Advanced Strategies for Articular Cartilage Defect Repair
Abstract
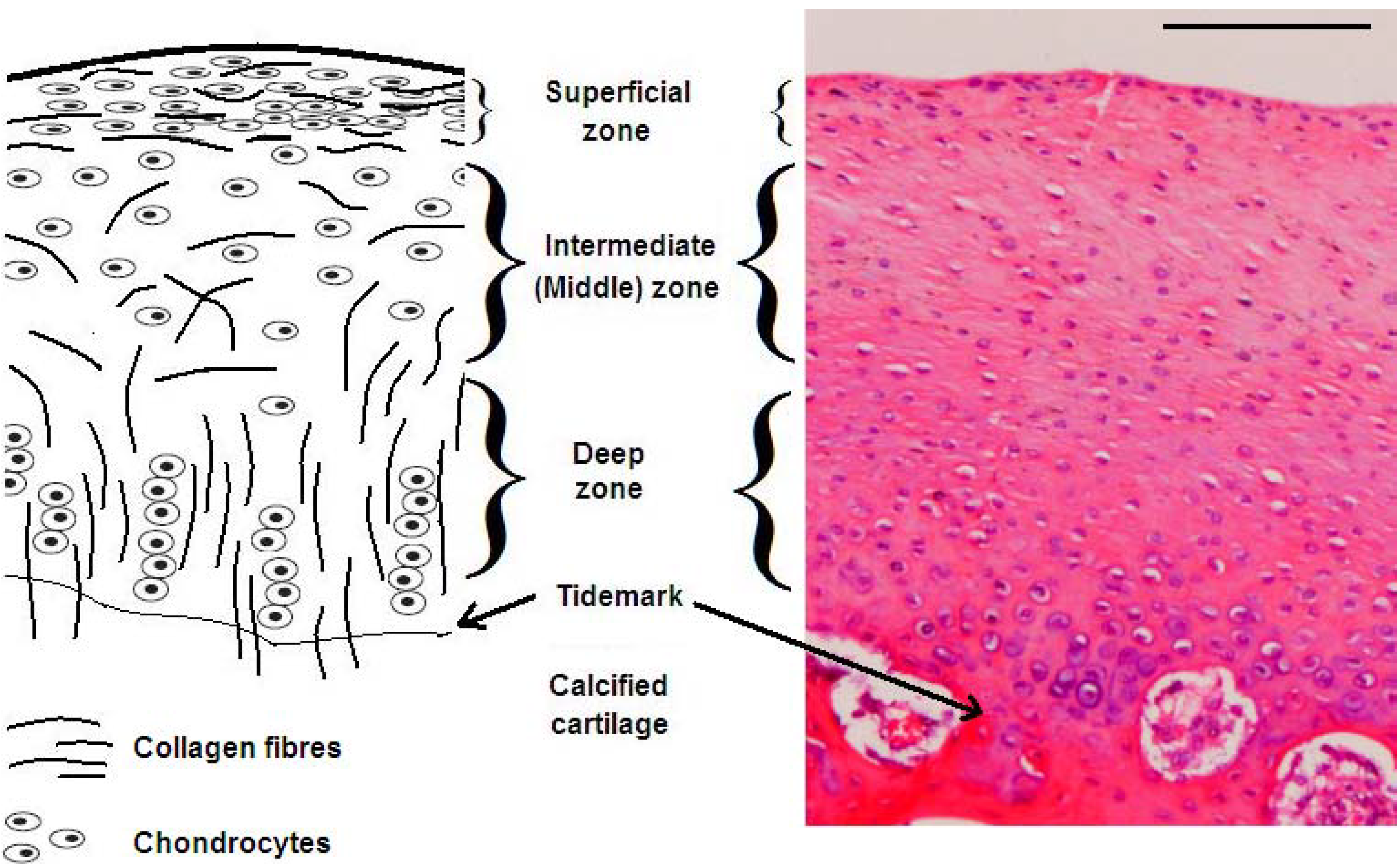
:1. Articular Cartilage Structure and Function
2. Articular Cartilage Damage
3. Cartilage Treatment Strategies: Current State of the Art
3.1. Debridement and Lavage
3.2. Microfracture
3.3. Autografts
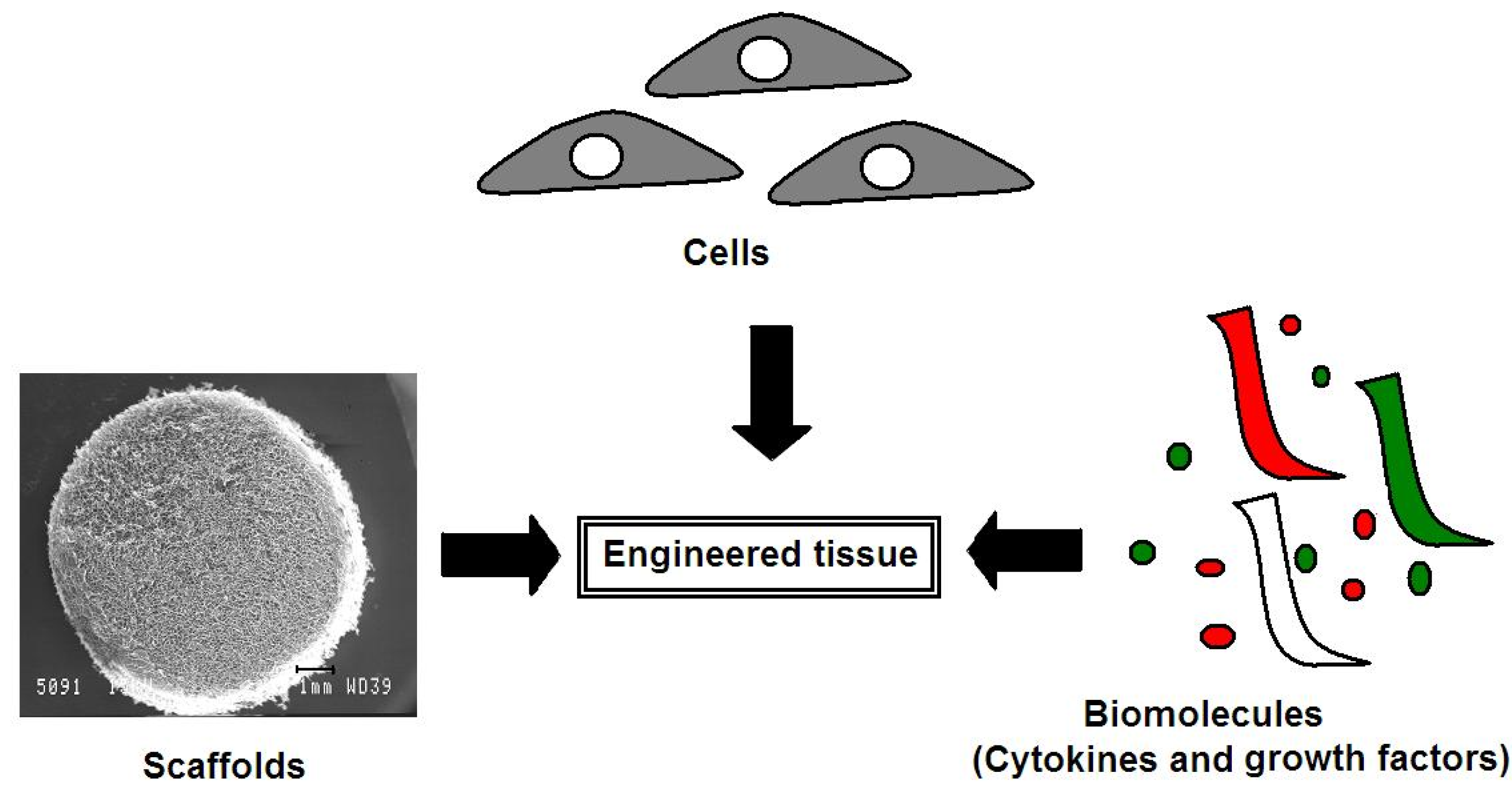
4. Advances in Articular Cartilage Repair Using a Tissue Engineering Approach
4.1. Cells for Cartilage Defect Repair
4.1.1. Chondrocytes
Autologous Chondrocyte Implantation (ACI)
Collagen-Covered Autologous Chondrocyte Implantation (CACI)
Matrix-Induced Autologous Chondrocyte Implantation (MACI)
Drawbacks Associated with the Use of Chondrocytes in Cartilage Defect Repair
4.1.2. Mesenchymal Stem Cells
| MSC type | Advantages | Disadvantages |
|---|---|---|
| Bone marrow stem cells [70,71] | Easily isolated from bone marrow High chondrogenic potential Broadly characterized and investigated Homogeneous population | Extracting bone marrow is a very painful and invasive procedure Low yield (approximate 1 in 1 × 105 cells in the marrow) Decline in proliferative and differentiation capacity with age |
| Adipose-derived stem cells [72] | Abundance of tissue High yield (approximate 5000 stem cells per gram of aspirate) Low donor tissue morbidity | Inhomogeneous cell population |
| Infrapatellar fat pad-derived stem cells [73] | High chondrogenic potential Low donor site morbidity | Limited source of tissue |
| Synovium-derived stem cells [74] | High yield High proliferative rate High chondrogenic potential | Limited source of tissue |
Bone Marrow-Derived Stem Cells
Adipose-Derived Stem Cells
Infrapatellar Fat Pad-Derived Stem Cells
Synovium-Derived Stem Cells
Comparison of the Chondrogenic Potential of MSCs from Various Sources
| Article | MSC Type Investigated | Outcome | ||||
|---|---|---|---|---|---|---|
| Adipose | Bone marrow | Muscle | Synovium | Periosteum | ||
| Sakaguchi et al., 2005 [93] | √ | √ | √ | √ | √ | Synovium-derived MSCs displayed greater chondrogenic response |
