Structural and Luminescence Properties of Lu2O3:Eu3+ F127 Tri-Block Copolymer Modified Thin Films Prepared by Sol-Gel Method
Abstract
:1. Introduction
2. Results and Discussion
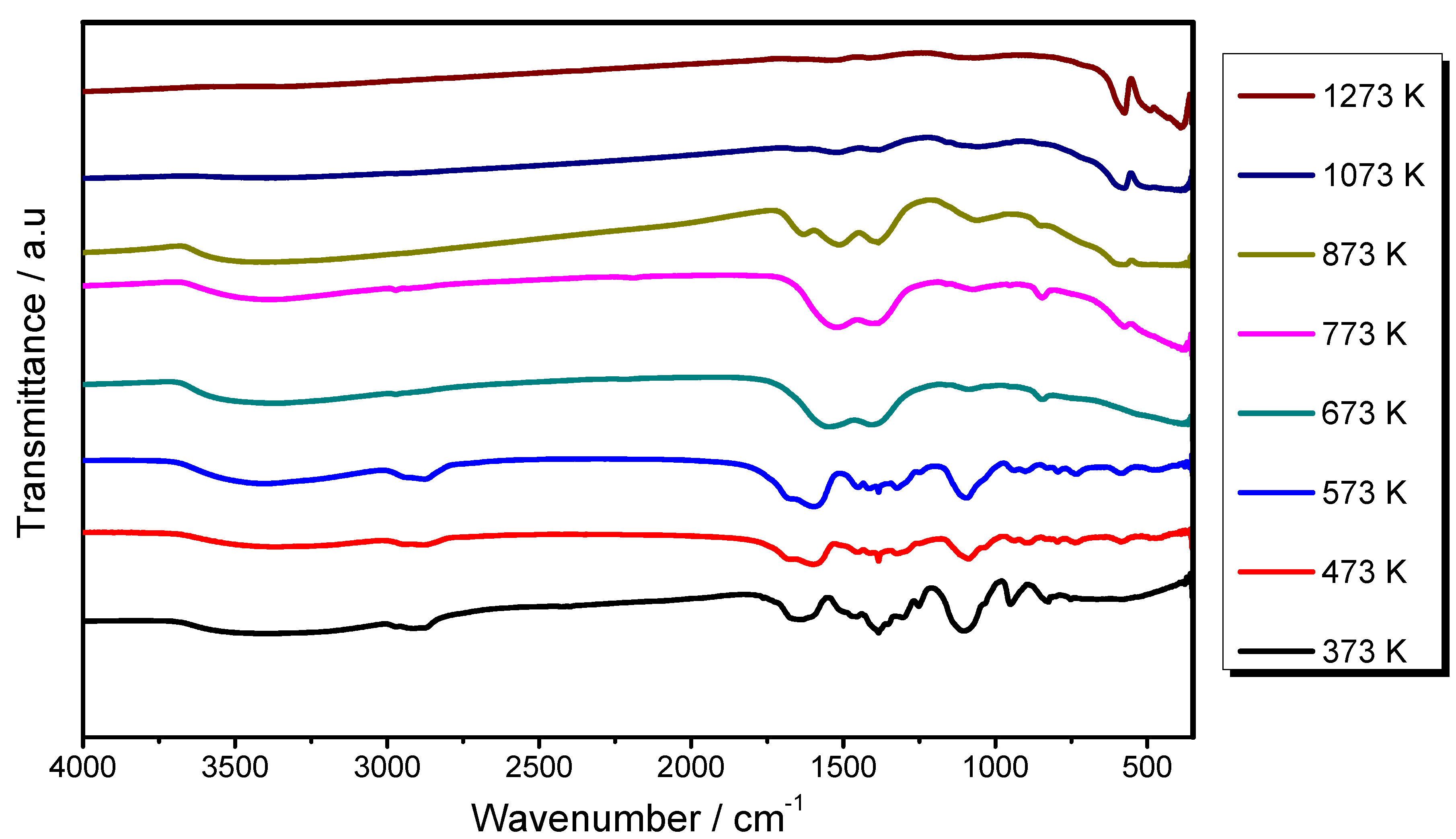
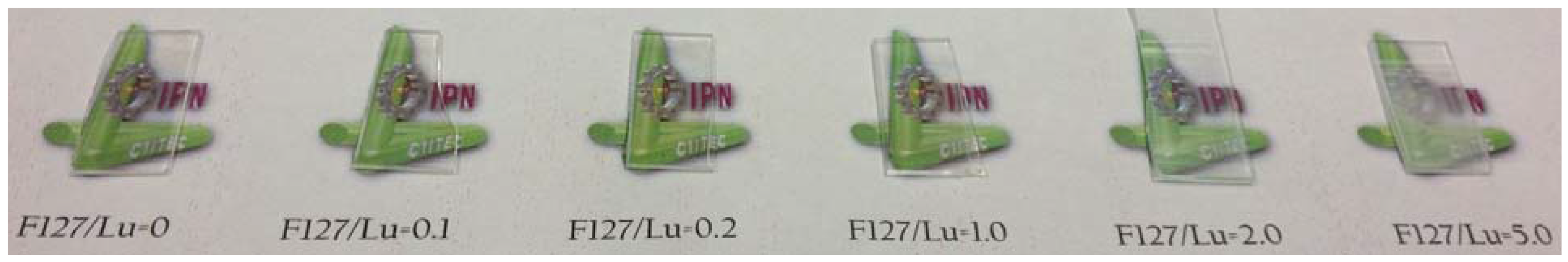
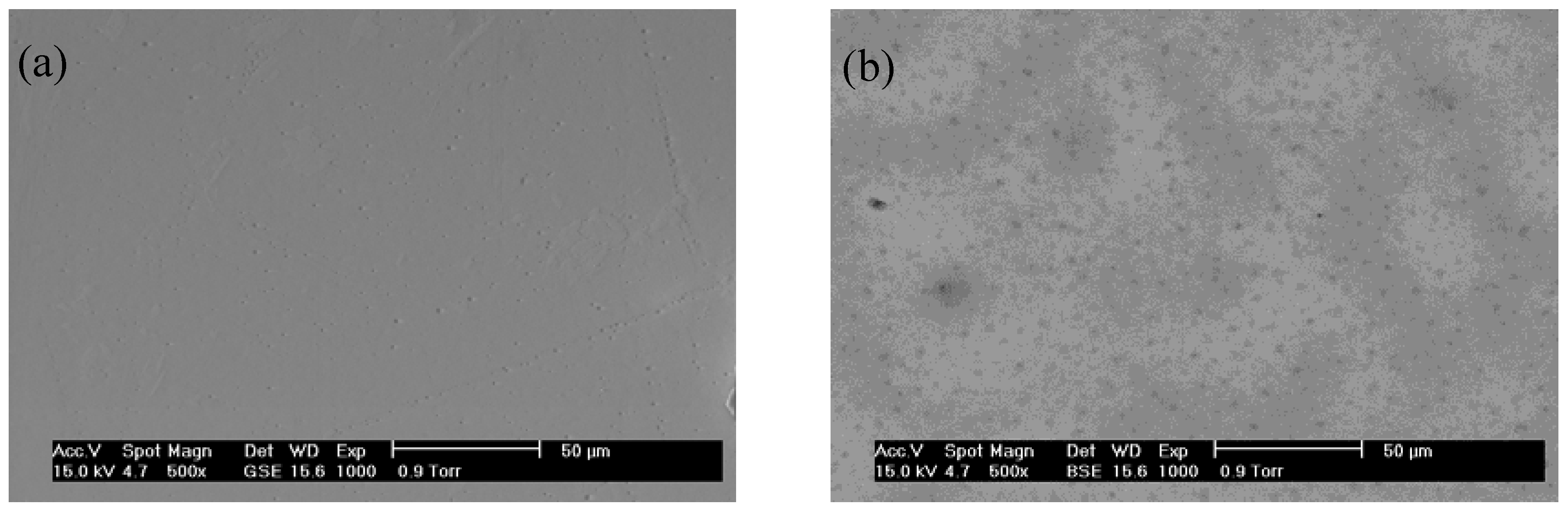
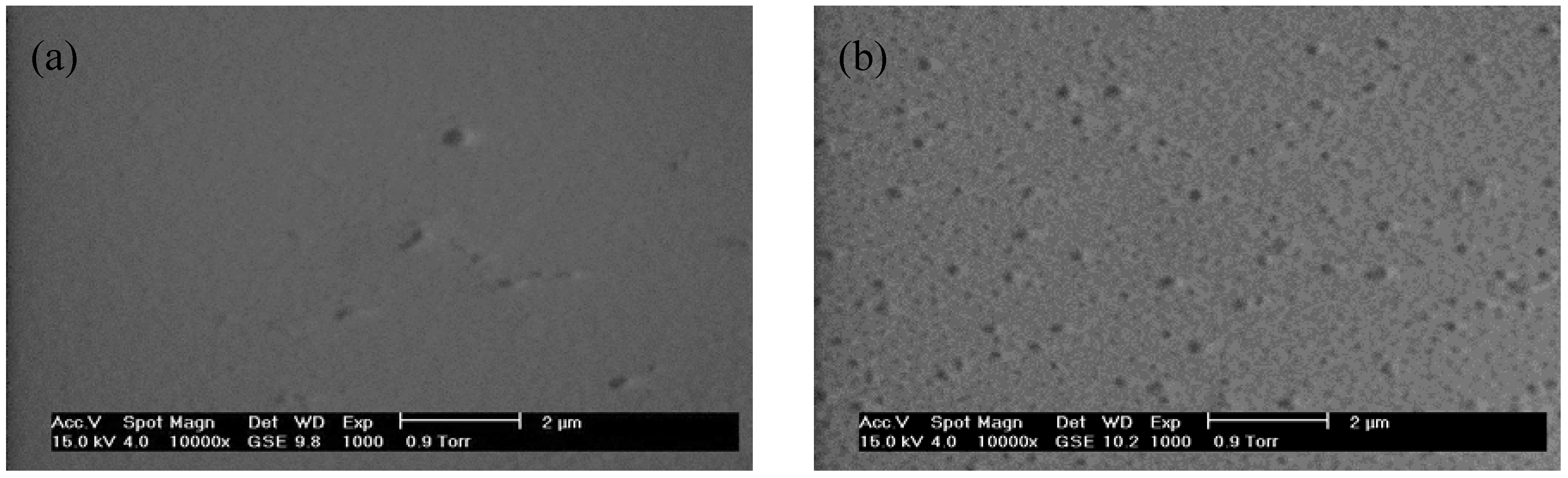
2.1. Structural and Morphological Studies

| Temperature (K) | F127/Lu = 1.0 | F127/Lu = 2.0 | ||
|---|---|---|---|---|
| Crystallite size (nm) | <111> orientation | Crystallite size (nm) | <111> orientation | |
| 873 | 9.0 | 84.1 | 14.0 | 79.9 |
| 1073 | 12.0 | 95.1 | 15.0 | 81.3 |
| 1273 | 16.0 | 97.4 | 17.0 | 84.1 |
2.2. m-Lines Spectroscopy
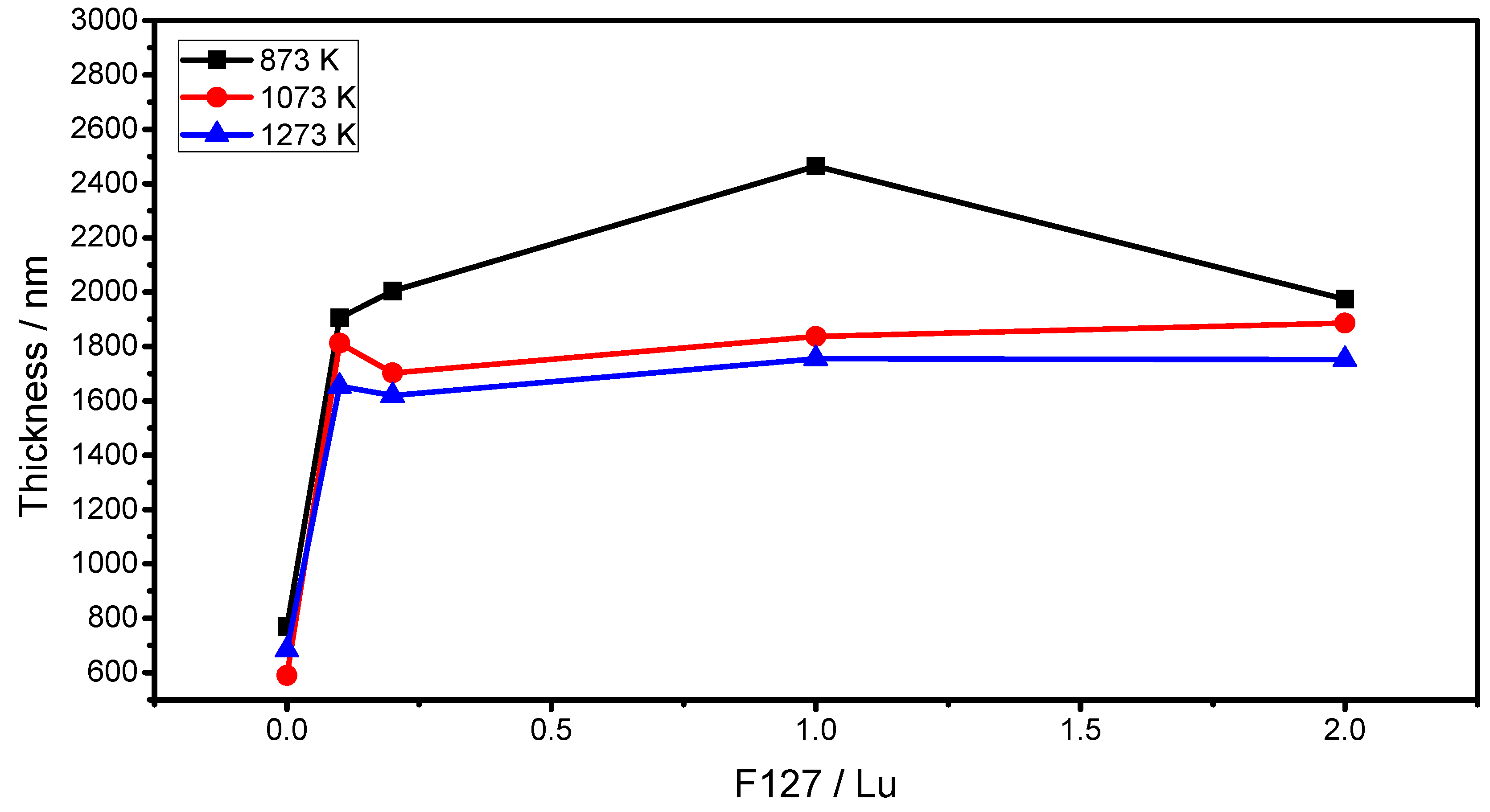
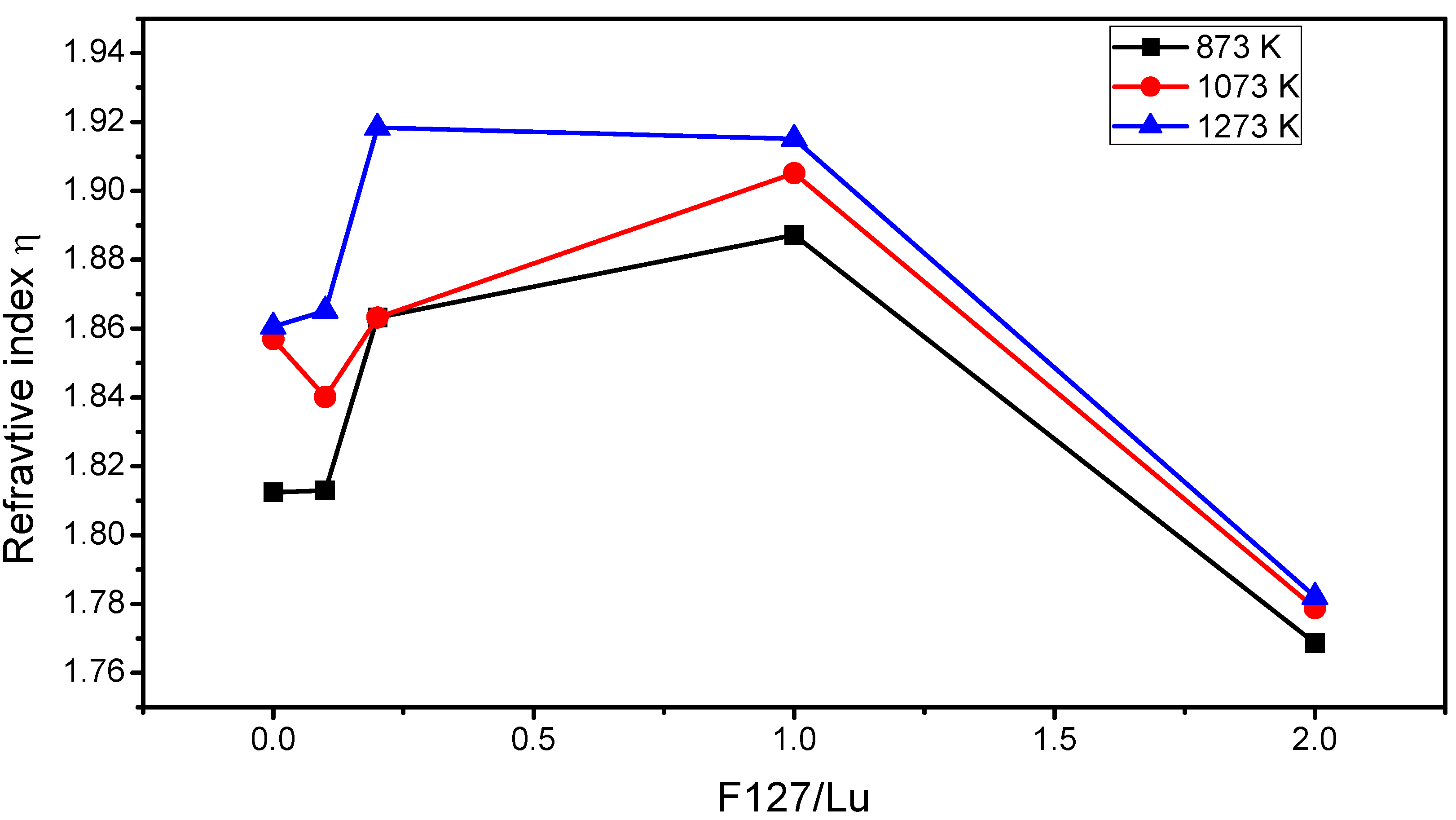
| Temperature (K) | F127/Lu=0 | F127/Lu=0.1 | F127/Lu=0.2 | F127/Lu=1.0 | F127/Lu=2.0 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Density (g cm−3) | Porosity (%) | Density (g cm−3) | Porosity (%) | Density (g cm−3) | Porosity (%) | Density (g cm−3) | Porosity (%) | Density (g cm−3) | Porosity (%) | |
| 873 | 8.56 | 16.1 | 8.60 | 15.2 | 8.93 | 9.3 | 9.11 | 6.03 | 8.21 | 21.94 |
| 1073 | 8.89 | 10.2 | 8.76 | 12.4 | 9.33 | 1.0 | 9.24 | 3.54 | 8.29 | 20.61 |
| 1273 | 8.91 | 9.7 | 8.95 | 9.0 | 9.36 | 1.6 | 9.31 | 2.14 | 8.31 | 20.18 |
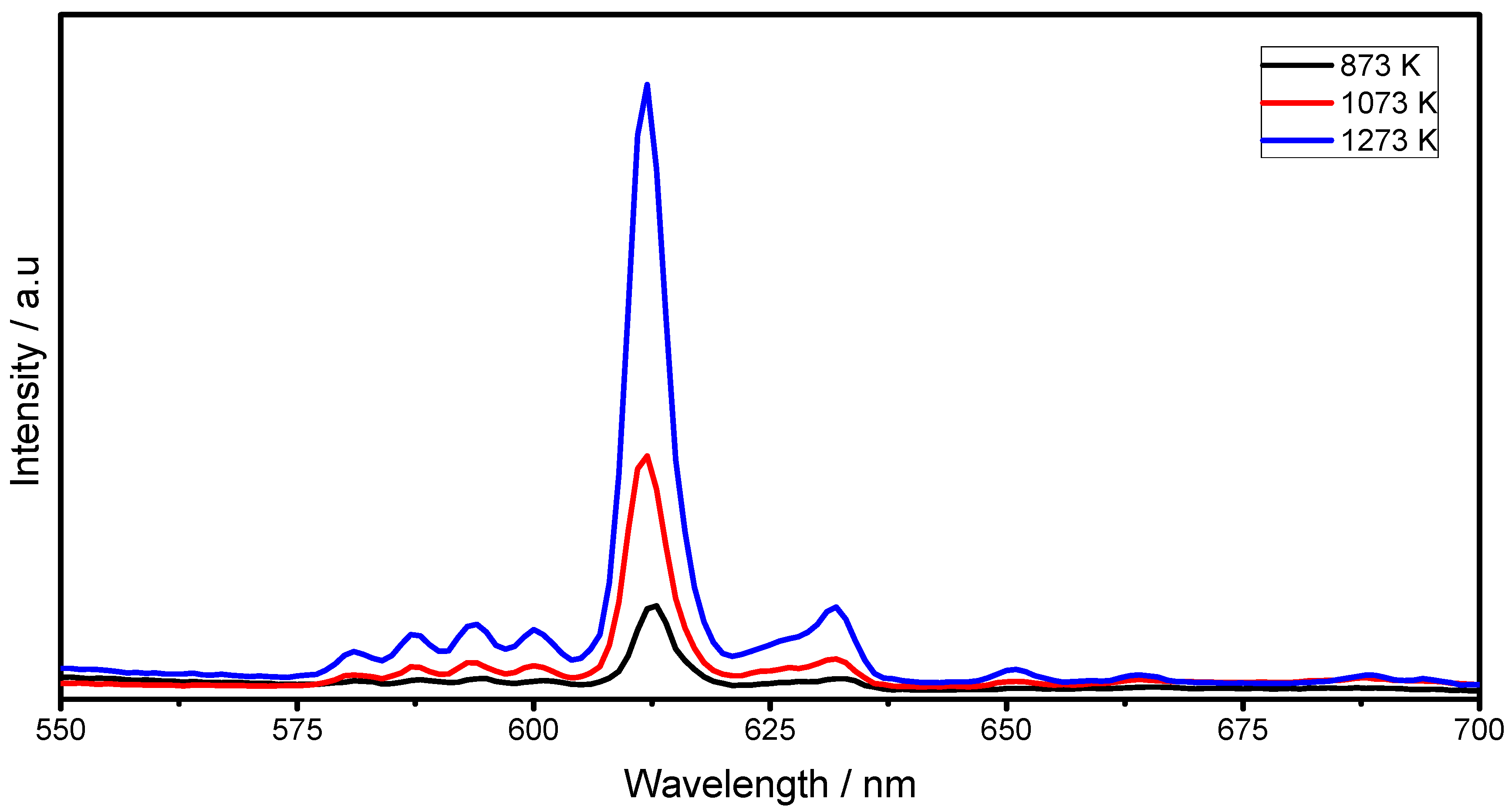
2.3. Luminescent Properties
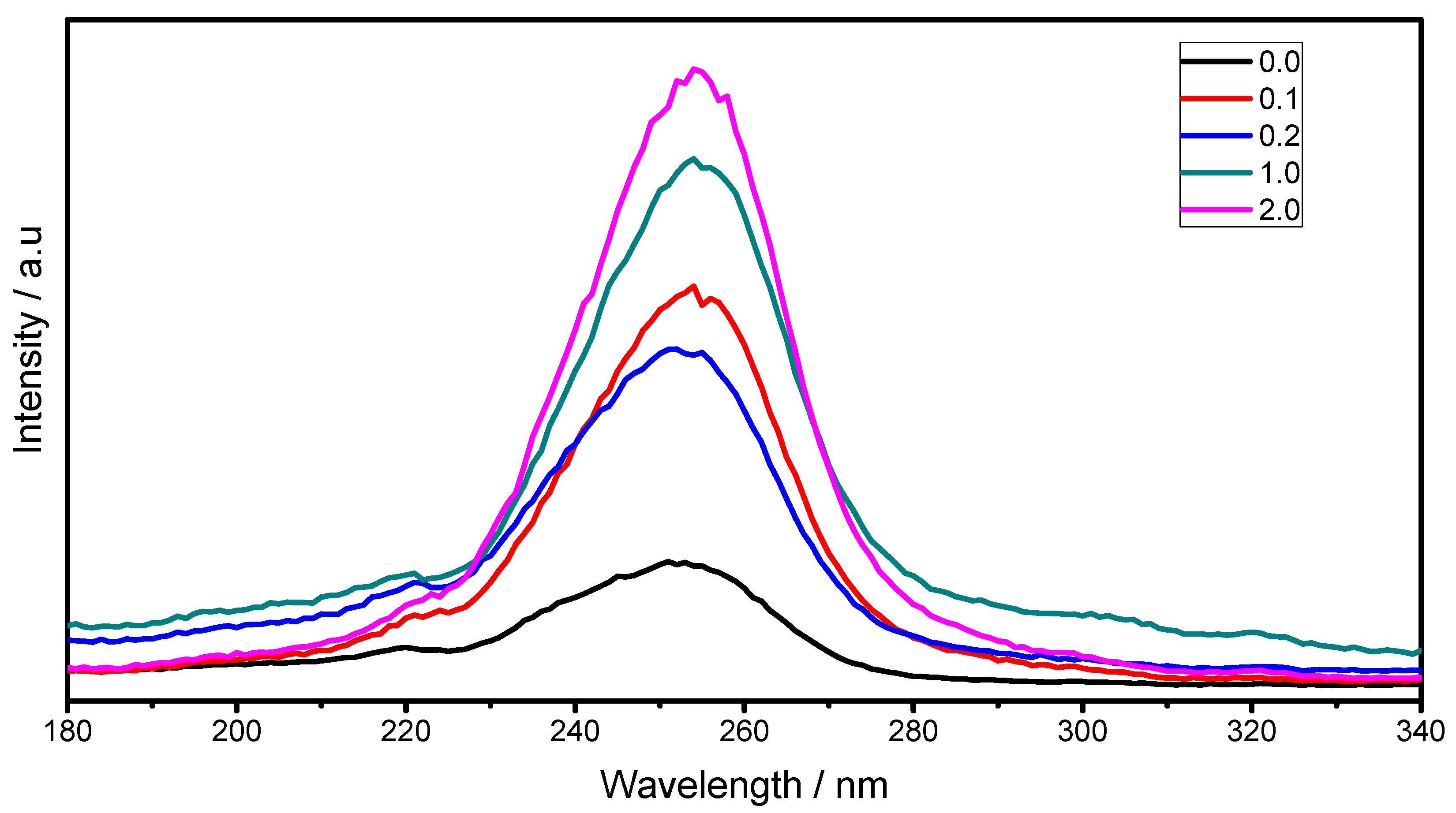
