Graphene versus Multi-Walled Carbon Nanotubes for Electrochemical Glucose Biosensing
Abstract
:1. Introduction
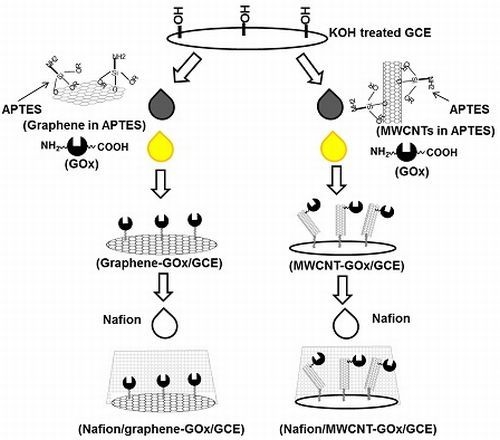
2. Results and Discussion
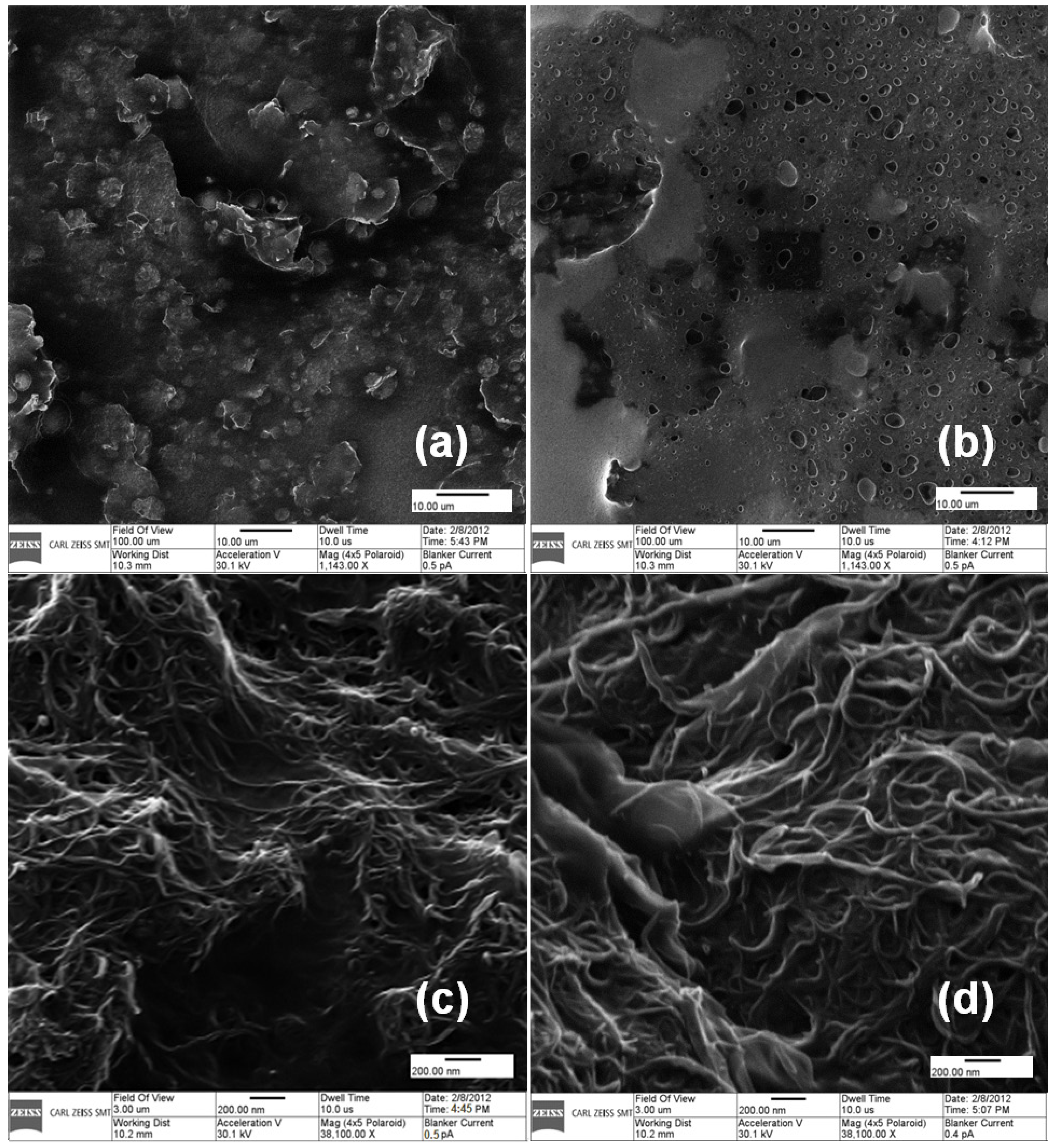
2.1. Development of GOx-Bound Graphene- and MWCNT-Functionalized Glassy Carbon Electrodes (GCEs)
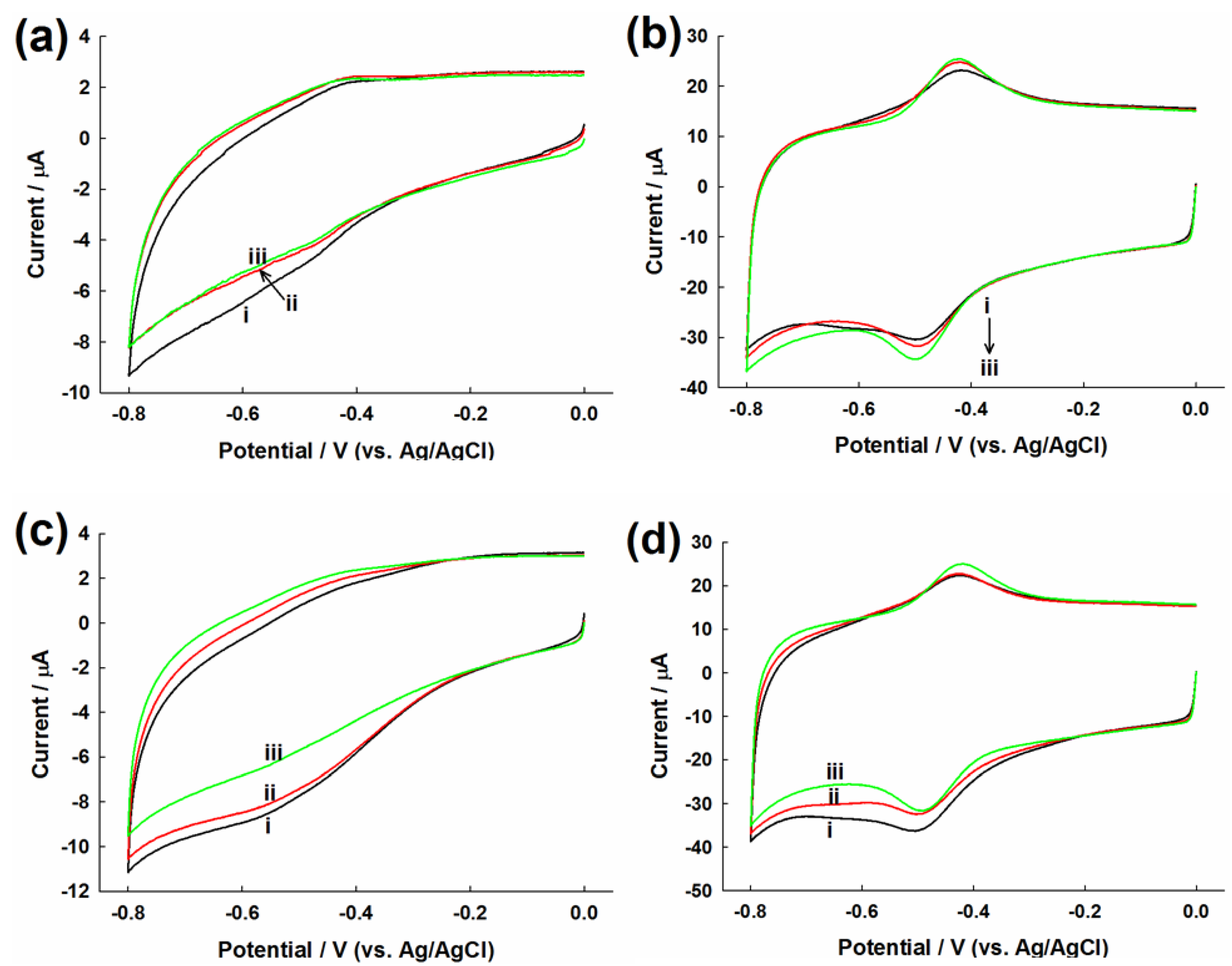
2.2. Evaluation of Direct Electron Transfer
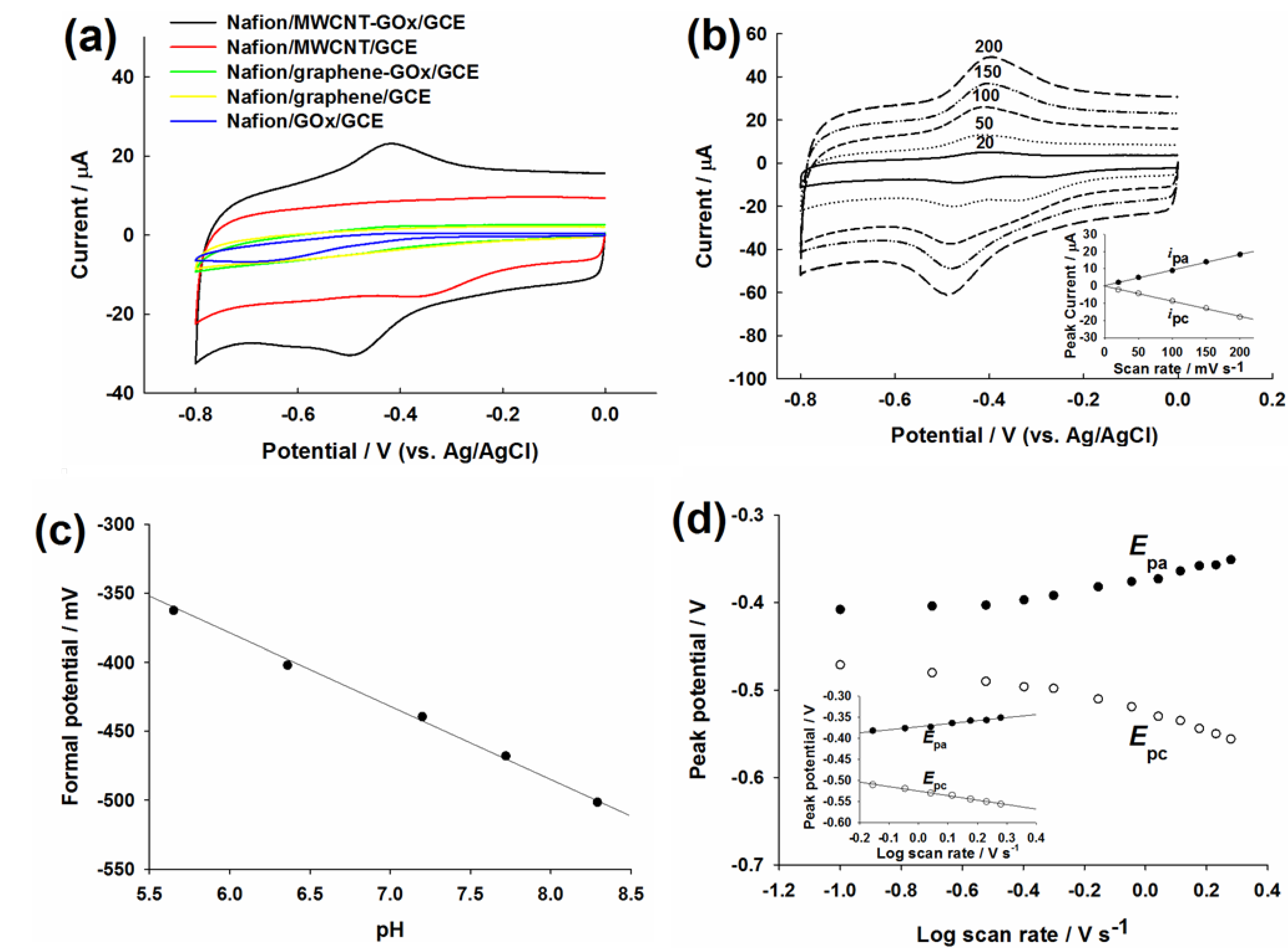
2.3. Evaluation of Glucose Oxidation
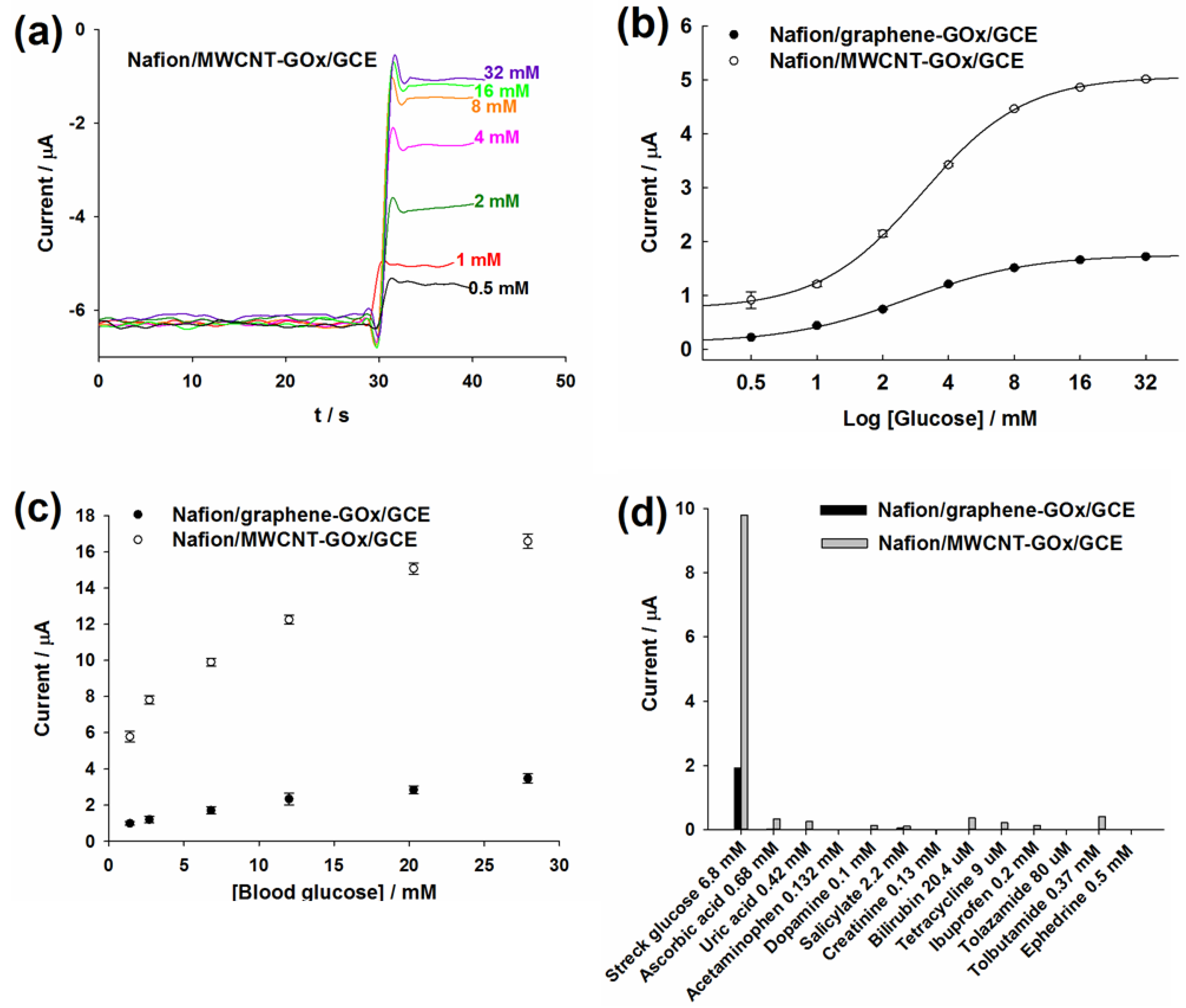
2.4. Amperometric Detection of Commercial and Blood Glucose
| Graphene- or CNT-based Glucose Biosensor | Glucose Linear Range (mM) | Real Sample Study | Interfering Study | Reference |
|---|---|---|---|---|
| Nafion/graphene-GOx/GCE | 0.5–4 (dynamic range: 0.5–16) | Detect 1.4–27.9 mM blood glucose in diluted Streck samples | No interference from physiological levels of interfering substances | This work |
| Graphene oxide-chitosan-GOx/GCE | 4 × 10−4–2 | Detect 5 and 10 mM glucose added into serum samples | No interference from 2 mM ascorbic acid, uric acid, citric acid and acetaminophen; not testing for other interfering substances | [54] |
| Carboxyl-long-chain-graphene oxide modified with Fe3O4, polyaniline and GOx | 1–1.4 | Detect blood glucose (0.2–1.4 mM) in diluted serum samples | No interference from 0.3 mM ascorbic acid and uric acid and 0.01 mM immunoglobulin G; not testing for other interfering substances | [55] |
| Palladium nanoparticle/chitosan-grafted graphene/GCE | 1 × 10−3–1 | Detect blood glucose in diluted blood samples (recovery: 92.5%–105.3%) | No interference from 0.2mM ascorbic acid and 0.5 mM uric acid; not testing for other interfering substances | [56] |