| Yoshimura et al., 2006 [74] | √ | √ | √ | √ | √ | Synovium-derived MSCs displayed greater chondrogenic response |
| Koga et al., 2008 [88] | √ | √ | √ | √ | – | Bone marrow and synovium-derived MSCs displayed greater chondrogenic response |
| Havlas et al., 2011 [94] | √ | √ | – | – | – | No difference between adipose and bone marrow MSC chondrogenic response |
| Vidal et al., 2008 [95] | √ | √ | – | – | – | Bone marrow-derived MSCs displayed greater chondrogenic response |
| Reich et al., 2012 [96] | √ | √ | – | – | – | Bone marrow-derived MSCs displayed greater chondrogenic response |
4.1.3. Co-Culture Systems
4.2. Advances in Scaffolds for Cartilage Defect Repair
4.2.1. The Effect of Scaffold Composition on Chondrogenesis
Synthetic Materials Utilized in Cartilage Tissue Engineering
Naturally-Derived Materials
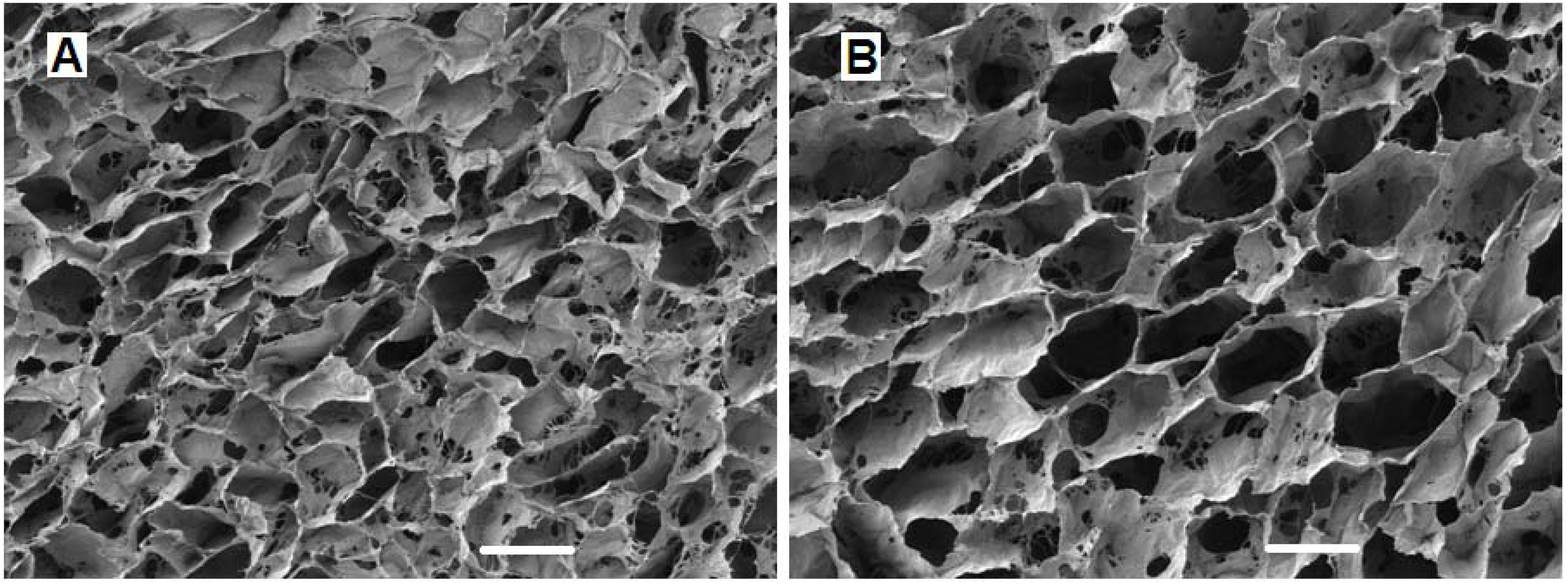
4.2.2. The Effect of Scaffold Geometry on Chondrogenesis
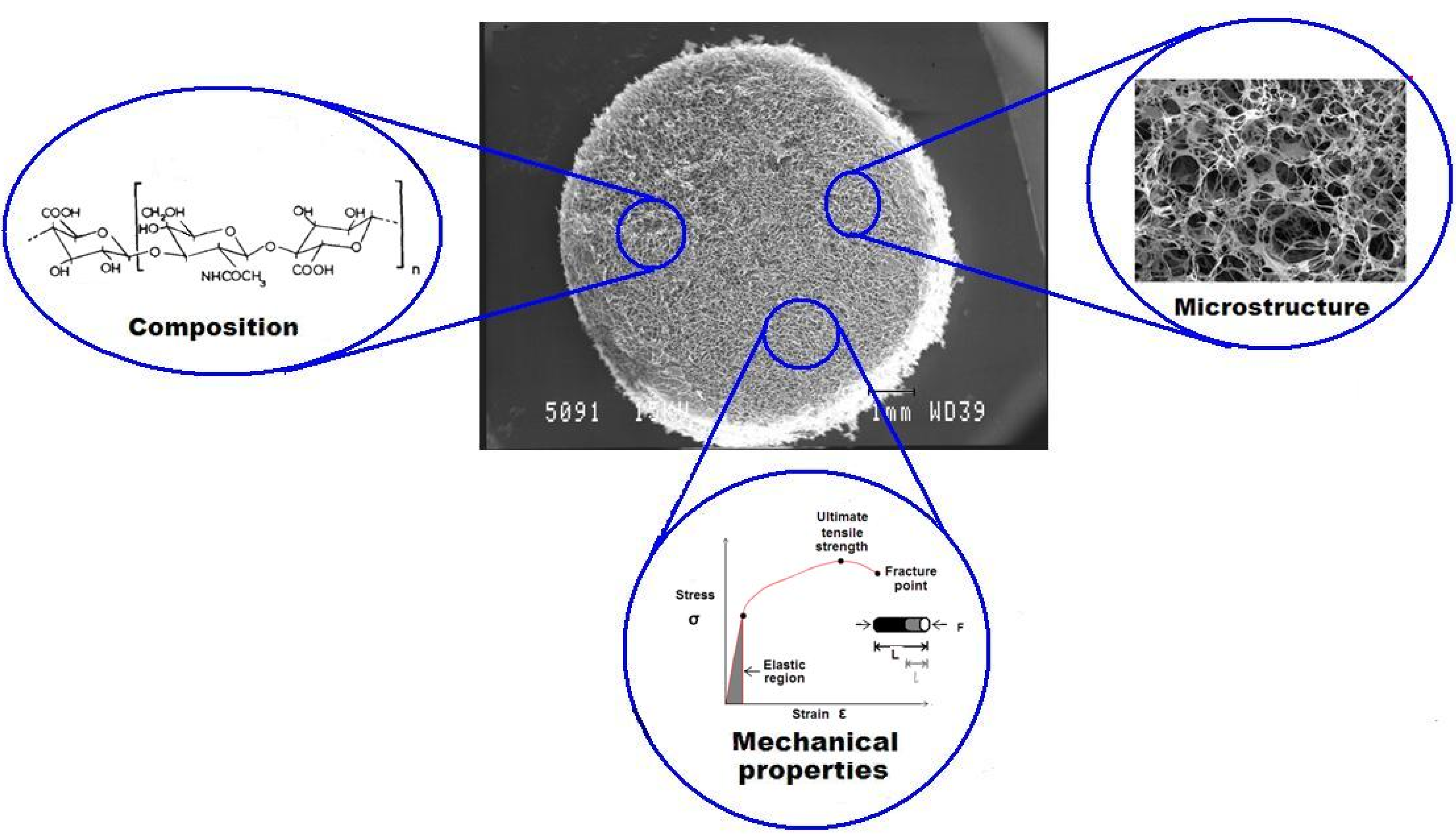
4.2.3. The Effect of Scaffold Mechanical Properties on Cell Behavior
4.3. Biomolecules for Cartilage Defect Repair
4.3.1. Growth Factors
4.3.2. The Use of Scaffolds for Delivery of Growth Factors

4.4. Gene Therapy in Tissue Engineering
5. Concluding Remarks
Acknowledgements
References
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Eckstein, F.; Milz, S.; Schulte, E.; Becker, C.; Putz, R. The distribution of cartilage thickness in the knee-joints of old-aged individuals—Measurement by A-mode ultrasound. Clin. Biomech. 1998, 13, 1–10. [Google Scholar] [CrossRef]
- Kovach, I.S. A molecular theory of cartilage viscoelasticity. Biophys. Chem. 1996, 59, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 2000, 28, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D. Collagen of articular cartilage. Arthritis Res. 2002, 4, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.P. Articular cartilage repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [PubMed]
- Hunziker, E.B. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartil. 1999, 7, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Joint Surg. 1993, 75, 532–553. [Google Scholar] [PubMed]
