2. Results and Discussion
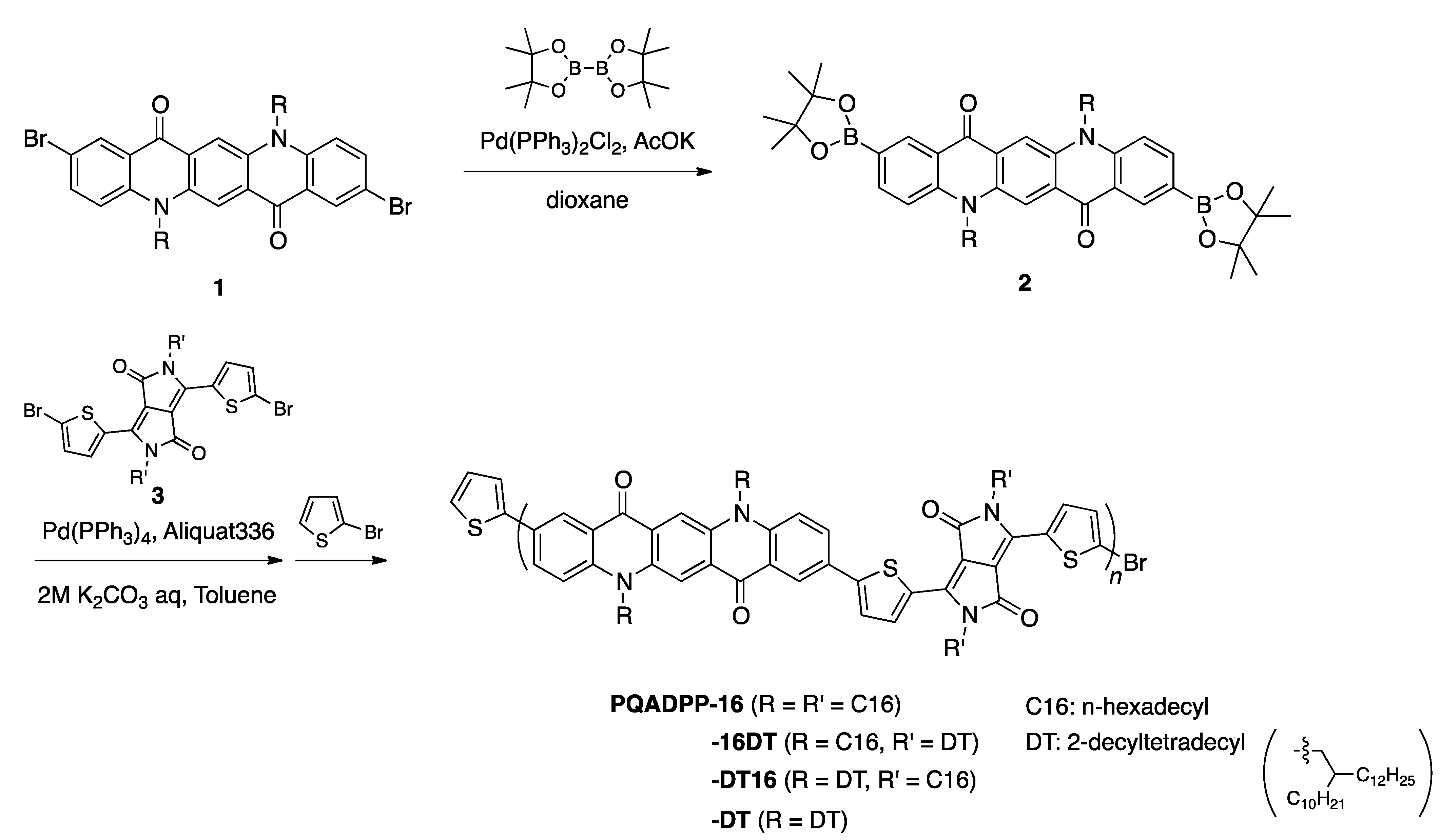
Scheme 1 shows the synthesis of QA-DPP polymers. 2,9-Dibromo
-N,
N-dialkylquino[2,3-
b]acridine-7,14-dione (
1) [
24] was reacted with bis(pinacolato)diboron in the presence of Pd(PPh
3)
2Cl
2 to afford 5,12-dialkyl-2,9-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinolino[2,3-
b]acridine-7,14(5
H,12
H)-dione (
2) as the comonomer.