| Nafion/MWCNT-GOx/GCE | 0.5–4 (dynamic range: 0.5–16) | Detect 1.4–27.9 mM blood glucose in diluted Streck samples | Negligible interference from interfering substances | This work |
| A mixture of GOx and a CNT film sandwiched with 10 nm thick PPFs | 0.025–2.2 | Not testing for real samples | No interference from 0.5 mM ascorbic acid | [57] |
| Incorporation of GOx into the colloidal Au-CNT composite matrix | 0.05–1 | Not testing for real samples | No interference from 1 μM cysteine and 0. 1 μM uric acid; significant interference from 1 μM ascorbic acid; not testing for other interfering substances | [58] |
| GOx-platinum nanoparticle-CNT-titania nanotube array modified electrode | 6 × 10−3–1.5 | Not testing for real samples | Not testing for the effect of interfering substances | [59] |
2.5. Effect of Interfering Substances
3. Experimental Section
3.1. Chemicals
3.2. Apparatus and Measurement
3.3. Biosensor Fabrication
3.4. Amperometric Glucose Detection
3.5. Evaluating the Effect of Interferences on Glucose Detection
4. Conclusions
Supplementary material
Supplementary File 1Acknowledgments
References
- Wang, X.; Zhi, L.J.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Vivekchand, S.R.C.; Rout, C.S.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, C.N.R. Graphene-Based electrochemical supercapacitors. J. Chem. Sci. 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, P.K.; Chen, W.; Wee, A.T.S.; Loh, K.P. Solution-Gated epitaxial graphene as pH sensor. J. Am. Chem. Soc. 2008, 130, 14392–14393. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416:1–125416:6. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.M.; Tang, L.H.; Lu, J.; Li, J.H. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, S.N.; Wu, P.; Cai, C.X. Detection of glucose based on direct electron transfer reaction of glucose oxidase immobilized on highly ordered polyaniline nanotubes. Anal. Chem. 2009, 81, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Munro, L.J.; Kampouris, D.K.; Banks, C.E. Electrochemistry of graphene: Not such a beneficial electrode material? RSC Adv. 2011, 1, 978–988. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.S.; Cui, H.F.; Liu, X.; Lim, T.M.; Zhang, W.D.; Sheu, F.S. Preparation and characterization of aligned carbon nanotube-ruthenium oxide nanocomposites for supercapacitors. Small 2005, 1, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.S.; Liu, X.; Cui, H.F.; Zhang, W.D.; Sheu, F.S.; Lim, T.M. Electrochemical oxidation of multi-walled carbon nanotubes and its application to electrochemical double layer capacitors. Electrochem. Commun. 2005, 7, 249–255. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- D’Souza, F.; Ito, O. Photosensitized electron transfer processes of nanocarbons applicable to solar cells. Chem. Soc. Rev. 2012, 41, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A.; Surman, C.; Nagraj, N.; Burns, A. Materials and transducers toward selective wireless gas sensing. Chem. Rev. 2011, 111, 7315–7354. [Google Scholar] [CrossRef] [PubMed]
- Wanekaya, A.K. Applications of nanoscale carbon-based materials in heavy metal sensing and detection. Analyst 2011, 136, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Zheng, D.; Pastorin, G.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Delivery of drugs and biomolecules using carbon nanotubes. Carbon 2011, 49, 4077–4097. [Google Scholar] [CrossRef]