- Beris, A.E.; Lykissas, M.G.; Papageorgiou, C.D.; Georgoulis, A.D. Advances in articular cartilage repair. Injury 2005, 36, S14–S23. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Verdonk, P.; Condello, V.; Delcogliano, M.; Dhollander, A.; Filardo, G.; Pignotti, E.; Marcacci, M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee. Am. J. Sports Med. 2009, 37, 156S–166S. [Google Scholar] [CrossRef] [PubMed]
- Plewes, L.W. Osteo-arthritis of the hip. Br. J. Surg. 1940, 27, 682–695. [Google Scholar] [CrossRef]
- Ghadially, J.A.; Ghadially, R.; Ghadially, F.N. Long-term results of deep defects in articular cartilage. Virchows Arch. B 1977, 25, 125–136. [Google Scholar]
- Mankin, H.J. The response of articular cartilage to mechanical injury. J. Bone Joint Surg. Ser. A 1982, 64, 460–466. [Google Scholar]
- Buckwalter, J.A. Chondral and osteochondral injuries: Mechanisms of injury and repair responses. Oper. Tech. Orthop. 1997, 7, 263–269. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; de Windt, T.S.; Brittberg, M.; Saris, D.B.F. Cartilage repair in football (soccer) athletes: What evidence leads to which treatment? A critical review of the literature. Cartilage 2012, 3, 43S–49S. [Google Scholar] [CrossRef]
- Cole, B.J.; Pascual-Garrido, C.; Grumet, R.C. Surgical management of articular cartilage defects in the knee. J. Bone Joint Surg. 2009, 91, 1778–1790. [Google Scholar] [PubMed]
- Detterline, A.J.; Goldberg, S.; Bach, B.R., Jr.; Cole, B.J. Treatment Options for Articular Cartilage Defects of the Knee. Orthop. Nurs. 2005, 24, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.D.; Stickles, B.J.; Balikian, P.; Busconi, B.D. Prospective analysis of radiofrequency versus mechanical debridement of isolated patellar chondral lesions. Arthroscopy 2002, 18, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, G.; Engebretsen, L.; Ludvigsen, T.C.; Drogset, J.O.; Grøntvedt, T.; Solheim, E.; Strand, T.; Roberts, S.; Isaksen, V.; Johansen, O. Autologous chondrocyte implantation compared with microfracture in the knee: A randomized trial. J. Bone Joint Surg. 2004, 86, 455–464. [Google Scholar] [PubMed]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy 2003, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Gobbi, A.; Filardo, G.; Delcogliano, M.; Zaffagnini, S.; Marcacci, M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Am. J. Sports Med. 2009, 37, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Saris, D.B.F.; Vanlauwe, J.; Victor, J.; Almqvist, K.F.; Verdonk, R.; Bellemans, J.; Luyten, F.P. Treatment of symptomatic cartilage defects of the knee. Am. J. Sports Med. 2009, 37, 10S–19S. [Google Scholar] [CrossRef] [PubMed]
- Minas, T. Nonarthroplasty management of knee arthritis in the young individual. Curr. Opin. Orthop. 1998, 9, 46–52. [Google Scholar] [CrossRef]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Erggelet, C.; Endres, M.; Neumann, K.; Morawietz, L.; Ringe, J.; Haberstroh, K.; Sittinger, M.; Kaps, C. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J. Orthop. Res. 2009, 27, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Enea, D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthros. Tech. 2012, 1, e175–e180. [Google Scholar] [CrossRef]
- Bentley, G.; Biant, L.C.; Vijayan, S.; Macmull, S.; Skinner, J.A.; Carrington, R.W.J. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J. Bone Joint Surg. Br. 2012, 94B, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.S.; Simonian, P.T.; Norman, A.G.; Clark, J.M. Effects of small incongruities in a sheep model of osteochondral autografting. Am. J. Sports Med. 2004, 32, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Cascio, B.M.; Sharma, B. The future of cartilage repair. Oper. Tech. Sports Med. 2008, 16, 221–224. [Google Scholar] [CrossRef]
- Getgood, A.; Bhullar, T.P.S.; Rushton, N. Current concepts in articular cartilage repair. Orthop. Trauma 2009, 23, 189–200. [Google Scholar] [CrossRef]
- Fischman, J. How to build a body part. Time Magazine. 1 March 1999. Available online: http://www.time.com/time/magazine/article/0,9171,20592,00.html (Accessed on 1 December 2012).