2 was then copolymerized with 3,6-bis-(5-bromo-thiophen-2-yl)-
N,
N′-bis(alkyl)-1,4-dioxopyrrolo[3,4-
c]pyrrole (
3) via the Suzuki-Miyaura coupling reaction, yielding the desired polymers. The alkyl groups introduced in
2 and
3 are
n-hexadecyl (C16) and 2-decyltetradecyl (DT), and thus four polymers with different side chain combination were prepared (PQADPP-16, -16DT, -DT16, and DT). Molecular weight of the polymers is summarized in
Table 1. While PQADPP-16DT, -DT16, and DT are soluble in chloroform, PQADPP-16 is only soluble in hot chlorobenzene or
o-dichlorobenzene, which is likely as a result of the reduced solubility due to the introduction of linear alkyl groups in both the QA and DPP units. Whereas relatively high molecular weights of
Mn > 15 kDa are obtained for PQADPP-16DT, -DT16, and -DT, PQADPP-16 gives low molecular weight of
Mn = 7.4 kDa. This is probably due to the low solubility of the polymer as evidenced by the fact that the polymer had precipitated during the polymerization.
Scheme 1.
Synthesis and chemical structure of the polymers based on quinacridone (QA) and diketopyrrolopyrrole (DPP).
Scheme 1.
Synthesis and chemical structure of the polymers based on quinacridone (QA) and diketopyrrolopyrrole (DPP).
Table 1.
Chemical properties of the polymers.
Table 1.
Chemical properties of the polymers.
| Polymer | Mn (kDa) a | Mw (kDa) a | PDI a | DPn a | λmax (nm) b | EHOMO (eV) c | µ (cm2/Vs) d |
|---|
| solution | film |
|---|
| PQADPP-16 | 7.4 | 10.5 | 1.4 | 4.9 | 645, 704 | 653, 710 | –5.2 | 6.8 × 10−3 |
| PQADPP-16DT | 28.1 | 91.7 | 3.3 | 16.2 | 624, 688 | 640, 696 | –5.2 | 5.3 × 10−4 |
| PQADPP-DT16 | 19.4 | 34.7 | 1.8 | 11.2 | 627, 689 | 642, 708 | –5.2 | 2.3 × 10−4 |
| PQADPP-DT | 14.8 | 59.4 | 3.3 | 7.6 | 623, 686 | 630, 692 | –5.2 | N.D. |
Photoelectron spectroscopy (PESA) revealed that the HOMO level (
EHOMO) of the polymers is ca. −5.2 eV.
Figure 2 depicts the UV-Vis absorption spectra of the polymers in the chlorobenzene solution and in the film. Note that the spectrum for PQADPP-16 in the solution was measured at 100 °C, due to its low solubility. All polymers show similar spectra with the absorption maxima (
λmax) and absorption edge (
λedge) of around 690 ~ 700 nm and 720 ~ 750 nm, respectively, in the solution. The
λmax slightly red-shifts by ca. 10 nm in the thin film. These
λmax in the present polymers are far red-shifted as compared to PQA2T,
λmax ≈ 470 nm and
λedge ≈ 600 nm, but blue-shifted as compared to the common DPP-based polymers, e.g., for PDQT,
λmax ≈ 790 nm and
λedge ≈ 900 nm.
Figure 2.
UV-Vis absorption spectra of the polymers in chlorobenzene solution (a) and in thin film (b).
Figure 2.
UV-Vis absorption spectra of the polymers in chlorobenzene solution (a) and in thin film (b).
