“Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications
Abstract
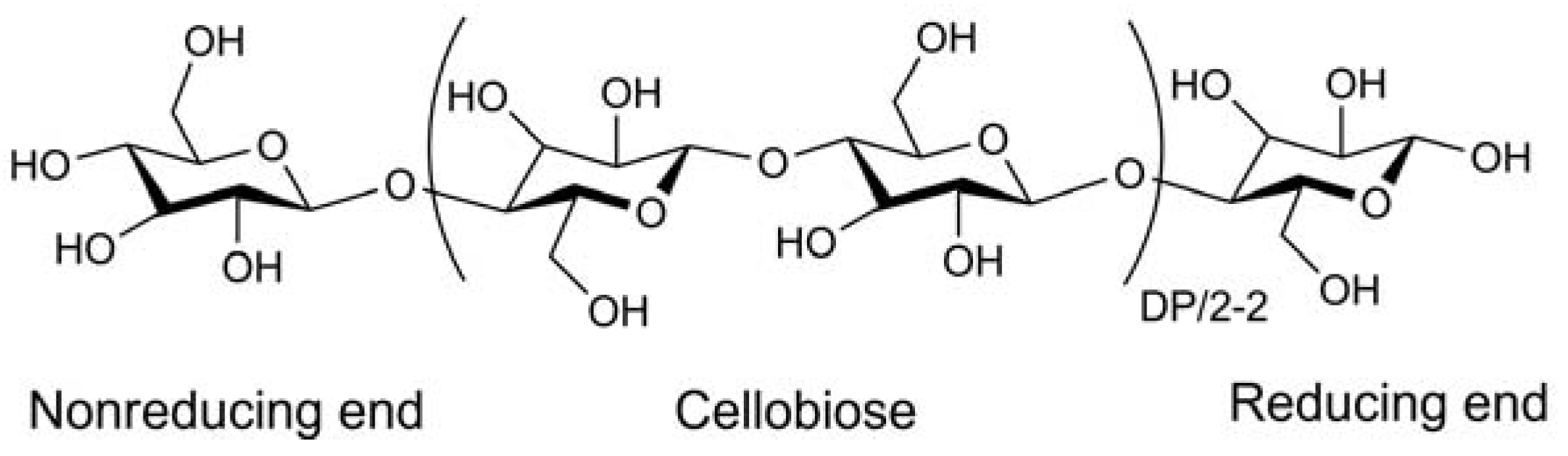
:1. Introduction
| Material forms | Applications | References |
|---|---|---|