- Ye, J.S.; Sheu, F.S. Functionalization of CNTs: New routes towards the development of novel electrochemical sensors. Curr. Nanosci. 2006, 2, 319–327. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Aboutalebi, S.H.; Chidembo, A.T.; Salari, M.; Konstantinov, K.; Wexler, D.; Liu, H.K.; Dou, S.X. Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ. Sci. 2011, 4, 1855–1865. [Google Scholar] [CrossRef]
- Biswas, C.; Lee, Y.H. Graphene versus carbon nanotubes in electronic devices. Adv. Funct. Mater. 2011, 21, 3806–3826. [Google Scholar] [CrossRef]
- Henwood, D.; Carey, J.D. Ab initio investigation of molecular hydrogen physisorption on graphene and carbon nanotubes. Phys. Rev. B 2007, 75, 245413:1–245413:10. [Google Scholar] [CrossRef]
- Yang, W.R.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Edit. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Graphene versus carbon nanotubes for chemical sensor and fuel cell applications. Analyst 2010, 135, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Christ, K.V.; Sadeghpour, H.R. Energy dispersion in graphene and carbon nanotubes and molecular encapsulation in nanotubes. Phys. Rev. B 2007, 75, 195418:1–195418:7. [Google Scholar]
- Xie, S.H.; Liu, Y.Y.; Li, J.Y. Comparison of the effective conductivity between composites reinforced by graphene nanosheets and carbon nanotubes. Appl. Phys. Lett. 2008, 92, 243121:1–243121:3. [Google Scholar]
- Du, J.H.; Zhao, L.; Zeng, Y.; Zhang, L.L.; Li, F.; Liu, P.F.; Liu, C. Comparison of electrical properties between multi-walled carbon nanotube and graphene nanosheet/high density polyethylene composites with a segregated network structure. Carbon 2011, 49, 1094–1100. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tang, Z.R.; Fu, X.; Xu, Y.J. Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: What advantage does graphene have over its forebear carbon nanotube? ACS Nano 2011, 5, 7426–7435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Q.; Cui, T.H. Ultra-Sensitive suspended graphene nanocomposite cancer sensors with strong suppression of electrical noise. Biosens. Bioelectron. 2012, 31, 105–109. [Google Scholar] [CrossRef] [PubMed]