- Rawe, J. What will be the 10 hottest jobs? Time Magazine. 22 May 2000. Available online: http://www.time.com/time/magazine/article/0,9171,997028,00.html (Accessed on 1 December 2012).
- Lyons, F.; Partap, S.; O’Brien, F.J. Part 1: Scaffolds and surfaces. Technol. Health Care 2008, 16, 305–317. [Google Scholar] [PubMed]
- Fritz, J.R.; Pelaez, D.; Cheung, H.S. Current challenges in cartilage tissue engineering: A review of current cellular-based therapies. Curr. Rheumatol. Rev. 2009, 5, 8–14. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J.; Gleeson, J.P. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Appelman, T.P.; Mizrahi, J.; Elisseeff, J.H.; Seliktar, D. The influence of biological motifs and dynamic mechanical stimulation in hydrogel scaffold systems on the phenotype of chondrocytes. Biomaterials 2011, 32, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Schuh, E.; Hofmann, S.; Stok, K.; Notbohm, H.; Müller, R.; Rotter, N. Chondrocyte redifferentiation in 3D: The effect of adhesion site density and substrate elasticity. J. Biomed. Mater. Res. A 2012, 100A, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Bowman, S.M.; Colhoun, H.A.; DiCarlo, E.F.; Kawcak, C.E.; McIlwraith, C.W. Evaluation of autologous chondrocyte transplantation via a collagen membrane in equine articular defects – results at 12 and 18 months. Osteoarthr. Cartil. 2008, 16, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Driesang, I.M.; Hunziker, E.B. Delamination rates of tissue flaps used in articular cartilage repair. J. Orthop. Res. 2000, 18, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Pestka, J.M.; Kreuz, P.C.; Erggelet, C.; Schmal, H.; Suedkamp, N.P.; Steinwachs, M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am. J. Sports Med. 2008, 36, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Zaslav, K.; Cole, B.; Brewster, R.; DeBerardino, T.; Farr, J.; Fowler, P.; Nissen, C. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: Results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am. J. Sports Med. 2009, 37, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; Hambly, K.; Villa, S.D.; Silvers, H.; Mandelbaum, B.R. Return to sports participation after articular cartilage repair in the knee. Am. J. Sports Med. 2009, 37, 167S–176S. [Google Scholar] [CrossRef] [PubMed]
- Gooding, C.R.; Bartlett, W.; Bentley, G.; Skinner, J.A.; Carrington, R.; Flanagan, A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee 2006, 13, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.P.; Skinner, J.A.; Carrington, R.W.; Flanagan, A.M.; Briggs, T.W.; Bentley, G. Collagen-covered autologous chondrocyte implantation for osteochondritis dissecans of the knee: Two- to seven-year results. J. Bone Joint Surg. Br. 2006, 88, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.B.; Fick, D.; Wood, D.J.; Linklater, J.M.; Zheng, M.H.; Ackland, T.R. MRI and clinical evaluation of collagen-covered autologous chondrocyte implantation (CACI) at two years. Knee 2007, 14, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Haddo, O.; Mahroof, S.; Higgs, D.; David, L.; Pringle, J.; Bayliss, M.; Cannon, S.R.; Briggs, T.W. The use of chondrogide membrane in autologous chondrocyte implantation. Knee 2004, 11, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Briggs, T.W.; Mahroof, S.; David, L.A.; Flannelly, J.; Pringle, J.; Bayliss, M. Histological evaluation of chondral defects after autologous chondrocyte implantation of the knee. J. Bone Joint Surg. Br. 2003, 85, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Steinwachs, M.R.; Guggi, T.; Kreuz, P.C. Marrow stimulation techniques. Injury 2008, 39, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Vannini, F.; Marcacci, M.; Andriolo, L.; Ferruzzi, A.; Giannini, S.; Kon, E. Matrix-assisted autologous chondrocyte transplantation for cartilage regeneration in osteoarthritic knees. Am. J. Sports Med. 2012, 41, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.H.; Willers, C.; Kirilak, L.; Yates, P.; Xu, J.; Wood, D.; Shimmin, A. Matrix-induced autologous chondrocyte implantation (MACI): Biological and histological assessment. Tissue Eng. 2007, 13, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, E.M.; Fuss, M.; Rohwedel, J.; Russlies, M.; Kuhnel, W.; Behrens, P. Development of a biocomposite to fill out articular cartilage lesions. Light, scanning and transmission electron microscopy of sheep chondrocytes cultured on a collagen I/III sponge. Ann. Anat. 1999, 181, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Cherubino, P.; Grassi, F.A.; Bulgheroni, P.; Ronga, M. Autologous chondrocyte implantation using a bilayer collagen membrane: A preliminary report. J. Orthop. Surg. 2003, 11, 10–15. [Google Scholar]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Berruto, M.; Brocchetta, D.; Delcogliano, A.; Ghinelli, D.; Gobbi, A.; Kon, E.; Pederzini, L.; Rosa, D.; Sacchetti, G.L.; Stefani, G.; Zanasi, S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin. Orthop. Relat. Res. 2005, 96–105. [Google Scholar]
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.H.; Noth, U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 2007, 9. [Google Scholar] [CrossRef] [PubMed]