| fiber | fiber, reinforcement material, biomaterial, magnetic paper et al. | [3,4,5,6,7] |
| film/membrane | drug delivery, separation, water treatment, package, optical media, biomembrane, adsorption, etc. | [3,4,5,6,7] |
| nanocomposite | biomaterials, drug delivery, reinforcement material, barrier film, membrane, conductive material, adhesion, etc. | [14,15,16,17,18,19,20] |
| npolymer | polymer drug delivery, biomaterial, water treatment, thickener, stabilizer, etc. | [14,15,16,17,18,19,20] |
2. Preparation Strategies
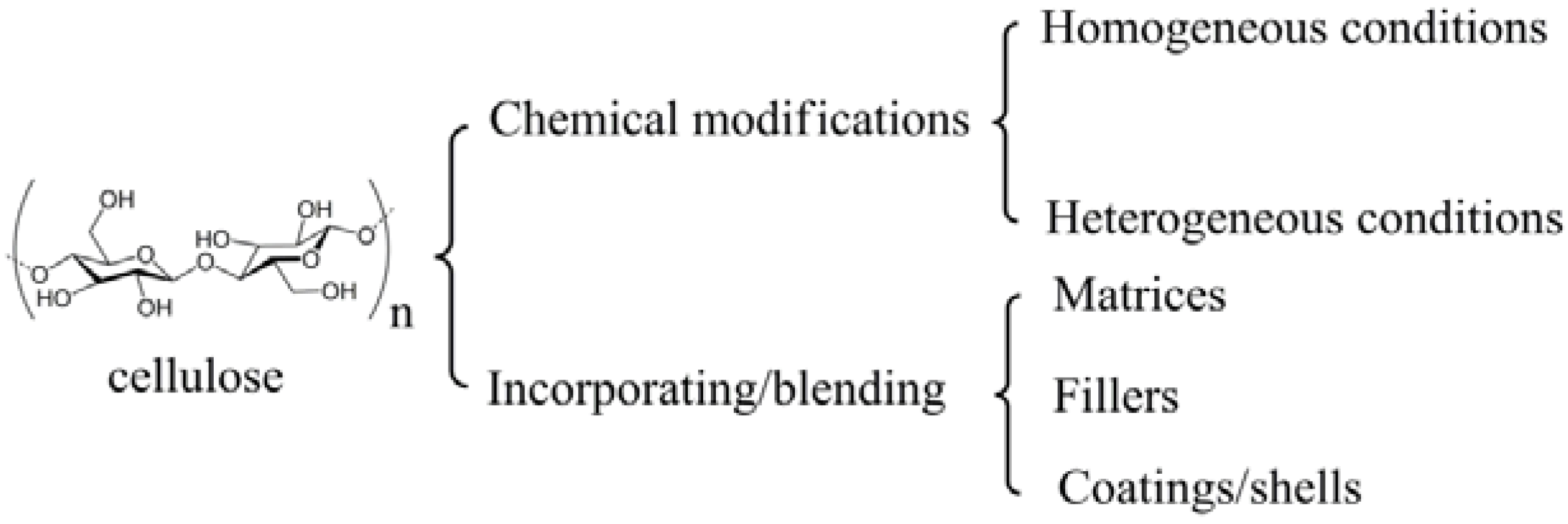
2.1. Chemical Modifications