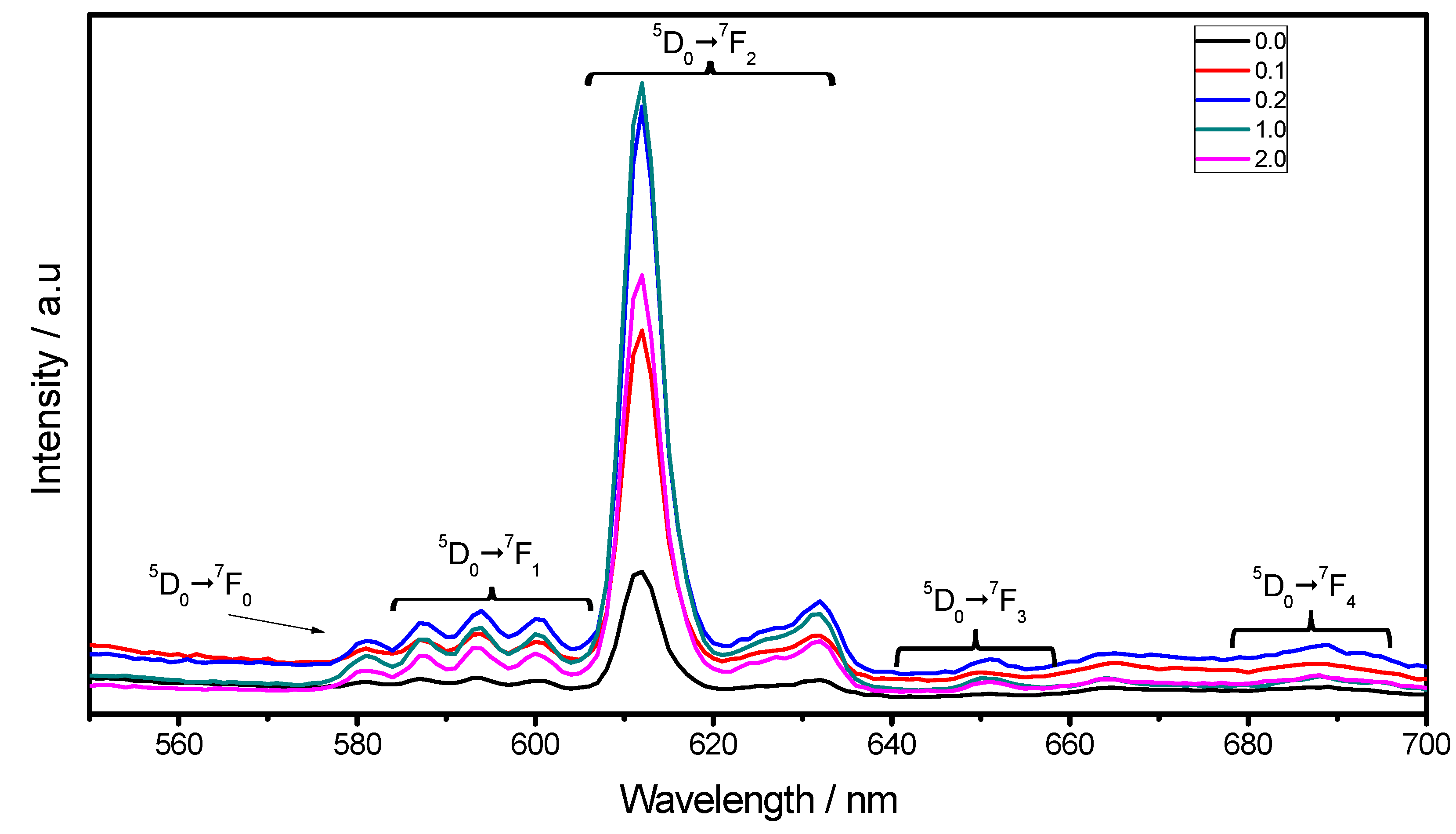
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Wang, J.; Lu, Q.; Liu, Q. Synthesis and luminescence properties of Eu or Tb doped Lu2O3squarenanosheets. Opt. Mater. 2007, 29, 593–597. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Dong, N.; Jiang, Y.; Tao, Y.; Yin, M. Preparation and characterization of optical spectroscopy of Lu2O3:Eu3+ nanocrystals. J. Solid State Chem. 2005, 178, 477–482. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Guo, H. Electro spinning synthesis and luminescent properties of Lu2O3:Eu3+ nanofiber. J. Rare Earths 2010, 28, 232–235. [Google Scholar] [CrossRef]
- Zych, E. Luminescence and scintillation of inorganic phosphor materials. In Handbook of luminescence, Display Materials and devices; Nalwa, H.S., Rohwer, L.S., Eds.; American Scientific Publishers: Valencia, CA, USA, 2003. [Google Scholar]
- Greskovich, C.; Duclos, S. Ceramic Scintillator. Annu. Rev. Mater. Sci. 1997, 27, 69–88. [Google Scholar] [CrossRef]
- Zych, E.; Trojan-Piegza, J.; Dorenbos, P. Radioluminescence of Lu2O3:Eu nanocrystalline powder and vacuum-sintered ceramic. Radiat. Meas. 2004, 38, 471–474. [Google Scholar] [CrossRef]
- Boyer, J.C.; Vitrone, F.; Capobianco, J.A.; Speghini, A.; Bettinelli, M. Variation of fluorescence lifetime and Judd-Ofelt parameters between Eu3+ doped bulk and nanocrystalline cubic Lu2O3. J. Phys. Chem. B 2004, 108, 20137–20143. [Google Scholar] [CrossRef]