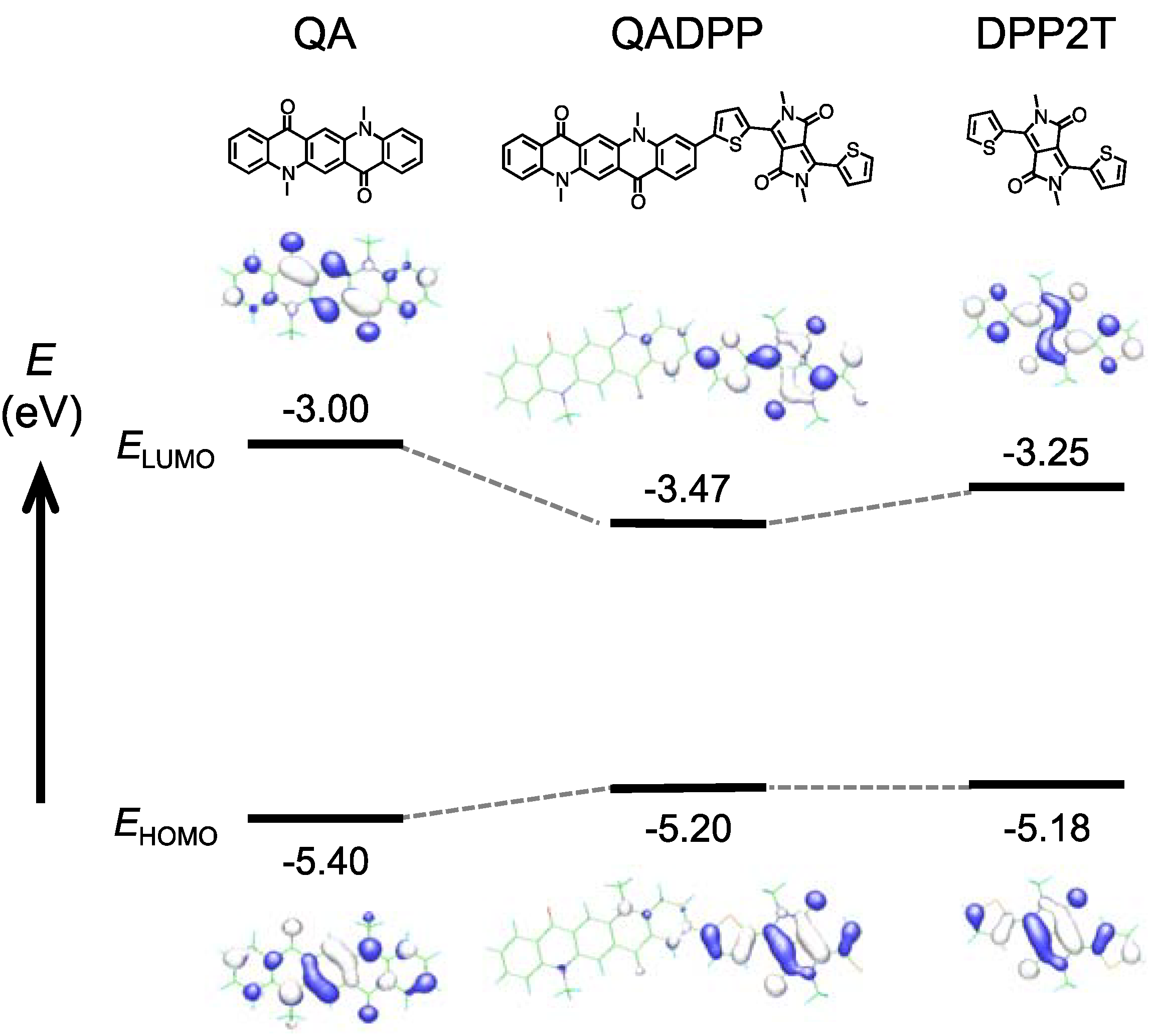
Figure 3 shows the computation (DFT at B3LYP/6-31(d) level) of the HOMO and LUMO geometry of quinacridone (QA, left), polymer repeat unit (QADPP, middle), and dithienyl diketopyrrolopyrrole (DPP2T).
EHOMO and LUMO level (
ELUMO) for each compound are also shown.
EHOMO and
ELUMO for QA and DPP2T were determined by electrochemistry.