2.1.1. Modifications in Homogeneous Conditions
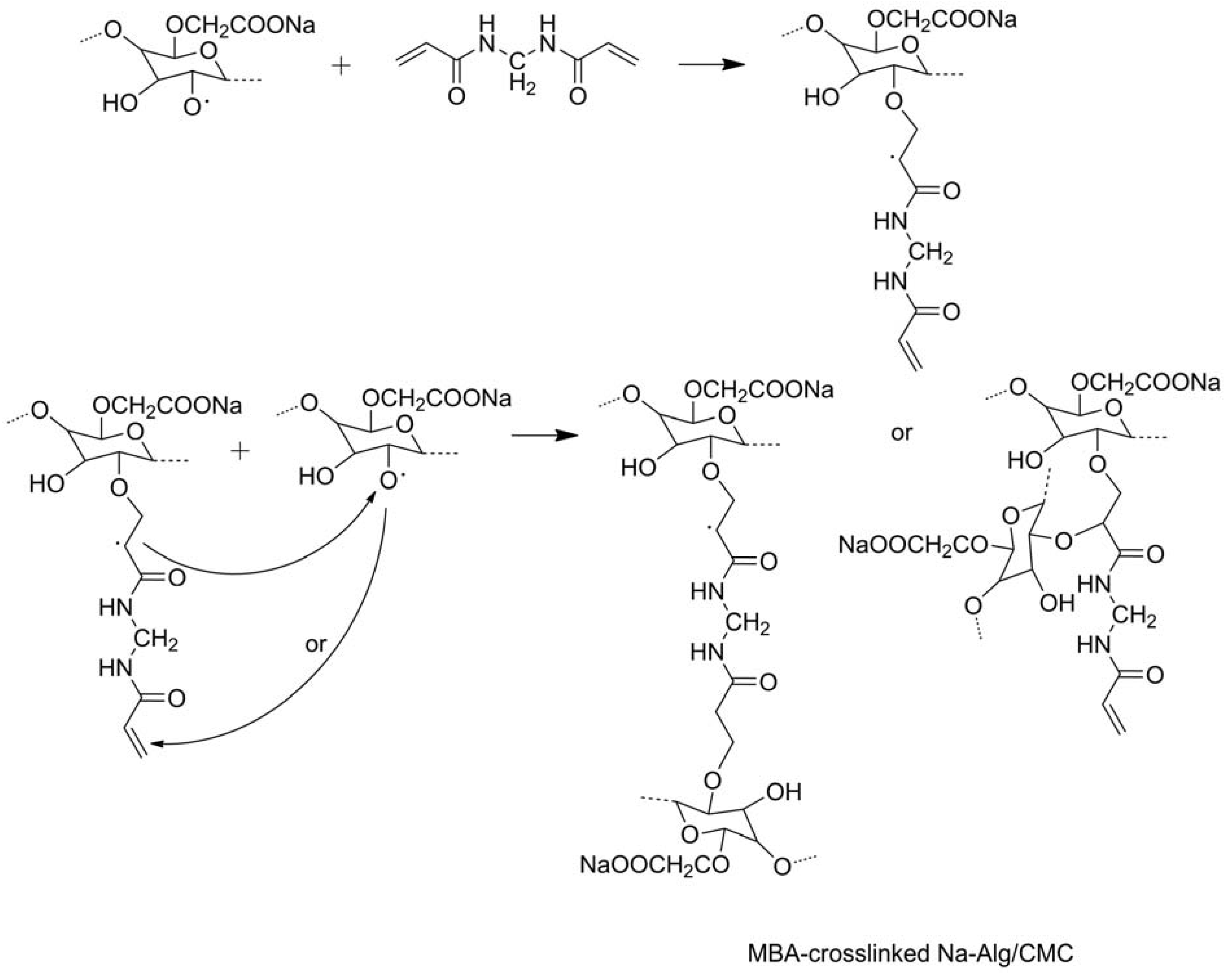
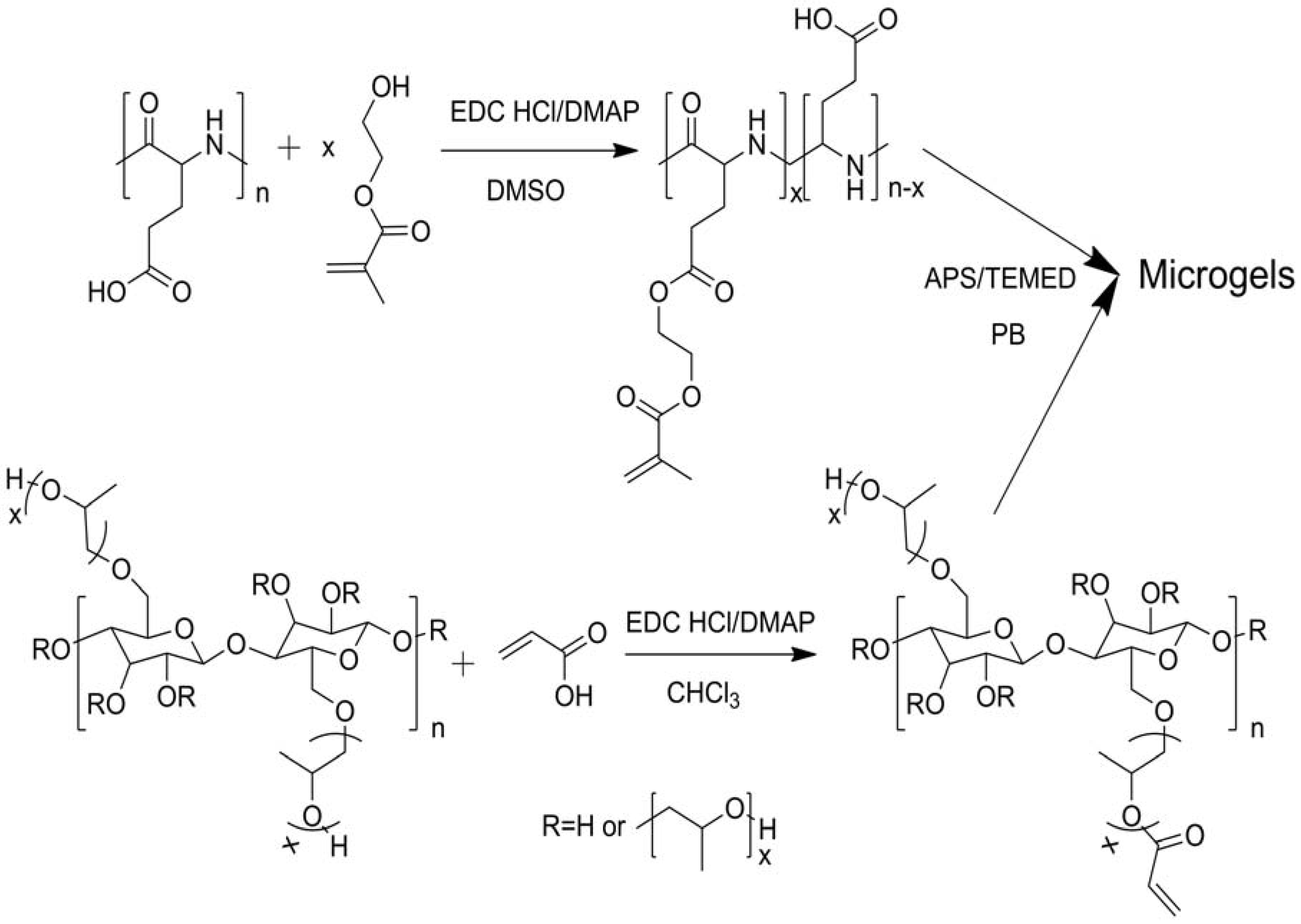

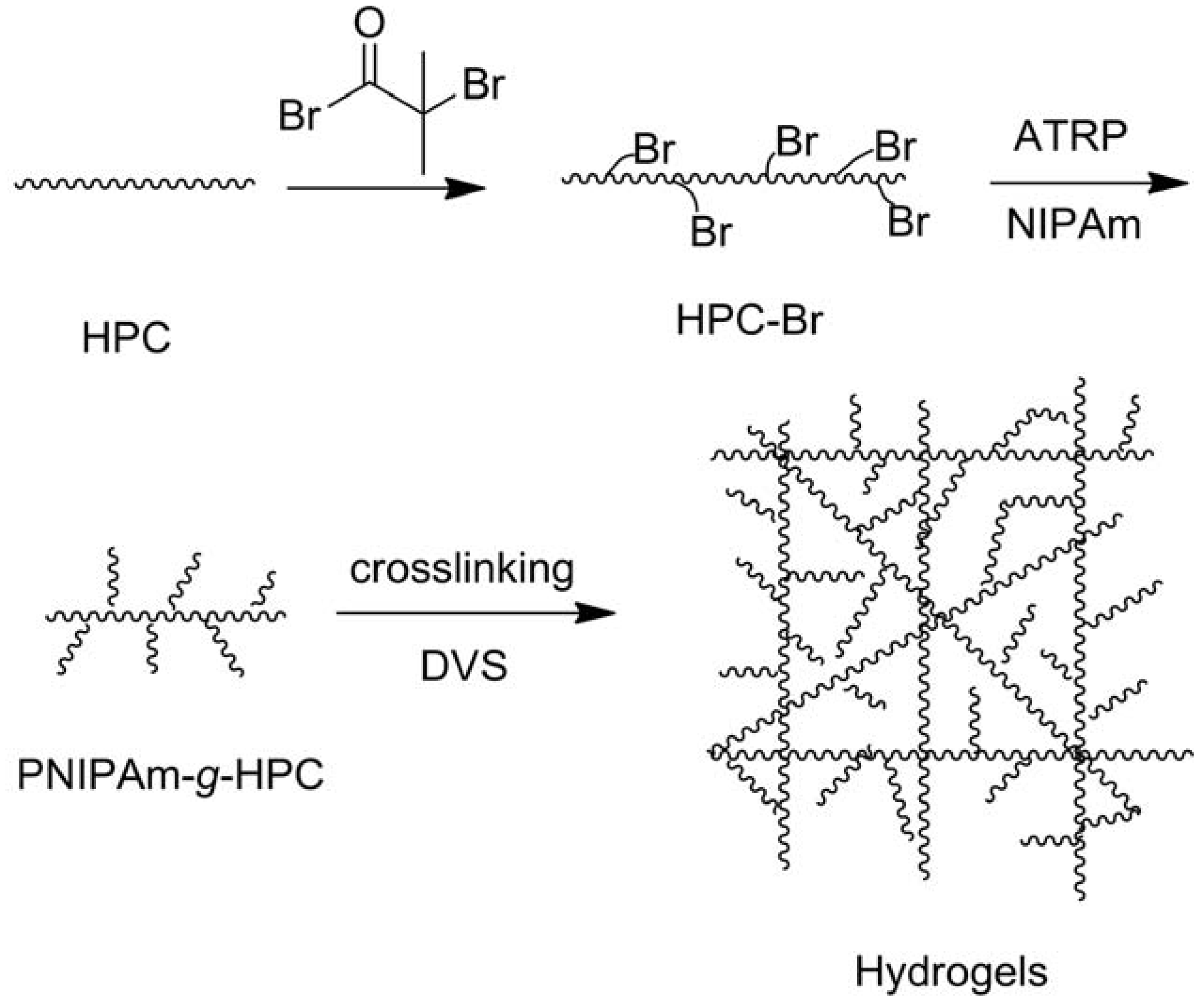
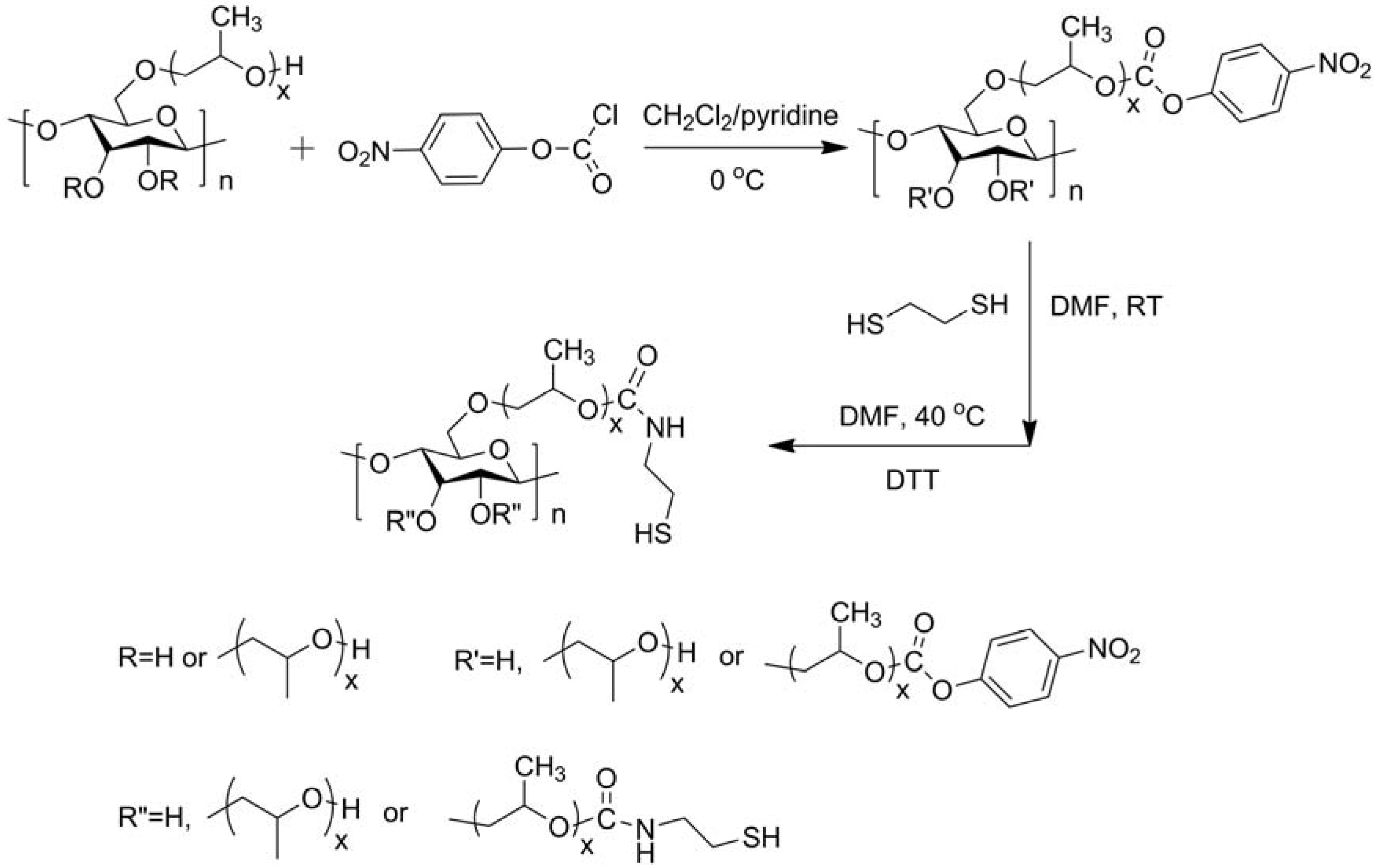