- Zych, E.; Hreniak, D.; Strek, W. Lu2O3:Eu, a new X-ray phosphor. Mater. Sci. 2002, 20, 111–122. [Google Scholar]
- Martinet, C.; Pillonet, A.; Lancok, J.; Garapon, G. Optical, structural and fluorescence properties of nanocrystalline cubic or monoclinic Eu:Lu2O3 films prepared by pulsed laser deposition. J. Lumin. 2007, 126, 807–816. [Google Scholar] [CrossRef]
- Garcia-Murillo, A.; Le Luyer, C.; Dujardin, C.; Martin, T.; Garapon, C.; Pedrini, C.; Mugnier, J. Elaboration and scintillator properties of Eu3+ doped Gd2O3 and Lu2O3 sol-gel films. Nucl. Instrum. Methods Phys. Res. A 2002, 486, 181–185. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Gu, M.; Xiao, L.; Xu, X. Highly enhanced photoluminescence and X-ray excited luminescence of Li doped Gd2O3:Eu3+ thin films. Solid State Commun. 2006, 137, 162–165. [Google Scholar] [CrossRef]
- Topping, S.G.; Sarin, V.K. CVD Lu2O3:Eu coatings for Advanced Scintillators. Int. J. Refract. Met. 2009, 27, 498–501. [Google Scholar] [CrossRef]
- Topping, S.G.; Park, C.H.; Rangan, S.K.; Sarin, V.K. Lutetium Oxide coatings by PVD. Mater. Res. Soc. Symp. Proc. 2007, 1038, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Babayevskaya, N.V.; Deyneka, T.G.; Mateychenko, P.V.; Matveevskaya, N.A.; Tolmachev, A.V.; Yavetskiy, R.P. Fabrication and characterization of Lu2O3:Eu3+ nanopowder and X-ray films. J. Alloys Compd. 2010, 507, L26–L31. [Google Scholar] [CrossRef]