EHOMO and
ELUMO used for QADPP are those of the polymer (PQADPP-16DT), in which
ELUMO is determined by the addition of the optical bandgap calculated from the absorption onset (1.73 eV) to
EHOMO (−5.2 eV). It is interesting to note that although HOMOs and LUMOs of both QA and DPP2T are distributed along the molecule long axes, those of QADPP localizes only on the DPP unit. It is likely that since
EHOMO and
ELUMO of DPP2T are higher and lower than those of QA, respectively, as depicted in
Figure 3, the electronic structure of DPP2T is dominant in that of QADPP. This speculation may be supported by the fact that both
EHOMO and
ELUMO of the polymers are close to those of DPP2T.
OFETs are fabricated using a bottom-gate top-contact configuration, in which the polymer solution was spin-coated on the hexamethyldisilazane (HMDS)-treated Si/SiO
2 substrate and Au source and drain electrodes are vacuum-deposited after annealing the resulting polymer film at 150 °C for 30 min.
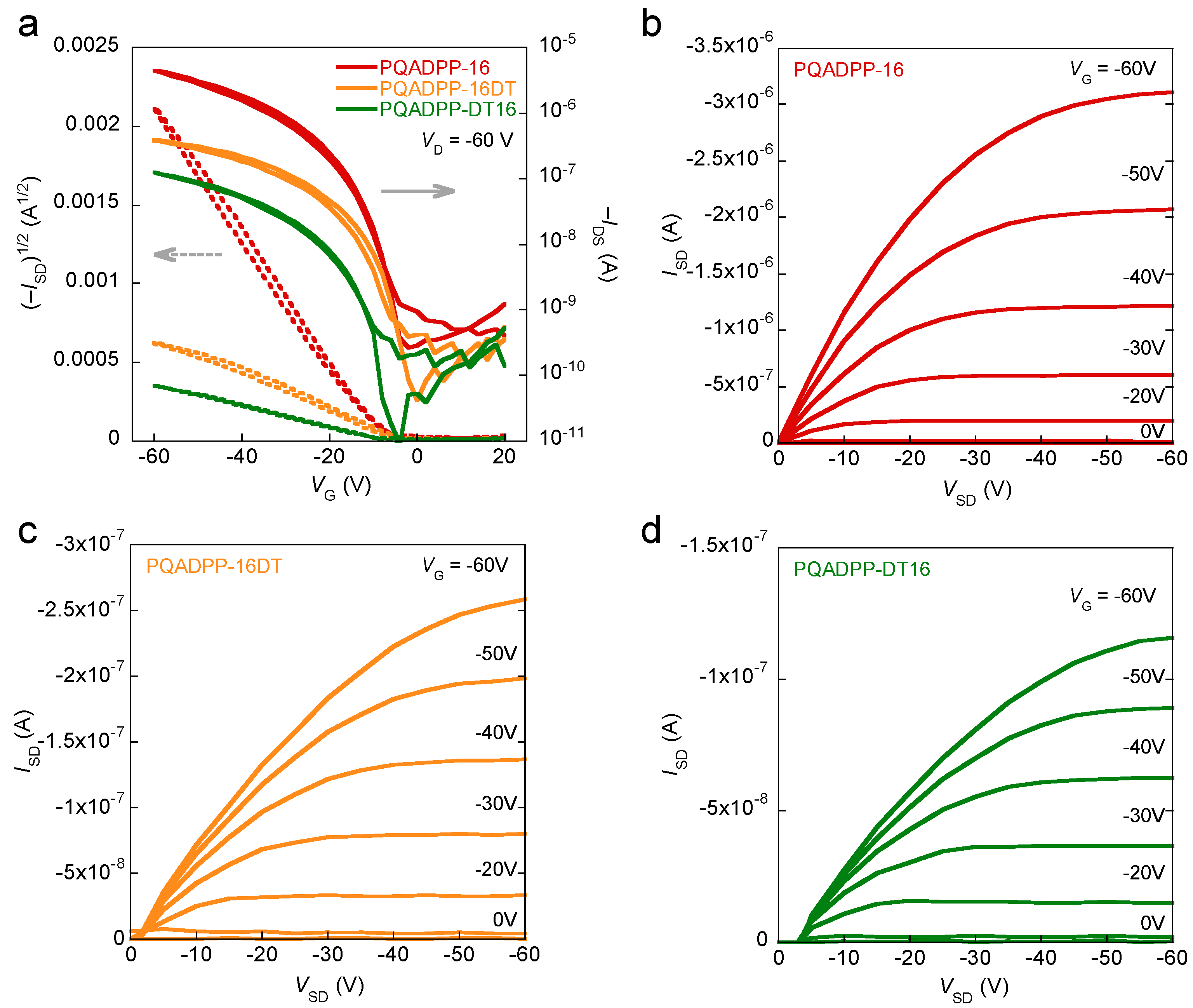
Figure 4 shows the transfer curves and output curves of the polymer devices. While PQADPP-16, -16DT, -DT16 showed p-channel characteristics, PQADPP-DT was inactive. The maximum mobilities evaluated from the saturation regime are summarized in
Table 1. Whereas modest mobilities, up to 6.8 × 10
−3 cm
2/Vs, are obtained for PQADPP-16, lower mobilities with the order of 10
−4 cm
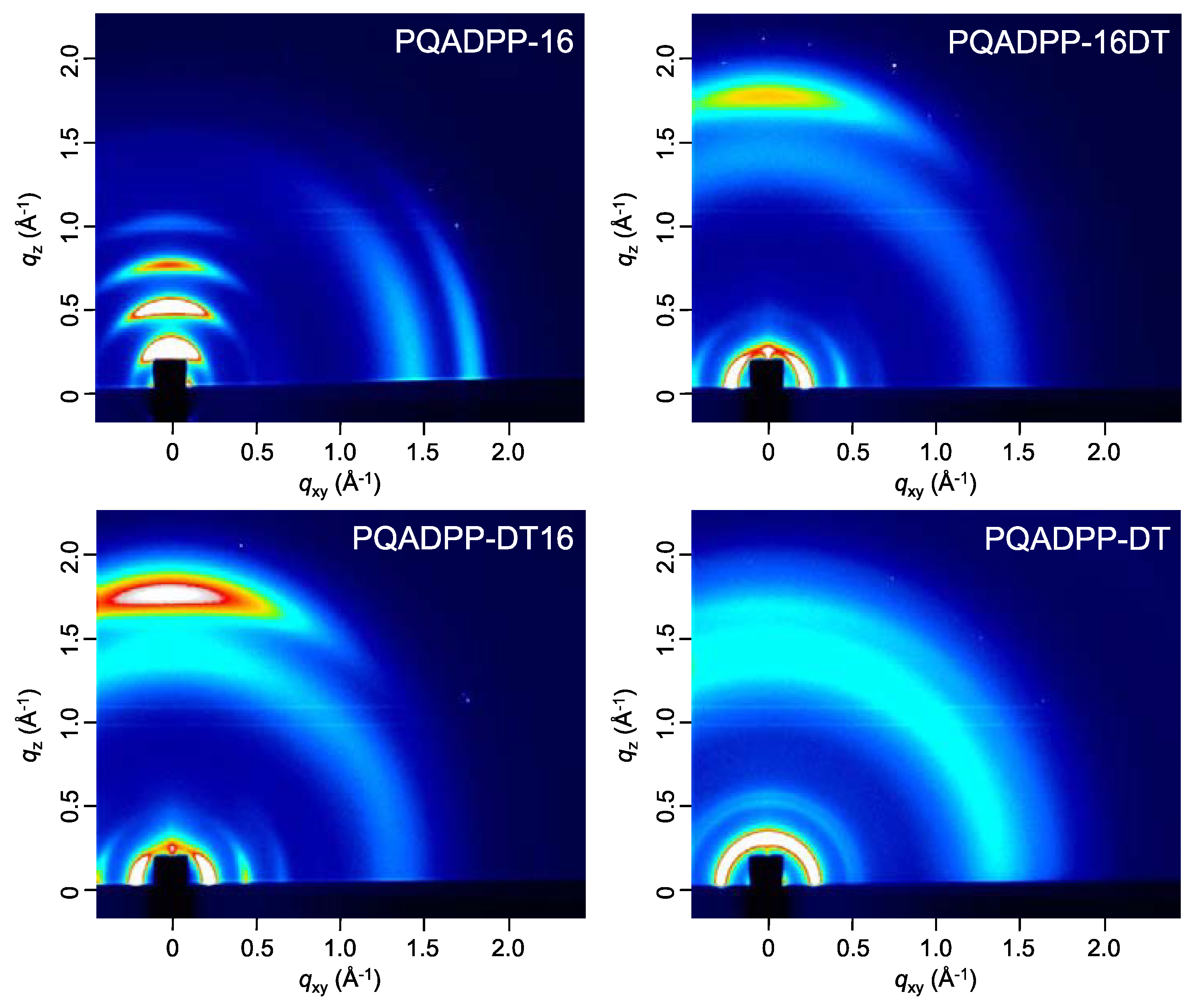
2/Vs are obtained for PQADPP-16DT and -DT16. All mobilities obtained here are relatively low, and these results may relate to the localized HOMOs. Since the HOMO distribution play an important role in the charge (hole) transport both along the backbone and the intermolecular interaction, this localization might prevent the efficient hole transport. The variation of the mobility depending on the side chain composition can be well explained by the structural study using the two-dimensional grazing incidence X-ray diffraction (2D-GIXD) as described below.