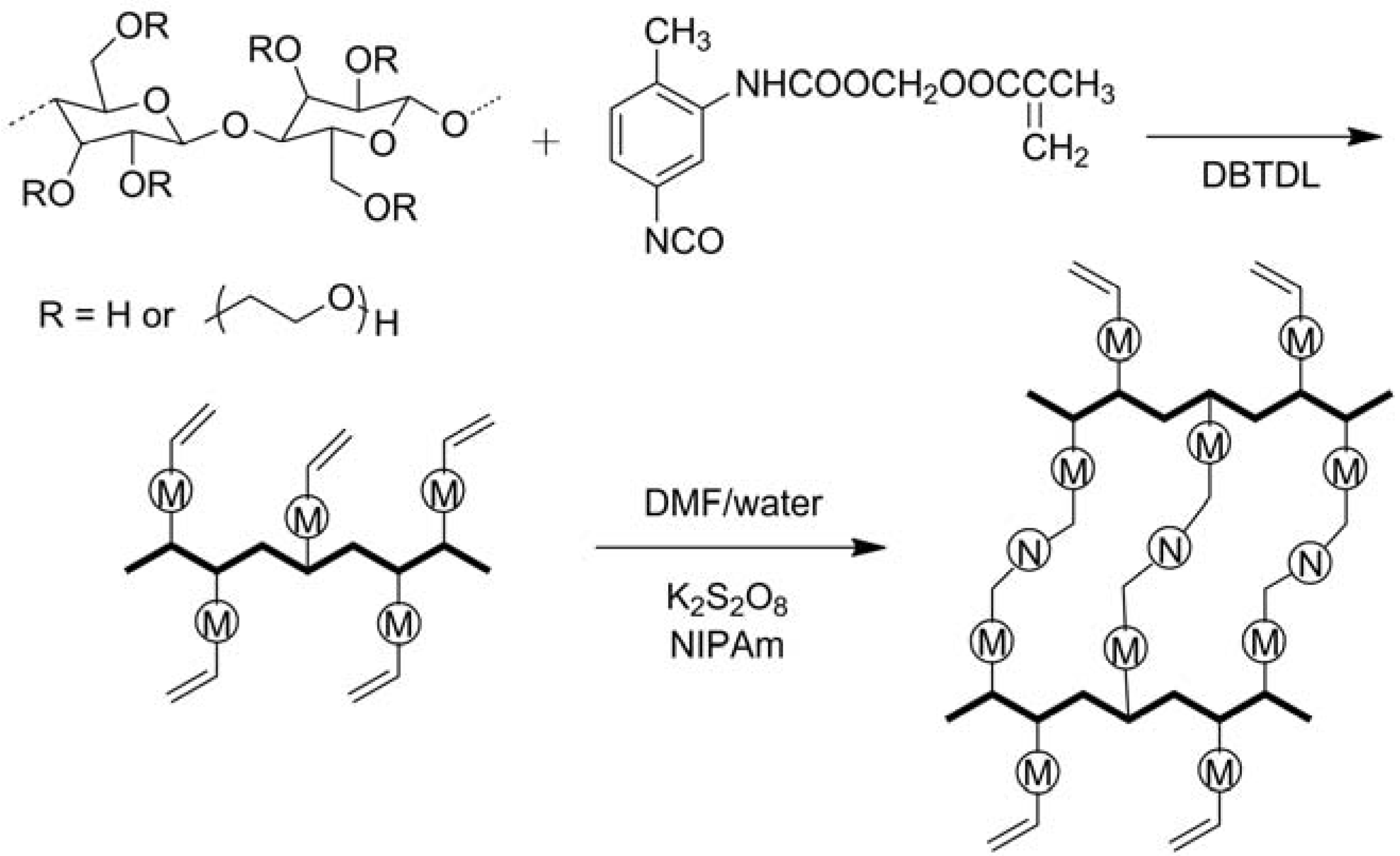
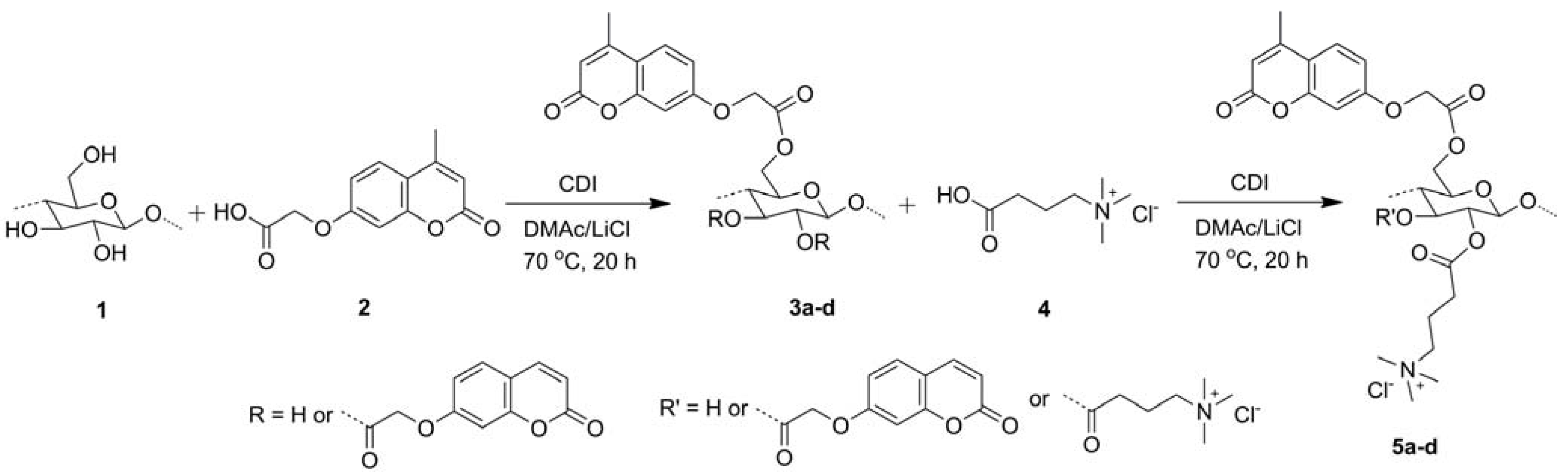
2.1.2. Modifications in Heterogeneous Conditions
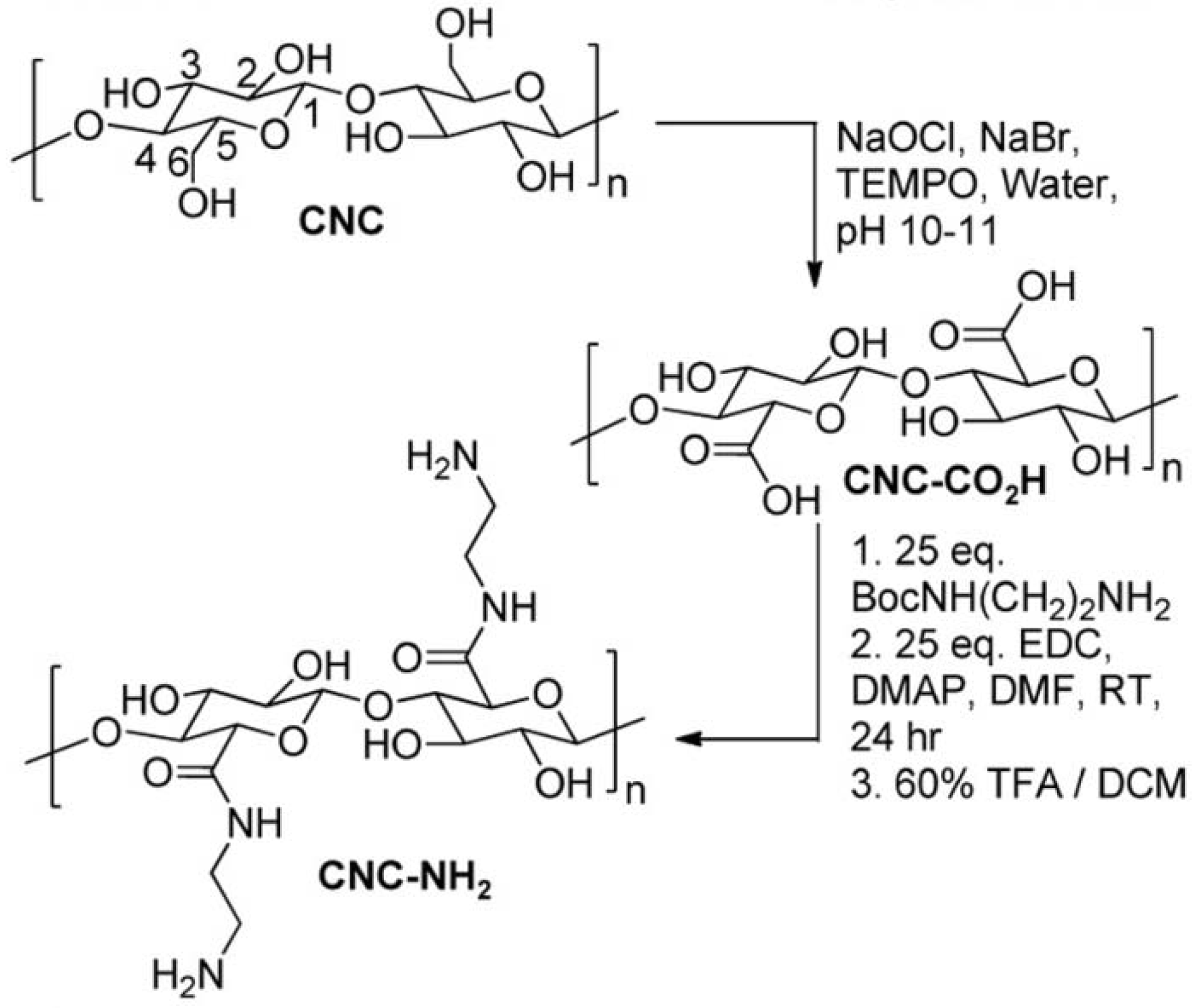