- Lempicki, A.; Brecher, C.; Szupryczynski, P.; Lingertat, H.; Nagarkar, V.V.; Tippins, S.V.; Miller, S.R. A new lutetia-based ceramic scintillator for X-ray imaging. Nucl. Instrum. Methods A 2002, 488, 579–590. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Q.W.; Shi, J.L. Processing and scintillation properties of Eu3+ doped Lu2O3 transparent Ceramics. Opt. Mater. 2009, 31, 729–733. [Google Scholar] [CrossRef]
- Malvestuto, M.; Scarel, G.; Wiemer, C.; Fanciulli, M.; D'Acapito, F.; Boscherini, F. X-ray absorption spectroscopy study of Yb2O3 and Lu2O3 thin films deposited on Si(100) by atomic layer deposition. Nucl. Instrum. Methods B 2006, 246, 90–95. [Google Scholar] [CrossRef]
- Galceran, M.; Pujol, M.C.; Aguiló, M.; Díaz, F. Synthesis and characterization of nanocrystalline Yb:Lu2O3 by modified Pechini method. Mater. Sci. Eng. B 2008, 146, 7–15. [Google Scholar] [CrossRef]
- García-Murillo, A.; Carrillo-Romo, F.J.; Le Luyer, C.; Morales-Ramírez, A.J.; García-Hernández, M.; Moreno-Palmerin, J. Sol-gel elaboration and structural investigations of Lu2O3:Eu3+ planar waveguides. J. Sol-Gel Sci. Technol. 2009, 50, 359–367. [Google Scholar] [CrossRef]