- You, J.M.; Kim, D.; Jeon, S. Electrocatalytic reduction of H2O2 by Pt nanoparticles covalently bonded to thiolated carbon nanostructures. Electrochim. Acta 2012, 65, 288–293. [Google Scholar] [CrossRef]
- Liu, C.; Alwarappan, S.; Chen, Z.F.; Kong, X.X.; Li, C.Z. Membraneless enzymatic biofuel cells based on graphene nanosheets. Biosens. Bioelectron. 2010, 25, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Vashist, S.K.; O’Neill, F.T.; O’Reilly, B.; MacCraith, B.D.; O’Kennedy, R. Development of a high sensitivity rapid sandwich ELISA procedure and its comparison with the conventional approach. Anal. Chem. 2010, 82, 7049–7052. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Vashist, S.K.; MacCraith, B.D.; O’Kennedy, R. Multisubstrate-compatible ELISA procedures for rapid and high-sensitivity immunoassays. Nat. Protoc. 2011, 6, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Dixit, C.K.; MacCraith, B.D.; O’Kennedy, R. Effect of antibody immobilization strategies on the analytical performance of a surface plasmon resonance-based immunoassay. Analyst 2011, 136, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Mediatorless amperometric glucose biosensing using 3-aminopropyltriethoxysilane-functionalized graphene. Talanta 2012, 99, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Hrapovic, S.; Wang, D.; Bensebaa, F.; Simard, B. Solubilization of multiwall carbon nanotubes by 3-aminopropyltriethoxysilane towards the fabrication of electrochemical biosensors with promoted electron transfer. Electroanalysis 2004, 16, 132–139. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; p. 231. [Google Scholar]