Figure 5 shows the 2D-GIXD patterns of the polymer films after annealing at 150 °C. Interestingly, the polymer orientation is strongly dependent on the side chain composition. In the film of PQADPP-16, the diffractions corresponding to the lamellar and π–π stacking structures appear along the
qz and
qxy axes, respectively, indicating that the polymer chains are oriented in an edge-on manner. In the meantime, PQADPP-16DT and -DT16 exhibited the diffraction corresponding to the lamellar and π–π stacking structures along the
qxy and
qz axes, respectively, suggesting that the polymers form the face-on orientation. While the π–π stacking diffraction for PQADPP-16, -16DT, and -DT16 appears strong, that for PQADPP-DT is very weak. The π–π stacking distance (
dπ) for PQADPP-16 is found to be 3.5 Å, which is relatively narrow for semiconducting polymers. The
dπ for PQADPP-16DT and -DT16, 3.6 Å, and -DT, 3.8 Å, are wider than that for PQADPP-16. These differences in the π–π stacking crystallinity can relate to the ratio of bulky branched group in the side chain. The long branched alkyl group may prevent the interchain interaction in the solid state, though it affords good solubility. Since the charge transport path in the semiconducting polymer films is primarily through the π–π interaction of face-to-face stacked polymer chains [
5], such differences in the orientational motif and the π–π stacking is fairly consistent with the trend of the mobility.
Figure 3.
Computation (DFT at B3LYP/6-31(d) level) of the HOMO and LUMO geometry of quinacridone (QA, left), polymer repeat unit (QADPP, middle), and dithienyl diketopyrrolopyrrole (DPP2T), in which the methyl group is used as the substituents. HOMO (EHOMO) and LUMO levels (ELUMO) for each compound are also shown. EHOMO and ELUMO for QA and DPP2T were determined by electrochemistry. EHOMO denoted as QADPP is that of the polymer (PQADPP-16DT) obtained by photoelectron spectroscopy in air (PESA), and ELUMO is determined by the addition of optical bandgap to EHOMO.
Figure 3.
Computation (DFT at B3LYP/6-31(d) level) of the HOMO and LUMO geometry of quinacridone (QA, left), polymer repeat unit (QADPP, middle), and dithienyl diketopyrrolopyrrole (DPP2T), in which the methyl group is used as the substituents. HOMO (EHOMO) and LUMO levels (ELUMO) for each compound are also shown. EHOMO and ELUMO for QA and DPP2T were determined by electrochemistry. EHOMO denoted as QADPP is that of the polymer (PQADPP-16DT) obtained by photoelectron spectroscopy in air (PESA), and ELUMO is determined by the addition of optical bandgap to EHOMO.
Figure 4.
Transfer characteristics of OFETs based on PQADPP-16, -16DT, and -DT16 (a) and output characteristics of OFETs based on PQADPP-16 (b); -16DT (c); and -DT16 (d).
Figure 4.
Transfer characteristics of OFETs based on PQADPP-16, -16DT, and -DT16 (a) and output characteristics of OFETs based on PQADPP-16 (b); -16DT (c); and -DT16 (d).
Figure 5.
2D-GIXD patterns of the polymer films. GIXD: Grazing incidence X-ray diffraction.
