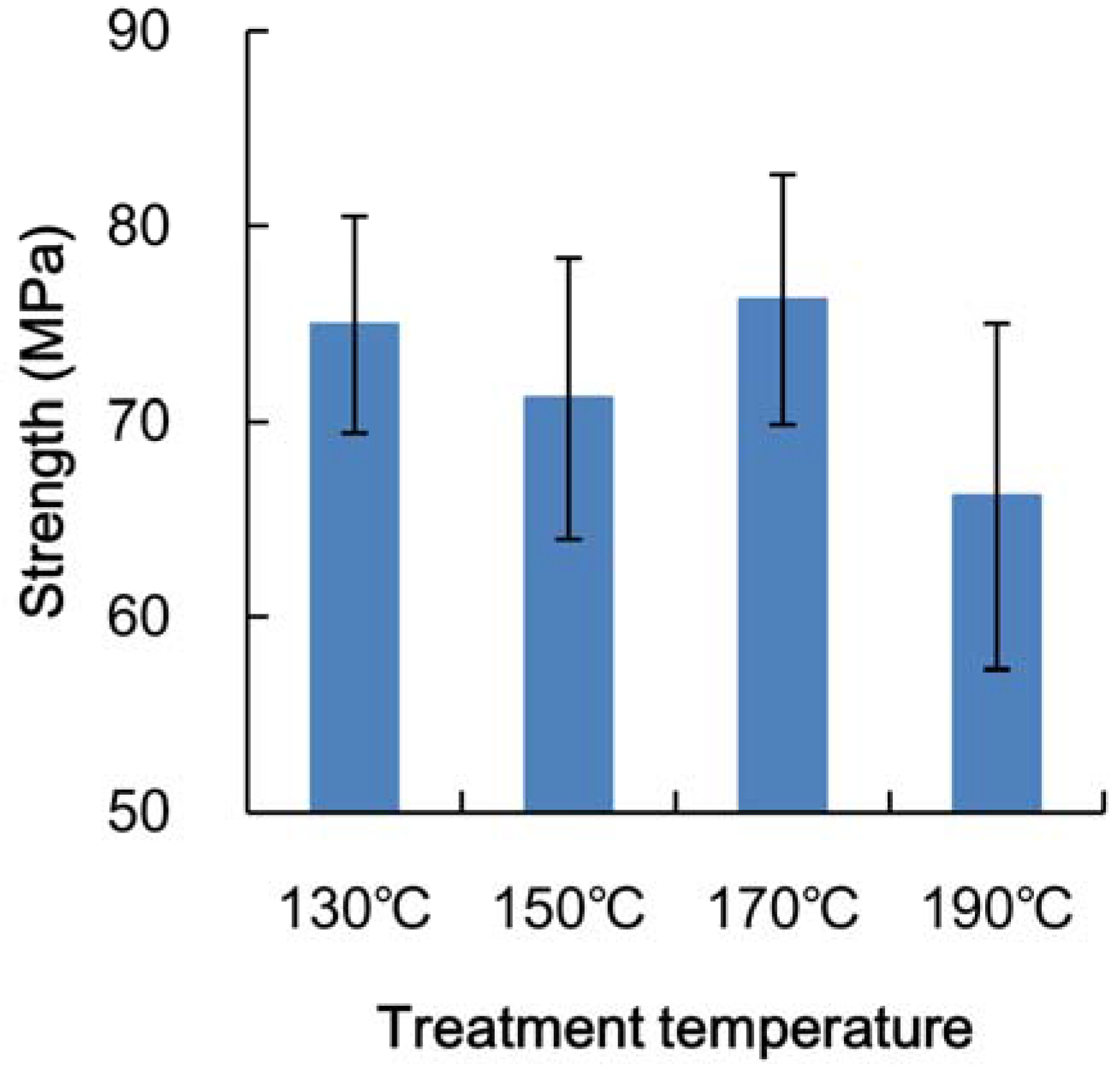
3.2. Mechanical Properties of Heated Veneer
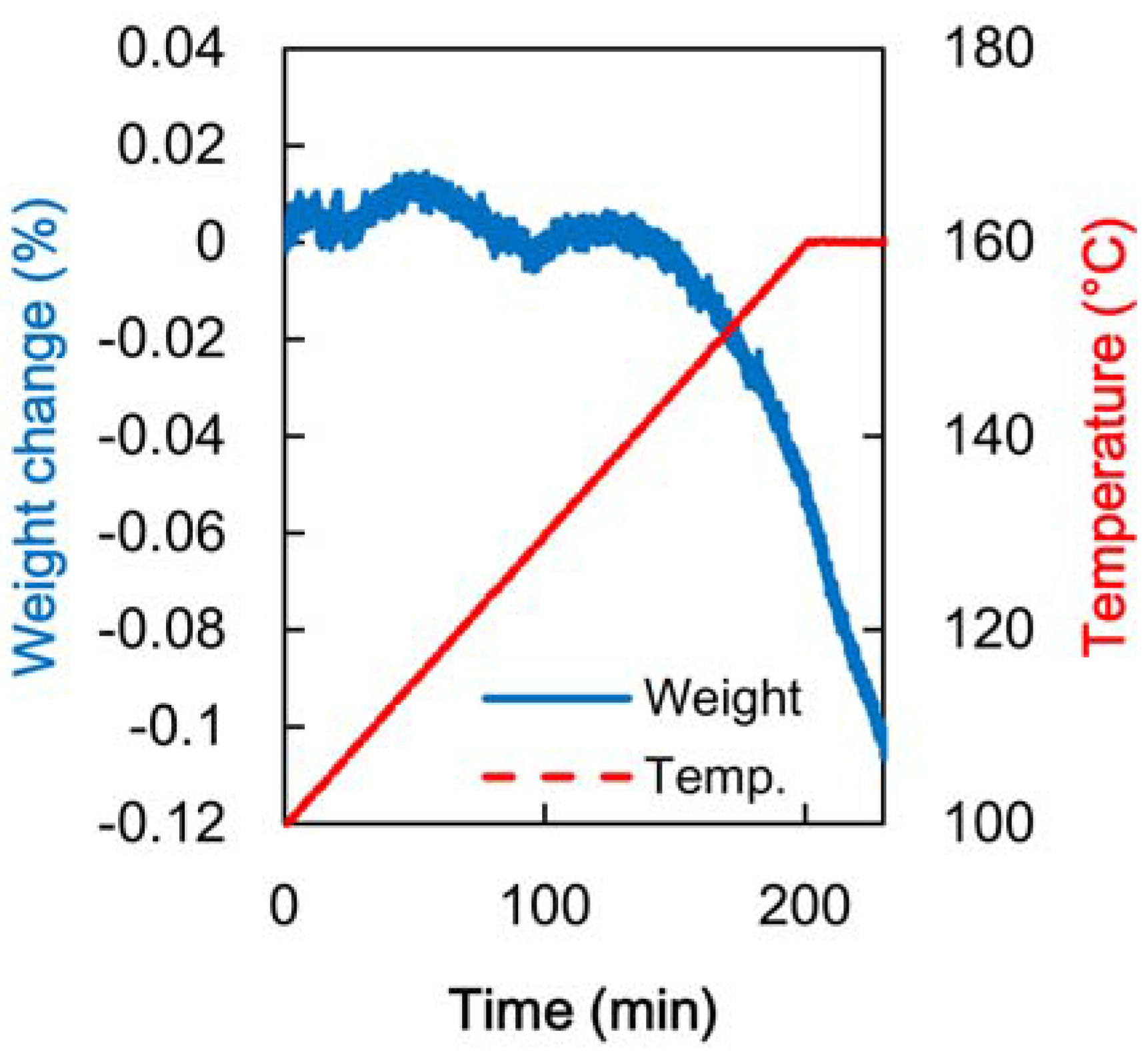
3.3. Adsorption Properties of Heated Veneer