- Deng, C.Y.; Chen, J.H.; Nie, Z.; Si, S.H. A sensitive and stable biosensor based on the direct electrochemistry of glucose oxidase assembled layer-by-layer at the multiwall carbon nanotube-modified electrode. Biosens. Bioelectron. 2010, 26, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Liu, L.; Li, M.G.; Xu, S.D.; Gao, F. Multifunctional carbon nanotubes for direct electrochemistry of glucose oxidase and glucose bioassay. Biosens. Bioelectron. 2011, 30, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Li, C.M. Direct electron transfer of glucose oxidase and biosensing of glucose on hollow sphere-nanostructured conducting polymer/metal oxide composite. Phys. Chem. Chem. Phys. 2010, 12, 12153–12159. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.F.; Zheng, J.B. Amine-terminated ionic liquid functionalized carbon nanotube-gold nanoparticles for investigating the direct electron transfer of glucose oxidase. Electrochem. Commun. 2009, 11, 608–611. [Google Scholar] [CrossRef]
- Zhang, H.F.; Meng, Z.C.; Wang, Q.; Zheng, J.B. A novel glucose biosensor based on direct electrochemistry of glucose oxidase incorporated in biomediated gold nanoparticles-carbon nanotubes composite film. Sens. Actuators B Chem. 2011, 158, 23–27. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Hashemnia, S.; Khayatzadeh, S.; Moosavi-Movahedi, A.A.; Ghourchian, H. Direct Electrochemistry of catalase in multiwall carbon nanotube/dodecyl trimethylammonium bromide film covered with a layer of Nafion on a glassy carbon electrode. Int. J. Electrochem. Sc. 2011, 6, 581–595. [Google Scholar]