Figure 5.
2D-GIXD patterns of the polymer films. GIXD: Grazing incidence X-ray diffraction.
3. Experimental Section
Synthesis. All chemicals and solvents are of reagent grade unless otherwise indicated. Chlorobenzene, and toluene were distilled prior to use. Molecular weights were determined by gel permeation chromatography (GPC) with a TOSOH HLC-8121GPC/HT at 140 °C using
o-dichlorobenzene as a solvent and calibrated with polystyrene standards. 2,9-dibromo
-N,
N-dialkylquino[2,3-
b]acridine-7,14-dione (1) [
24] and 3,6-bis-(5-bromo-thiophen-2-yl)-
N,
N′-bis(alkyl)-1,4-dioxopyrrolo[3,4-
c]pyrrole (3) [
14] were synthesized according to the reported procedure.
5,12-dihexadecyl-2,9-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinolino[2,3-b]acridine-7,14(5H,12H)-dione (2). 1 (1.0 g, 1.09 mmol), dichlorobis(triphenylphosphine)dipalladium(II) (35 mg, 0.05 mmol), potassium acetate (642 mg, 2.64 mmol) was added to 25 mL of dioxane in a 100 mL 3-neck flask and purged with N2 for 30 min. After stirring the mixture for 1 h, bis(pinacolato)diboron (267 mg, 1.06 mmol) was added, which was then refluxed for 15 h. After cooling to room temperature, water was added and the aqueous layer was extracted with dichloromethane. The organic layer was washed with water then with brine, and then dried over anhydrous MgSO4. After removing the solvent by vacuum evaporation, the residue was purified by the purification with preparative GPC (chloroform) and recrystallized in ethyl acetate, which gave 2 as an orange solid (454 mg, 41% yield). 1H NMR (400 MHz, CDCl3): δ 9.05 (s, 2H), 8.78 (d, 2H,), 8.12 (dd, 2H, J = 7.8 Hz), 7.48 (dd, 2H, J = 8.8Hz), 4.53 (m, 4H), 2.00 (m, 4H), 1.39 (s, 24H), 1.50–1.20 (m, 52H), 0.90–0.86 (m, 12H). 13C NMR (400 MHz, CDCl3): 13C NMR (400 MHz, CDCl3): δ 178.3, 145.0, 140.0, 136.8, 136.5, 126.9, 121.0, 115.0, 114.9, 84.2, 50.1, 37.1, 32.3, 32.2, 32.1, 30.3, 30.0, 30.0, 30.0, 30.0, 29.9, 29.7, 29.7, 27.0, 25.3, 23.0, 23.0, 14.5. HRMS: Calcd for C64H99B2N2O6, [M + H]+: 1013.76838. Found: 1013.77008.
5,12-di(2-decyltetradecyl)-2,9-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinolino[2,3-b]acridine-7,14(5H,12H)-dione (2). 1 (500 mg, 0.44 mmol), dichlorobis(triphenylphosphine)dipalladium(II) (15 mg, 0.02 mmol), potassium acetate (259 mg, 2.64 mmol) was added to 25 mL of dioxane in a 100 mL 3-neck flask and purged with N2 for 30 min. After stirring the mixture for 1 h, bis(pinacolato)diboron (267 mg, 1.06 mmol) was added, which was then refluxed for 15 h. After cooling to room temperature, water was added and the aqueous layer was extracted with dichloromethane. The organic layer was washed with water then with brine, and then dried over anhydrous MgSO4. After removing the solvent by vacuum evaporation, the residue was purified by preparative GPC (chloroform), which gave 2 as an orange solid (351 mg, 64% yield). 1H NMR (400 MHz, CDCl3): δ 9.08 (s, 2H), 8.87 (d, 2H),8.10 (dd, 2H, J = 8.8 Hz), 7.55 (dd, 2H, J = 8.7Hz), 4.53 (m, 4H), 2.25 (m, 2H), 1.38 (s, 24H), 1.50–1.20 (m, 80H), 0.90–0.84 (m, 12H).13C NMR (400 MHz, CDCl3): δ 178.3, 145.0, 140.0, 136.8, 136.5, 126.9, 121.0, 115.0, 114.9, 84.2, 50.1, 37.1, 32.3, 32.3, 32.1, 30.3, 30.0, 30.0, 29.9, 29.9, 29.8, 29.7, 29.7, 27.0, 23.0, 23.0, 14.5. HR-MS: Calcd for C80H130B2N2O6, [M + H]+: 1238.01878. Found: 1238.02075.