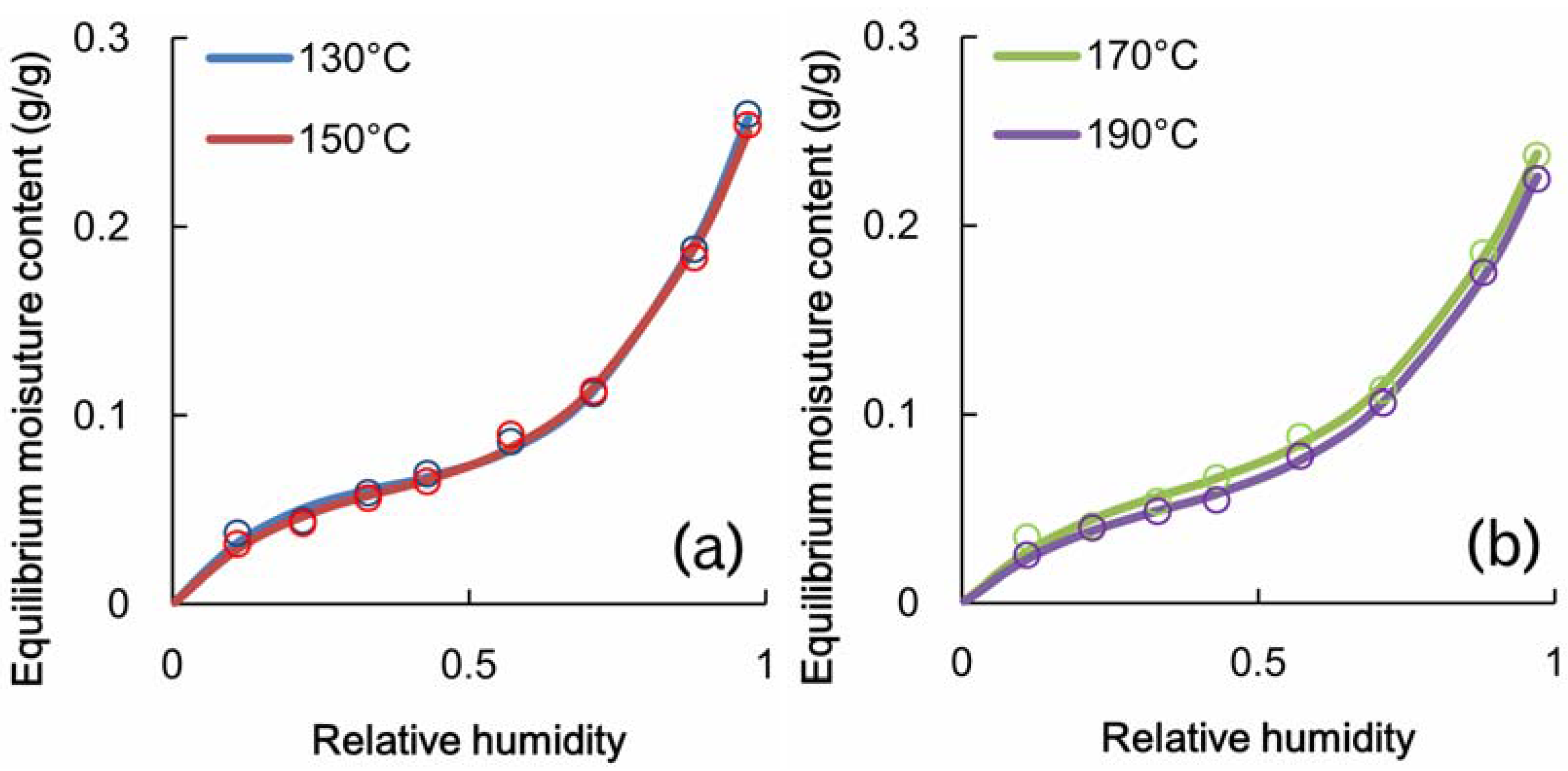
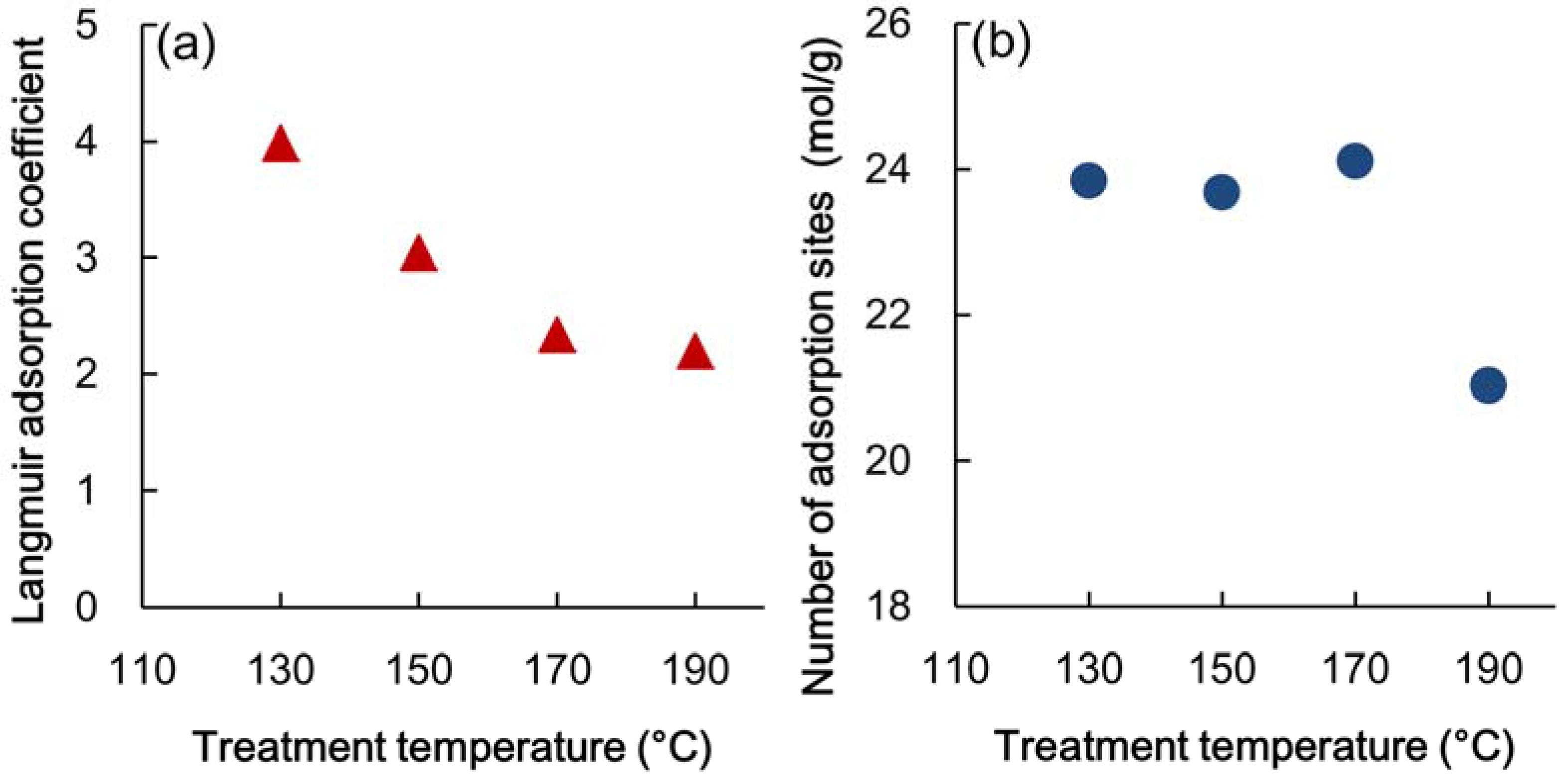
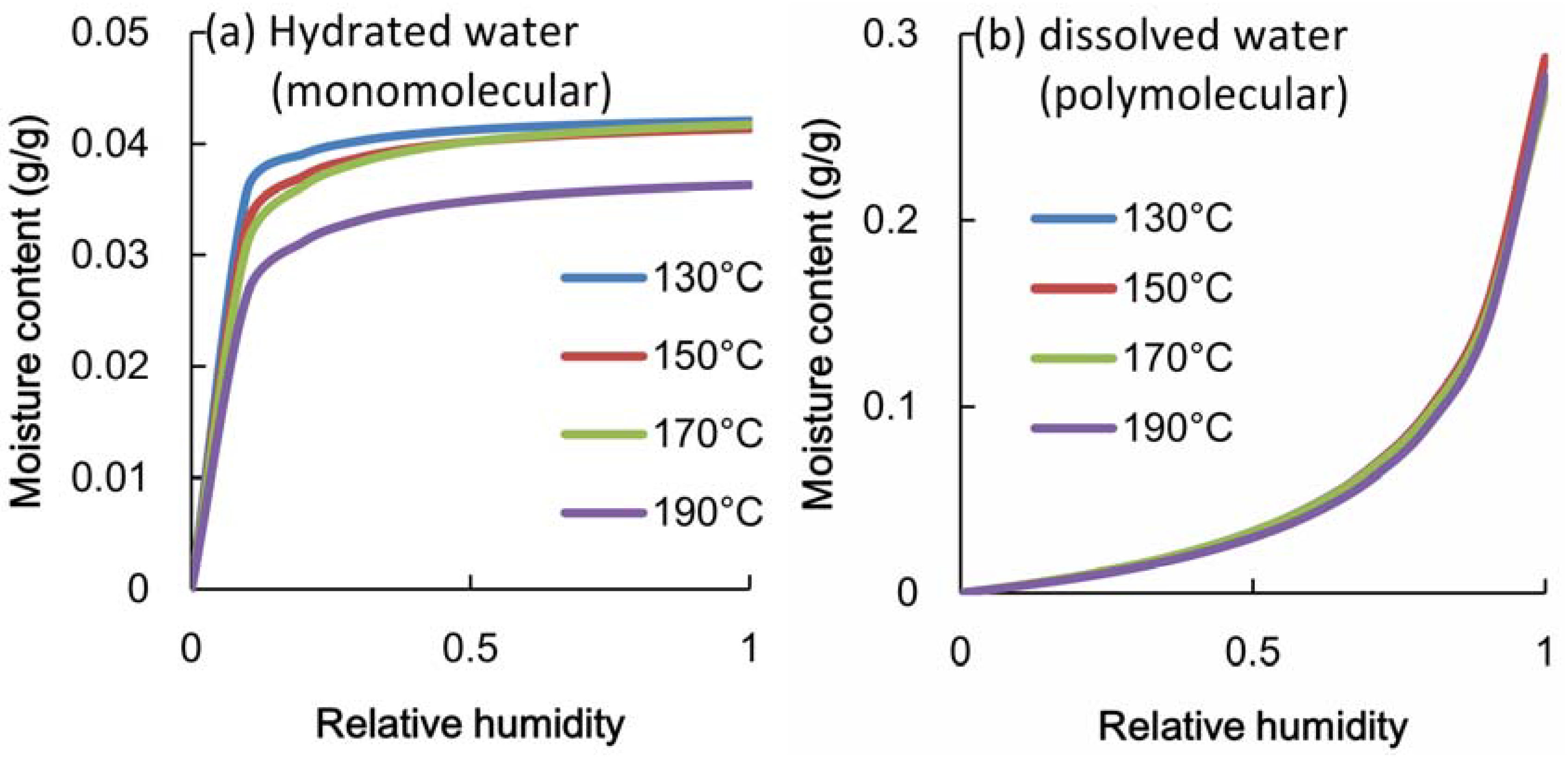
4. Relationship between Adsorption Properties and Formaldehyde Emission
5. Conclusions
Acknowledgments
References
- Zhang, M. Fast growing tree resource and utilization in China. In Fast Growing Tree—Plantation and Utilization; Iwasaki, M., Saka, S., Toma, T., Hayashi, T., Matsumura, J., Murata, K., Eds.; Kaiseisha: Ohtsu, Japan, 2012; pp. 205–213. (in Japanese) [Google Scholar]
- Forestry Agency. Annual Report on Forest and Forestry in Japan 2010. Available online: http://www.rinya.maff.go.jp/j/kikaku/hakusyo/22hakusho/pdf/22_e.pdf (accessed on 15 January 2013).