- Guo, H.; Yin, M.; Dong, N.; Xu, M.; Lou, L.; Zhang, W. Effect of heat-treatment temperature on the luminescent properties of Lu2O3:Eu film prepared by Pechini sol-gel method. Appl. Surf. Sci. 2005, 243, 245–250. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Bescher, E.P. Physical properties of sol-gel coatings. J. Sol-Gel Sci. Technol. 2000, 19, 23–29. [Google Scholar] [CrossRef]
- Xiaolin, L.; Feng, Z.; Shimming, H.; Bo, L.; Che, Ni. Fabrication of highly a-axis-oriented Gd2O3:Eu3+ thick film and its luminescence properties. Opt. Mater. 2008, 32, 126–130. [Google Scholar]
- Liu, X.; Han, K.; Huang, S.; Liu, B.; Ni, C. Optical properties of GdTaO4: Eu3+ thick films prepared from a PVP-containing solution. Surf. Sci. 2009, 255, 4680–4683. [Google Scholar] [CrossRef]
- García-Hernández, M.; García-Murillo, A.; Carrillo-Romo, F.; Jaramillo-Vigueras, D.; Chadeyron, G.; de la Rosa, E.; Boyer, D. Eu-Doped BaTiO3 Powder and Film from Sol-Gel Process with Polyvinylpyrrolidone Additive. Int. J. Mol. Sci. 2009, 10, 4088–4101. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-C.; Fung, K.-Z.; Hung, I.-M.; Hon, M.-H. Synthesis of highly ordered and worm-like mesoporous TiO2 assisted by tri-block copolymer. Solid State Ion. 2008, 179, 1300–1304. [Google Scholar] [CrossRef]
- Yang, J.; Peterlik, H.; Lomoschitz, M.; Schubert, U. Preparation of mesoporous titania by surfactant-assisted sol-gel processing of acetaldoxime-modified titanium alkoxides. J. Non-Cryst. Solids 2010, 356, 1217–1227. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, Y.H.; Lee, M.H.; Kim, H.J.; Pan, J.H.; Lim, G.I.; Choi, Y.S.; Kim, K.; Park, N.G.; Lee, C.; Lee, W.I. Formation of Efficient Dye-Sensitized Solar Cells by Introducing an Interfacial Layer of Long-Range Ordered Mesoporous TiO2 Thin Film. Langmuir 2008, 24, 13225–13230. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Chuang, S. Infrared and TPD studies of nitrates adsorbed on Tb4O7, La2O3, BaO, and MgO/γ-Al2O3. J. Phys. Chem. B 2000, 104, 673–683. [Google Scholar] [CrossRef]
- Arconada, N.; Castro, Y.; Durán, A. Photocatalytic properties in aqueous solution of porous TiO2 anatase films prepared by sol-gel process. Appl. Catal. A 2010, 385, 101–107. [Google Scholar] [CrossRef]
- McDevitt, N.T.; Baun, W.L. Infrared absorption study of metal oxides in the low frequency region (700–240 cm−1). Spectrochim. Acta 1964, 20, 799–808. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry; Oxford Science Publications: Oxford, UK, 1984. [Google Scholar]
- Jeong, L.; Kyo, H.; Tae, K. The Microscopic Origin of Residual Stress for Flat Self-Actuating Piezoelectric Cantilevers. Nanoscale Res. Lett. 2011, 6, 55–59. [Google Scholar] [CrossRef]
- Garcia-Murillo, A.; Le Luyer, C.; Garapon, C.; Dujardin, C.; Bernstein, E.; Pedrini, C.; Mugnier, J. Optical properties of europium doped Gd2O3 waveguiding thin films prepared by sol-gel method. Opt. Mater. 2002, 19, 161–168. [Google Scholar] [CrossRef]