- Dell’Accio, F.; Vanlauwe, J.; Bellemans, J.; Neys, J.; de Bari, C.; Luyten, F.P. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J. Orthop. Res. 2003, 21, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.J.; Malek, M.A.; Frassica, F.J.; Polder, J.A.; Mohan, A.K.; Bloom, E.T.; Braun, M.M.; Coté, T.R. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J. Bone Joint Surg. Am. 2006, 88, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Selmi, T.A.; Verdonk, P.; Chambat, P.; Dubrana, F.; Potel, J.F.; Barnouin, L.; Neyret, P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J. Bone Joint Surg. Br. 2008, 90, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Bartholomew, A.M.; Mahmud, N.; Nelson, M.; Patil, S.; Hardy, W.; Sturgeon, C.; Hewett, T.; Chung, T.; Stock, W.; Sher, D.; Weissman, S.; Ferrer, K.; Mosca, J.; Deans, R.; Moseley, A.; Hoffman, R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp. Hematol. 2001, 29, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; Huang, X.; Han, X.; Xie, N.; Ren, G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518. [Google Scholar] [CrossRef]
- Mueller, S.M.; Glowacki, J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J. Cell. Biochem. 2001, 82, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, H.-J.; Im, G.-I. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem. Biophys. Res. Commun. 2008, 373, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Garcia, M.; Ning, H.; Banie, L.; Guo, Y.L.; Lue, T.F.; Lin, C.S. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008, 17, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Tew, S.; Adesida, A.; Hardingham, T. Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res. Ther. 2008, 10, 1–11. [Google Scholar] [CrossRef]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Review: Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Im, G.I. Chondrogenesis using mesenchymal stem cells and PCL scaffolds. J. Biomed. Mater. Res. A 2010, 92, 659–666. [Google Scholar] [PubMed]
- Farrell, E.; O’Brien, F.J.; Doyle, P.; Fischer, J.; Yannas, I.; Harley, B.A.; O’Connell, B.; Prendergast, P.J.; Campbell, V.A. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006, 12, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Awad, H.A.; Fermor, B.; Leddy, H.A.; Gimble, J.M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 2004, 41, 389–399. [Google Scholar] [PubMed]
- Awad, H.A.; Quinn Wickham, M.; Leddy, H.A.; Gimble, J.M.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004, 25, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.R.; Gimble, J.M.; Franklin, D.M.; Rice, H.E.; Awad, H.; Guilak, F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophys. Research Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Samimi, B.; Zhu, M.; Hame, S.L.; Thomas, B.J.; Lieberman, J.R.; Hedrick, M.H.; Benhaim, P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J. Bone Joint Surg. Br. 2003, 85B, 740–747. [Google Scholar] [PubMed]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther. 2007, 9, R55. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Vinardell, T.; Thorpe, S.D.; Haugh, M.G.; Jones, E.; McGonagle, D.; Kelly, D.J. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J. Biomech. 2010, 43, 920–926. [Google Scholar] [CrossRef] [PubMed]
- de Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, S.; Sekiya, I.; Sakaguchi, Y.; Yagishita, K.; Ichinose, S.; Muneta, T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 2006, 97, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Varshney, R.R.; Ren, L.; Cai, D.; Wang, D.A. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng. B Rev. 2009, 15, 75–86. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Jitsuiki, J.I.; Ochi, M.; Ikuta, Y. Meniscal repair enhanced by an interpositional free synovial autograft: An experimental study in rabbits. Arthroscopy 1994, 10, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Joint Surg. 1996, 78, 721–733. [Google Scholar] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; Wolfe, M.S.; Archer, C.W. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Havlas, V.; Kos, P.; Jendelova, P.; Lesny, P.; Trc, T.; Sykova, E. Comparison of chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells with cultured chondrocytes and bone marrow mesenchymal stem cells. Acta Chir. Orthop. Traumatol. Cech. 2011, 78, 138–144. [Google Scholar] [PubMed]
- Vidal, M.A.; Robinson, S.O.; Lopez, M.J.; Paulsen, D.B.; Borkhsenious, O.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet. Surg. 2008, 37, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Reich, C.M.; Raabe, O.; Wenisch, S.; Bridger, P.S.; Kramer, M.; Arnhold, S. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells—a comparative study. Vet. Res. Commun. 2012, 36, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Vuoristo, J.T.; Larson, B.L.; Prockop, D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc. Natl. Acad. Soc. USA 2002, 99, 4397–4402. [Google Scholar] [CrossRef]
- Fischer, J.; Dickhut, A.; Rickert, M.; Richter, W. Human articular chondrocytes secrete parathyroid hormone–related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010, 62, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Mauck, R.L.; Burdick, J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. A 2011, 17, 1137–1145. [Google Scholar] [CrossRef]