General procedure for the polymerization. A solution of 2 (0.10 mmol), 3 (0.10 mmol), Pd(PPh3)4 (2.3 mg, 2 mol %), and 1 drop of Aliquat 336 was added in 4 mL of toluene and 3 mL of aqueous K2CO3 (2M) were added to vial. The vial was then purged with argon and sealed. The resulting mixture was heated 180 °C for 40 min using a μ-wave reactor. After cooling to room temperature, the reaction mixture was precipitated into mixture of methanol (100 mL) 1 h at room temperature. 2-Bromothiophene (17 mg, 0.1 mmol) was then added and 180 °C for 40 min using a µ-wave reactor. Then the precipitated solid was subjected to sequential Soxhlet extraction with methanol and hexane to remove low molecular weight fractions (for PQADPP-DT, the sample was collected with hexane). The residue was extracted with chloroform and then precipitated in 200 mL of methanol to yield the desired sample as red solids (yield = 23%–92%).
Measurements. UV-Vis absorption spectra were measured using a Shimadzu UV-3100 spectrometer. HOMO level (EHOMO) was determined from the onset of photoelectron spectra measured by using a RIKEN KEIKI CO., LTD photoelectron spectrometer MODEL AC-2 in air. Cyclic voltammograms (CVs) were recorded on a BAS electrochemical analyzer, model 612D, in dichloromethane containing tetrabutylammonium hexafluorophosphate (Bu4NPF6, 0.1 M) as the supporting electrolyte at a scan rate of 100 mV/s. The counter and working electrodes were made of Pt, and the reference electrode was Ag/AgCl. All the potentials were calibrated with the standard ferrocene/ferrocenium redox couple (Fc/Fc+): E1/2 = +0.43 V measured under identical conditions). MO calculations were carried out with the DFT method using Gaussian 03 program package; Frisch, M.J. et al., revision C.02; Gaussian, Inc., Wallingford, CT, 2004. Grazing incidence X-ray diffraction (GIXD) experiments were conducted at the SPring-8 on beamline BL19B2. The sample was irradiated at a fixed incident angle on the order of 0.12° through a HUBER diffractometer and the GIXD patterns were recorded with a 2-D image detector (PILATUS 300K). GIXD patterns were recorded with X-ray energy of 12.39 keV (λ = 1 Å).
Device fabrication and characterizations. OFET devices were fabricated in a “top-contact” configuration on heavily doped
n+-Si (100) wafers with 200-nm-thick thermally grown SiO
2 (
Ci = 17.3 nF cm
−2). The Si/SiO
2 substrates were carefully cleaned and then treated with hexamethyldisilazane (HMDS) to form a self-assembled monolayer (SAM), in which the silicon wafers were exposed to FDTS vapor at 100 °C in a closed desiccator for 3 h under nitrogen. Polymer layers were then spin-coated from warm (~80 °C) 3 g/L dichlorobenzene solution with 2500 rpm for 45 s, subsequently annealed at 150 °C for 30 min under nitrogen. On top of the polymer thin films, Au drain and source electrodes (thickness 80 nm) were deposited in vacuum through a shadow mask, where the drain-source channel length (
L) and width (
W) are 50 μm and ca. 1.5 mm, respectively. Current-voltage characteristics of the OFET devices were measured at room temperature in air with a Keithly 4200-SCS semiconductor characterization system. Field-effect mobilities were calculated in the saturation regime (
VSD = −60 V) of the
ISD using the following equation,
where
Ci is the capacitance of the SiO
2 dielectric,
ISD is the source-drain current, and
VSD,
VG and
Vth are the source-drain, gate and threshold voltages, respectively. Current on/off ratios (
Ion/Ioff) were determined from the minimum current at around
VG = 0–20 V (
Ioff) and the current at
VG = −60 V (
Ion). The mobility data were collected from more than 10 different devices.