- Janegitz, B.C.; Pauliukaite, R.; Ghica, M.E.; Brett, C.M.A.; Fatibello, O. Direct electron transfer of glucose oxidase at glassy carbon electrode modified with functionalized carbon nanotubes within a dihexadecylphosphate film. Sens. Actuators B Chem. 2011, 158, 411–417. [Google Scholar] [CrossRef]
- Zhao, Y.D.; Zhang, W.D.; Chen, H.; Luo, Q.M. Direct electron transfer of glucose oxidase molecules adsorbed onto carbon nanotube powder microelectrode. Anal. Sci. 2002, 18, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Hu, S.S. Direct electrochemistry of glucose oxidase in a colloid Au-dihexadecylphosphate composite film and its application to develop a glucose biosensor. Bioelectrochemistry 2007, 70, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Lu, Z.Z.; Luo, S.L.; Liu, C.B.; Tang, Y.H. Direct electrodeposition of a biocomposite consisting of reduced graphene oxide, chitosan and glucose oxidase on a glassy carbon electrode for direct sensing of glucose. Microchim. Acta 2013, 180, 127–135. [Google Scholar] [CrossRef]
- Yang, H.W.; Hua, M.Y.; Chen, S.L.; Tsai, R.Y. Reusable sensor based on high magnetization carboxyl-modified graphene oxide with intrinsic hydrogen peroxide catalytic activity for hydrogen peroxide and glucose detection. Biosens. Bioelectron. 2013, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cheng, J.S.; Liu, X.F.; Bai, H.T.; Jiang, J.H. Palladium nanoparticle/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor. Biosens. Bioelectron. 2011, 26, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, H.; Shibayama, Y.; Matsui, Y. An amperometric biosensor based on a composite of single-walled carbon nanotubes, plasma-polymerized thin film, and an enzyme. Biosens. Bioelectron. 2008, 23, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.D.; Zhou, W.M.; Guo, J.; Wang, R.; Liang, R.P. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.Y.; He, D.M.; Luo, S.L.; Cai, Q.Y. An amperometric glucose biosensor fabricated with Pt nanoparticle-decorated carbon nanotubes/TiO2 nanotube arrays composite. Sens. Actuators B Chem. 2009, 137, 134–138. [Google Scholar] [CrossRef]