- Wu, L.; Leijten, J.C.; Georgi, N.; Post, J.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng. A 2011, 17, 1425–1436. [Google Scholar] [CrossRef]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Capito, R.M.; Spector, M. Scaffold-based articular cartilage repair. IEEE Eng. Med. Biol. Mag. 2003, 22, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Coutts, R.D.; Healey, R.M.; Ostrander, R.; Sah, R.L.; Goomer, R.; Amiel, D. Matrices for cartilage repair. Clin. Orthop. Relat. Res. 2001, 391, S271–S279. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.S.; van Wachem, P.B.; van Luyn, M.J.A.; Brouwer, L.A.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials 2000, 21, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Schagemann, J.C.; Kurz, H.; Casper, M.E.; Stone, J.S.; Dadsetan, M.; Yu-Long, S.; Mrosek, E.H.; Fitzsimmons, J.S.; O’Driscoll, S.W.; Reinholz, G.G. The effect of scaffold composition on the early structural characteristics of chondrocytes and expression of adhesion molecules. Biomaterials 2010, 31, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Porous poly(α-hydroxyacid)/Bioglass® composite scaffolds for bone tissue engineering. I. Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, T.H.; Im, G.I.; Lee, J.H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.S.; van der Kraan, P.M.; Hafmans, T.; Kamp, J.; Buma, P.; van Susante, J.L.C.; van den Berg, W.B.; Veerkamp, J.H.; van Kuppevelt, T.H. Crosslinked type II collagen matrices: Preparation, characterization, and potential for cartilage engineering. Biomaterials 2002, 23, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 2009, 3, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.; Leung, J.H.; Silva, E.C.C.M.; Gibson, L.J. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater. 2007, 3, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O'Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Mueller, S.M.; Shortkroff, S.; Schneider, T.O.; Breinan, H.A.; Yannas, I.V.; Spector, M. Meniscus cells seeded in type I and type II collagen-GAG matrices in vitro. Biomaterials 1999, 20, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol. Bioeng. 2006, 93, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Tierney, C.M.; Jaasma, M.J.; O'Brien, F.J. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J. Biomed. Mater. Res. A 2009, 91A, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980, 14, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Louie, L.K.; Yannas, I.V.; Hsu, H.P.; Spector, M. Healing of tendon defects implanted with a porous collagen-GAG matrix: Histological evaluation. Tissue Eng. 1997, 3, 187–195. [Google Scholar] [CrossRef]
- Chamberlain, L.J.; Yannas, I.V.; Hsu, H.P.; Strichartz, G.; Spector, M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp. Neurol. 1998, 154, 315–329. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Alhag, M.; Farrell, E.; Toner, M.; Claffey, N.; Lee, T.; O’Brien, F. Evaluation of early healing events around mesenchymal stem cell-seeded collagen–glycosaminoglycan scaffold. An experimental study in Wistar rats. Oral Maxillofac. Surg. 2011, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- van Susante, J.L.C.; Pieper, J.; Buma, P.; van Kuppevelt, T.H.; van Beuningen, H.; van der Kraan, P.M.; Veerkamp, J.H.; van den Berg, W.B.; Veth, R.P.H. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 2001, 22, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Grigolo, B.; Lisignoli, G.; Piacentini, A.; Fiorini, M.; Gobbi, P.; Mazzotti, G.; Duca, M.; Pavesio, A.; Facchini, A. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAFF®11): Molecular, immunohistochemical and ultrastructural analysis. Biomaterials 2002, 23, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Kon, E.; Berruto, M.; Francisco, R.; Filardo, G.; Marcacci, M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C. Am. J. Sports Med. 2006, 34, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O'Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.K.; Stevens, R.; Pearson, R.G.; Davies, M.C.; Tendler, S.J.B.; Roberts, C.J.; Williams, P.M.; Shakesheff, K.M. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J. Biomed. Mater. Res. 2002, 61, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O'Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.R.; Engler, A.J. Stiffness gradients mimicking tissue variation regulate mesenchymal stem cell fate. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.D.; Sheehan, J.K. Structure for hyaluronic acid. Nat. New Biol. 1972, 235, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Sperling, J.W.; Sanyal, A.; Fitzsimmons, J.S.; Reinholz, G.G.; Conover, C.A.; O’Driscoll, S.W. Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr. Cartil. 2003, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ziran, N.; Goater, J.J.; Schwarz, E.M.; Puzas, J.E.; Rosier, R.N.; Zuscik, M.; Drissi, H.; O’Keefe, R.J. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-β delays hypertrophy and PGE2 inhibits terminal differentiation. Bone 2004, 34, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Kelly, D.J. Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J. Mech. Behav. Biomed. Mater. 2012, 11, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Estes, B.T.; Guilak, F. The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. J. Biomed. Mater. Res. A 2010, 93A, 994–1003. [Google Scholar] [PubMed]