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yang, T.H.; Lin, L.T.; Lin, C.J.; Tsai, M.J. Properties of low-formaldehyde-emission particleboard made from recycled wood-waste chips sprayed with PMDI/PF resin. Build. Environ. 2007, 42, 2472–2479. [Google Scholar] [CrossRef]
- Amazio, P.; Avella, M.; Errico, M.E.; Gentile, G.; Balducci, F.; Gnaccarini, A.; Moratalla, J.; Belanche, M. Low formaldehyde emission particleboard panels realized through a new acrylic binder. J. Appl. Polym. Sci. 2011, 122, 2779–2788. [Google Scholar] [CrossRef]
- Nikvash, N.; Kharazipour, A.; Euring, M. Effects of wheat protein as a biological binder in the manufacture of particleboards using a mixture of Canola, Hemp, Bagasse, and commercial wood. For. Prod. J. 2012, 62, 49–57. [Google Scholar]
- Meyer, B.; Boehme, C. Formaldehyde emission from solid wood. For. Prod. J. 1997, 47, 45–48. [Google Scholar]
- Roffael, E. Formaldehyde Release from Particleboard and Other Wood-Based Panels; Forest Research Institute: Kuala Lumpur, Malaysia, 1993; pp. 8–9. [Google Scholar]
- Bekhta, R.; Niemz, P.; Sedliacik, J. Effect of pre-pressing of veneer on the glueability and properties of veneer-based products. Eur. J. Wood Prod. 2012, 70, 99–106. [Google Scholar] [CrossRef]
- Obataya, E.; Tanaka, F.; Norimoto, M.; Tomita, B. Hygroscopicity of heat-treated wood. I. Effects of after-treatments on the hygroscopicity of heat-treated wood. J. Jpn. Wood Res. Soc. 2000, 46, 77–87. (in Japanese). [Google Scholar]
- Building Boards, Determination of The Formaldehyde Emissions—Desiccator Method; Japanese industrial standard JIS A 1460:2001; Japanese Standards Association: Tokyo, Japan, 2001.
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: London, UK, 1999; pp. 97–98. [Google Scholar]
- Nakano, T. Analysis of the temperature dependence of water sorption for wood on the basis of dual mode theory. J. Wood Sci. 2006, 52, 490–495. [Google Scholar] [CrossRef]
- Hailwood, A.J.; Horrobin, S. Absorption of water by polymers: Analysis in terms of a simple model. Trans. Faraday Soc. 1946, 84–102. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Hill, C.A.S. The sorption of water vapour by anhydride modified softwood. Wood Sci. Techonol. 2003, 37, 221–231. [Google Scholar] [CrossRef]
- Shiga, M.; Nakano, T. Effects of heating at lower temperature on water adsorption behavior for wood. J. Soc. Mater. Sci. Jpn. 2009, 58, 175–179. (in Japanese). [Google Scholar] [CrossRef]
- Hasegawa, A. The effect of drying temperature on chemical-substance emission from solid wood. J. Environ. Eng. AIJ 2009, 73, 1267–1273. (in Japanese). [Google Scholar] [CrossRef]
- Schäfer, M.; Roffael, E. On the formaldehyde release of wood. Holz als Roh- und Werkstoff 2000, 58, 259–264. [Google Scholar] [CrossRef]
- Shafizadeh, F.; McGinnis, G.D.; Philpot, C.W. Thermal degradation of xylan and related model compounds. Carbohyd. Res. 1972, 25, 23–33. [Google Scholar] [CrossRef]
- Shimizu, K.; Teratani, F.; Miyazaki, K. Effect of the thermal treatment on wood hemicellulose. IV. Mechanism in early stage of xylan phrolysis. Mokuzai Gakkaishi 1971, 17, 154–159. [Google Scholar]
- Kojiro, K.; Furata, Y.; Ishimaru, Y. Influence of heating and dry history on micropores in dry wood. J. Wood Sci. 2008, 54, 202–207. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murata, K.; Watanabe, Y.; Nakano, T. Effect of Thermal Treatment of Veneer on Formaldehyde Emission of Poplar Plywood. Materials 2013, 6, 410-420. https://doi.org/10.3390/ma6020410
Murata K, Watanabe Y, Nakano T. Effect of Thermal Treatment of Veneer on Formaldehyde Emission of Poplar Plywood. Materials. 2013; 6(2):410-420. https://doi.org/10.3390/ma6020410
Chicago/Turabian StyleMurata, Koji, Yashuhiro Watanabe, and Takato Nakano. 2013. "Effect of Thermal Treatment of Veneer on Formaldehyde Emission of Poplar Plywood" Materials 6, no. 2: 410-420. https://doi.org/10.3390/ma6020410