- Pang, M.L.; Lin, J.; Fu, J.; Xing, R.B.; Luo, C.X.; Han, Y.C. Preparation, patterning and luminescent properties of nanocrystalline Gd2O3:A (A = Eu3+, Dy3+, Sm3+, Er3+) phosphor films via Pechini sol-gel lithography. Opt. Mater. 2003, 23, 547–558. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sudarsan, V.; Vatsa, R.V.; Godbole, S.V.; Kadam, R.N.; Bhatta, U.M.; Tyagi, A.K. Effect of structure, particle size and relative concentration of Eu3+ and Tb3+ ions on the luminescence properties of Eu3+ co-doped Y2O3:Tb nanoparticles. Nanotechnology 2008, 19, 325704–325711. [Google Scholar] [CrossRef] [PubMed]
- Brusentsev, F.A.; Rebenko, N. Algol-60 program for calculating sin θ/λ values and the polarization-Lorentz factors LP. Struct. Chem. 1966, 8, 406–413. [Google Scholar]
- Tien, P.K.; Ulrich, R. Theory of prism-film coupler and thin-film light guides. J. Opt. Soc. Am. 1970, 60, 1325–1337. [Google Scholar] [CrossRef]
- Medina, D.Y.; Orozco, S.; Hernandez, I.; Hernandez, R.T.; Falcony, C. Characterization of europium doped lanthanum oxide films prepared by spray pyrolysis. J. Non-Cryst. Solids 2011, 357, 3740–3743. [Google Scholar] [CrossRef]
- Liu, J.; Fei, X.; Yu, X.; Tao, Z.; Yang, L.; Yang, S. Highly enhanced f–f transitions of Eu3+ in La2O3 phosphor via citric acid and poly (ethylene glycol) precursor route. J. Non-Cryst. Solids 2007, 353, 4697–4701. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Q.L.; Shao, S.F.; Liu, W.P.; Wan, S.M.; Yin, S.T. Phase Transition, structure and Luminescence of Eu:YAG nanophosphors by co-precipitation method. J. Alloys Compd. 2009, 470, 306–310. [Google Scholar] [CrossRef]