- Jones, J.I.; Gockerman, A.; Busby, W.H., Jr.; Camacho-Hubner, C.; Clemmons, D.R. Extracellular matrix contains insulin-like growth factor binding protein-5: Potentiation of the effects of IGF-I. J. Cell Biol. 1993, 121, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Suetterlin, R.; Baschong, W.; Heberer, M.; Vunjak-Novakovic, G.; Freed, L.E. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J. Cell. Biochem. 2001, 83, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Dickhut, A.; Dexheimer, V.; Martin, K.; Lauinger, R.; Heisel, C.; Richter, W. Chondrogenesis of human mesenchymal stem cells by local transforming growth factor-beta delivery in a biphasic resorbable carrier. Tissue Eng. A 2010, 16, 453–464. [Google Scholar] [CrossRef]
- Yaeger, P.C.; Masi, T.S.L.; de Ortiz, J.L.B.; Binette, F.O.; Tubo, R.; McPherson, J.M. Synergistic action of transforming growth factor-[beta] and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp. Cell Res. 1997, 237, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chung, U.-I.; Yang, D.; Karsenty, G.; Bringhurst, F.R.; Kronenberg, H.M. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev. Biol. 2006, 292, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Kieswetter, K.; Schwartz, Z.; Alderete, M.; Dean, D.D.; Boyan, B.D. Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation. Endocrine 1997, 6, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Angele, P.; Roll, C.; Prantl, L.; Kujat, R.; Kinner, B. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J. Orthop. Res. 2010, 28, 354–360. [Google Scholar] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Davidson, J.M.; Guelcher, S.A. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials 2009, 30, 3486–3494. [Google Scholar] [CrossRef] [PubMed]
- Buket Basmanav, F.; Kose, G.T.; Hasirci, V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials 2008, 29, 4195–4204. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Hinckfuss, A.; Bilgen, B.; Ciombor, D.M.; Aaron, R.; Mathiowitz, E. Sequential release of bioactive IGF-I and TGF-β1 from PLGA microsphere-based scaffolds. Biomaterials 2008, 29, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.E.; Kwon, I.C.; Ahn, H.J.; Lee, S.-H.; Cho, H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of the controlled-released TGF-[beta]1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 2004, 25, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Sgaglione, N.A. The future of cartilage restoration. J. Knee Surg. 2004, 17, 235–243. [Google Scholar] [PubMed]
- Palmer, G.D.; Steinert, A.; Pascher, A.; Gouze, E.; Gouze, J.-N.; Betz, O.; Johnstone, B.; Evans, C.H.; Ghivizzani, S.C. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol. Ther. 2005, 12, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999, 5, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.-A.; O'Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release 2012, 158, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Bolliet, C.; Bohn, M.C.; Spector, M. Non-viral delivery of the gene for glial cell line-derived neurotrophic factor to mesenchymal stem cells in vitro via a collagen scaffold. Tissue Eng. C Methods 2008, 14, 207–219. [Google Scholar] [CrossRef]
- Im, G.I.; Kim, H.J.; Lee, J.H. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials 2011, 32, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Doukas, J.; Chandler, L.A.; Gonzalez, A.M.; Gu, D.; Hoganson, D.K.; Ma, C.; Nguyen, T.; Printz, M.A.; Nesbit, M.; Herlyn, M.; Crombleholme, T.M.; Aukerman, S.L.; Sosnowski, B.A.; Pierce, G.F. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum. Gene Ther. 2001, 12, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Kaul, G.; Cucchiarini, M.; Stein, U.; Zurakowski, D.; Remberger, K.; Menger, M.D.; Kohn, D.; Trippel, S.B. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther. 2005, 12, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A. In vivo nonviral delivery factors to enhance bone repair. Clin. Orthop. Related Res. 2000, 379, S113–S119. [Google Scholar] [CrossRef]
- Capito, R.M.; Spector, M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007, 14, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Haupt, J.L.; Frisbie, D.D.; McIlwraith, C.W.; Robbins, P.D.; Ghivizzani, S.; Evans, C.H.; Nixon, A.J. Dual transduction of insulin-like growth factor-I and interleukin-l receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J. Orthop. Res. 2005, 23, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.J.; Haupt, J.L.; Frisbie, D.D.; Morisset, S.S.; McIlwraith, C.W.; Robbins, P.D.; Evans, C.H.; Ghivizzani, S. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I//interleukin-1 receptor antagonist therapy. Gene Ther. 2004, 12, 177–186. [Google Scholar] [CrossRef]
- Mason, J.M.; Breitbart, A.S.; Barcia, M.; Porti, D.; Pergolizzi, R.G.; Grande, D.A. Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin. Orthop. Relat. Res. 2000, 379, S171–S178. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, D.S.; Pruchnic, R.; Bosch, P.; Ziran, B.H.; Whalen, J.; Huard, J. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J. Bone Joint Surg. Br. 2002, 84B, 120–127. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsiko, A.; Levingstone, T.J.; O'Brien, F.J. Advanced Strategies for Articular Cartilage Defect Repair. Materials 2013, 6, 637-668. https://doi.org/10.3390/ma6020637
Matsiko A, Levingstone TJ, O'Brien FJ. Advanced Strategies for Articular Cartilage Defect Repair. Materials. 2013; 6(2):637-668. https://doi.org/10.3390/ma6020637
Chicago/Turabian StyleMatsiko, Amos, Tanya J. Levingstone, and Fergal J. O'Brien. 2013. "Advanced Strategies for Articular Cartilage Defect Repair" Materials 6, no. 2: 637-668. https://doi.org/10.3390/ma6020637