- Zheng, D.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Rapid and simple preparation of a reagentless glucose electrochemical biosensor. Analyst 2012, 137, 3800–3805. [Google Scholar] [CrossRef] [PubMed]
- Siegert, L.; Kampouris, D.K.; Kruusma, J.; Sammelsels, V.; Banks, C.E. The heterogeneity of multiwalled and single walled carbon nanotubes: Iron impurities can catalyze the electrochemical oxidation of glucose. Electroanalytical 2009, 21, 48–51. [Google Scholar] [CrossRef]
- Stuart, M.J.E.; Pumera, M. Signal transducers and enzyme cofactors are susceptible to oxidation by nanographite impurities in carbon nanotube materials. Chem. Eur. J. 2011, 17, 5544–5548. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, D.; Vashist, S.K.; Dykas, M.M.; Saha, S.; Al-Rubeaan, K.; Lam, E.; Luong, J.H.T.; Sheu, F.-S. Graphene versus Multi-Walled Carbon Nanotubes for Electrochemical Glucose Biosensing. Materials 2013, 6, 1011-1027. https://doi.org/10.3390/ma6031011
Zheng D, Vashist SK, Dykas MM, Saha S, Al-Rubeaan K, Lam E, Luong JHT, Sheu F-S. Graphene versus Multi-Walled Carbon Nanotubes for Electrochemical Glucose Biosensing. Materials. 2013; 6(3):1011-1027. https://doi.org/10.3390/ma6031011
Chicago/Turabian StyleZheng, Dan, Sandeep Kumar Vashist, Michal Marcin Dykas, Surajit Saha, Khalid Al-Rubeaan, Edmond Lam, John H.T. Luong, and Fwu-Shan Sheu. 2013. "Graphene versus Multi-Walled Carbon Nanotubes for Electrochemical Glucose Biosensing" Materials 6, no. 3: 1011-1027. https://doi.org/10.3390/ma6031011