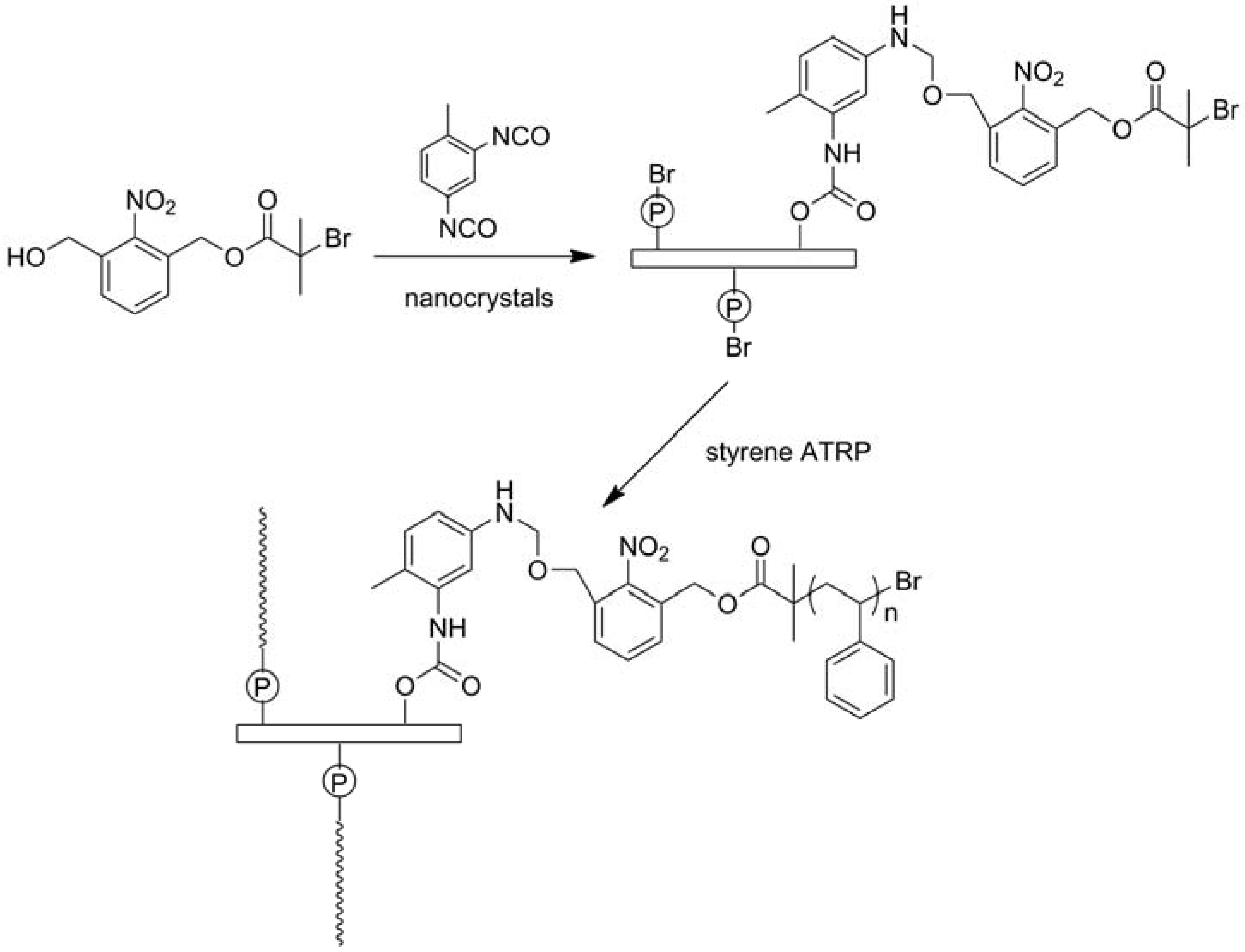
2.2. Physical Incorporating/Blending
2.2.1. Matrices
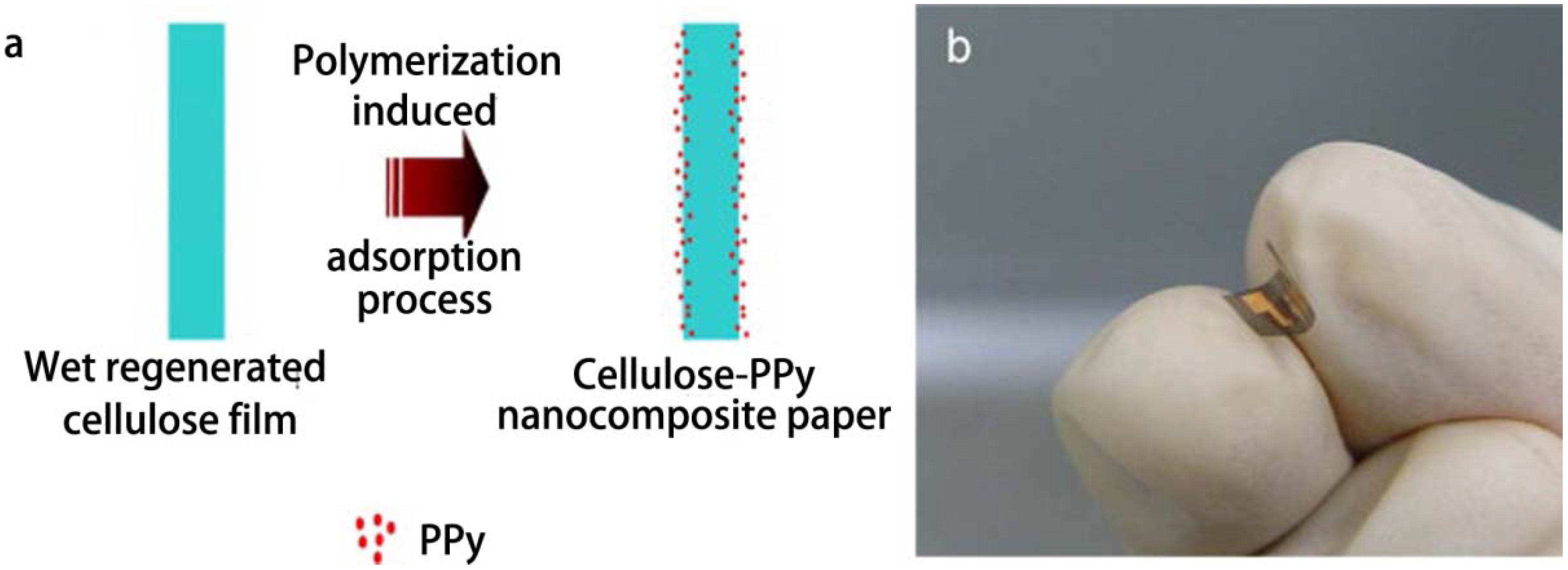
2.2.2. Fillers
2.2.3. Coatings/Shells
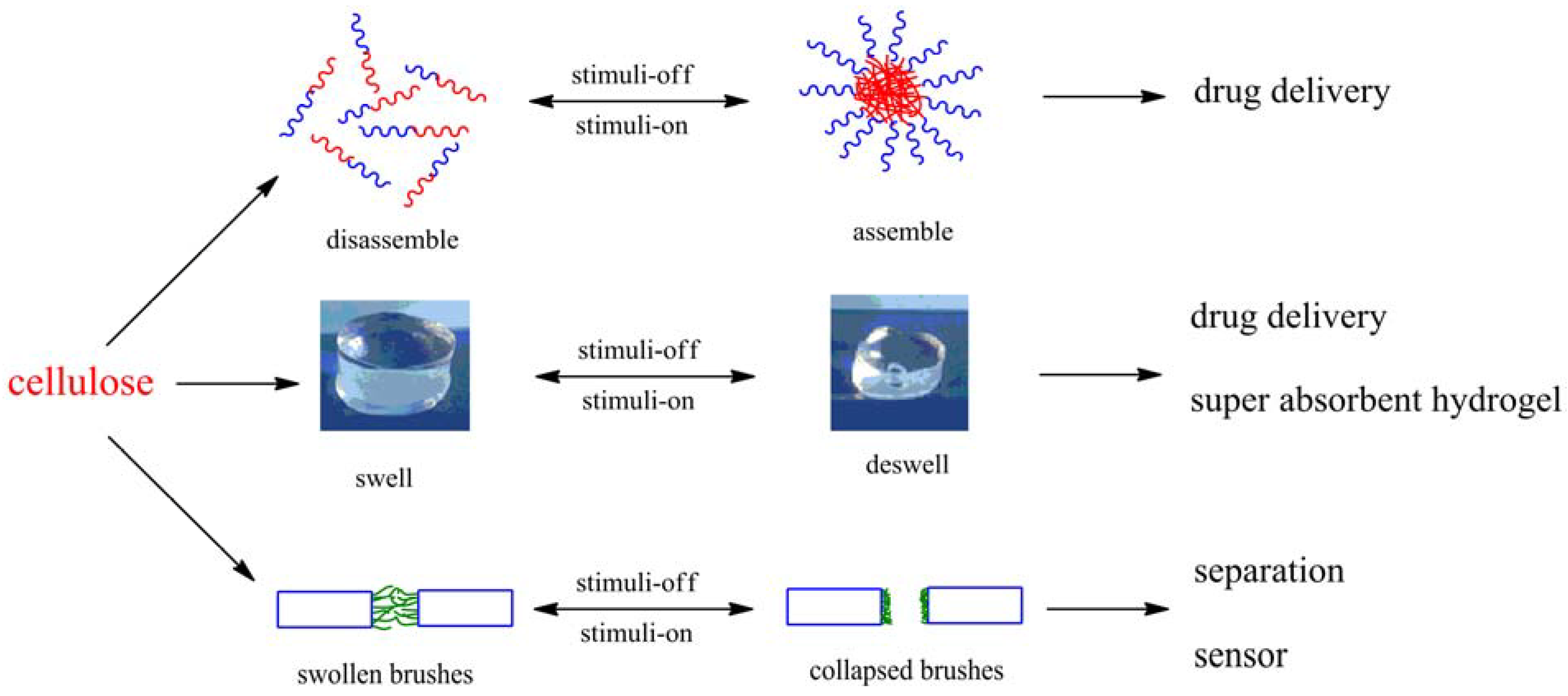