- Nedelec, J.M. Sol-gel processing of nanostructure inorganic scintillating materials. J. Nanomater. 2007. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Jesus Morales Ramírez, A.; Hernández, M.G.; Murillo, A.G.; De Jesús Carrillo Romo, F.; Palmerin, J.M.; Velazquez, D.Y.M.; Jota, M.L.C. Structural and Luminescence Properties of Lu2O3:Eu3+ F127 Tri-Block Copolymer Modified Thin Films Prepared by Sol-Gel Method. Materials 2013, 6, 713-725. https://doi.org/10.3390/ma6030713
De Jesus Morales Ramírez A, Hernández MG, Murillo AG, De Jesús Carrillo Romo F, Palmerin JM, Velazquez DYM, Jota MLC. Structural and Luminescence Properties of Lu2O3:Eu3+ F127 Tri-Block Copolymer Modified Thin Films Prepared by Sol-Gel Method. Materials. 2013; 6(3):713-725. https://doi.org/10.3390/ma6030713
Chicago/Turabian StyleDe Jesus Morales Ramírez, Angel, Margarita García Hernández, Antonieta García Murillo, Felipe De Jesús Carrillo Romo, Joel Moreno Palmerin, Dulce Yolotzin Medina Velazquez, and María Luz Carrera Jota. 2013. "Structural and Luminescence Properties of Lu2O3:Eu3+ F127 Tri-Block Copolymer Modified Thin Films Prepared by Sol-Gel Method" Materials 6, no. 3: 713-725. https://doi.org/10.3390/ma6030713