3. Properties and Applications
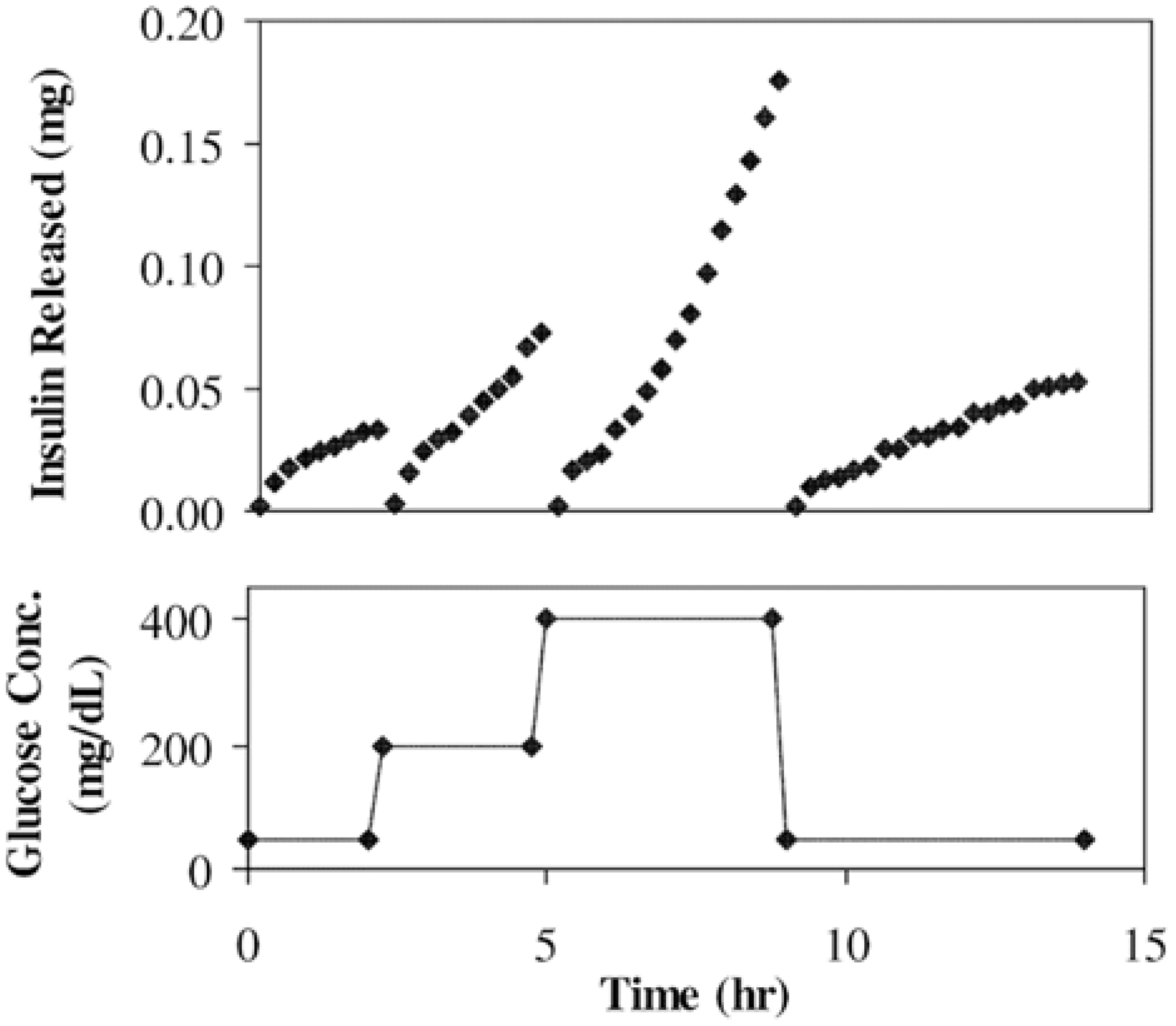
3.1. Drug Delivery Systems and Biomaterials
3.1.1. Aggregates and Hydrogels
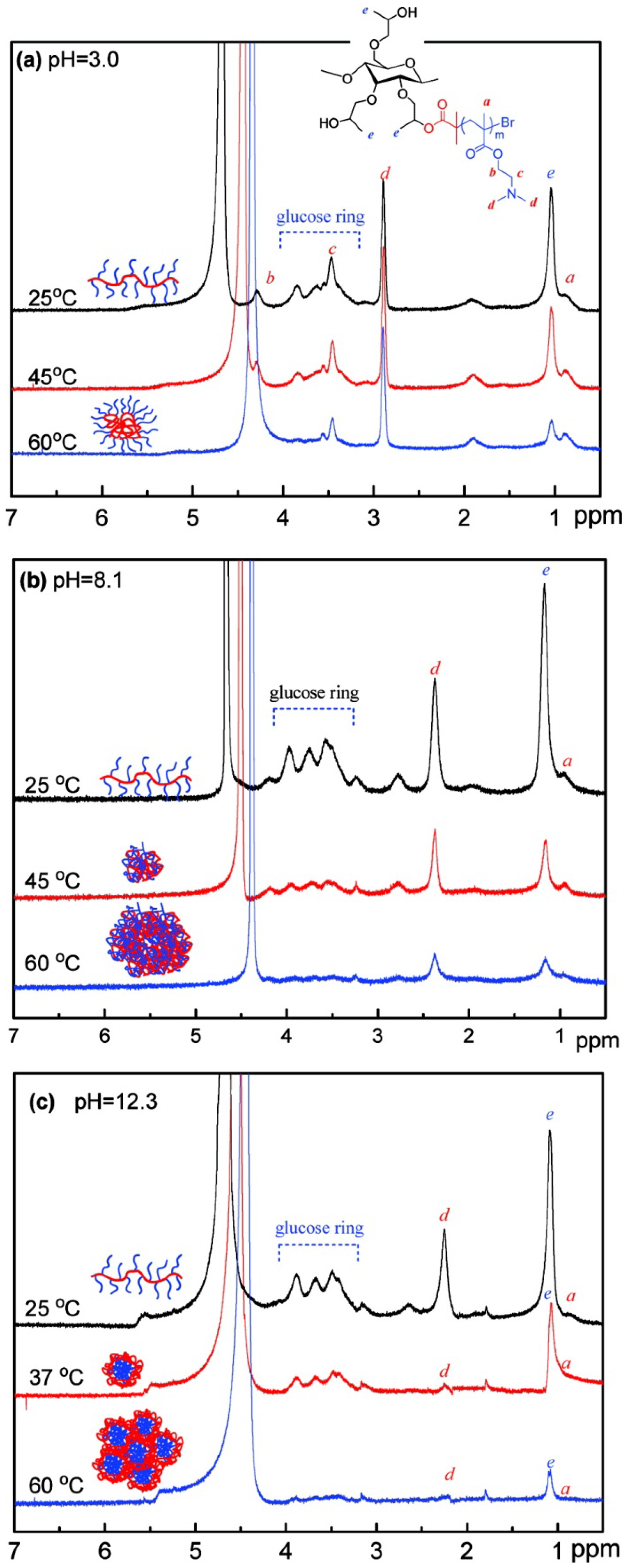
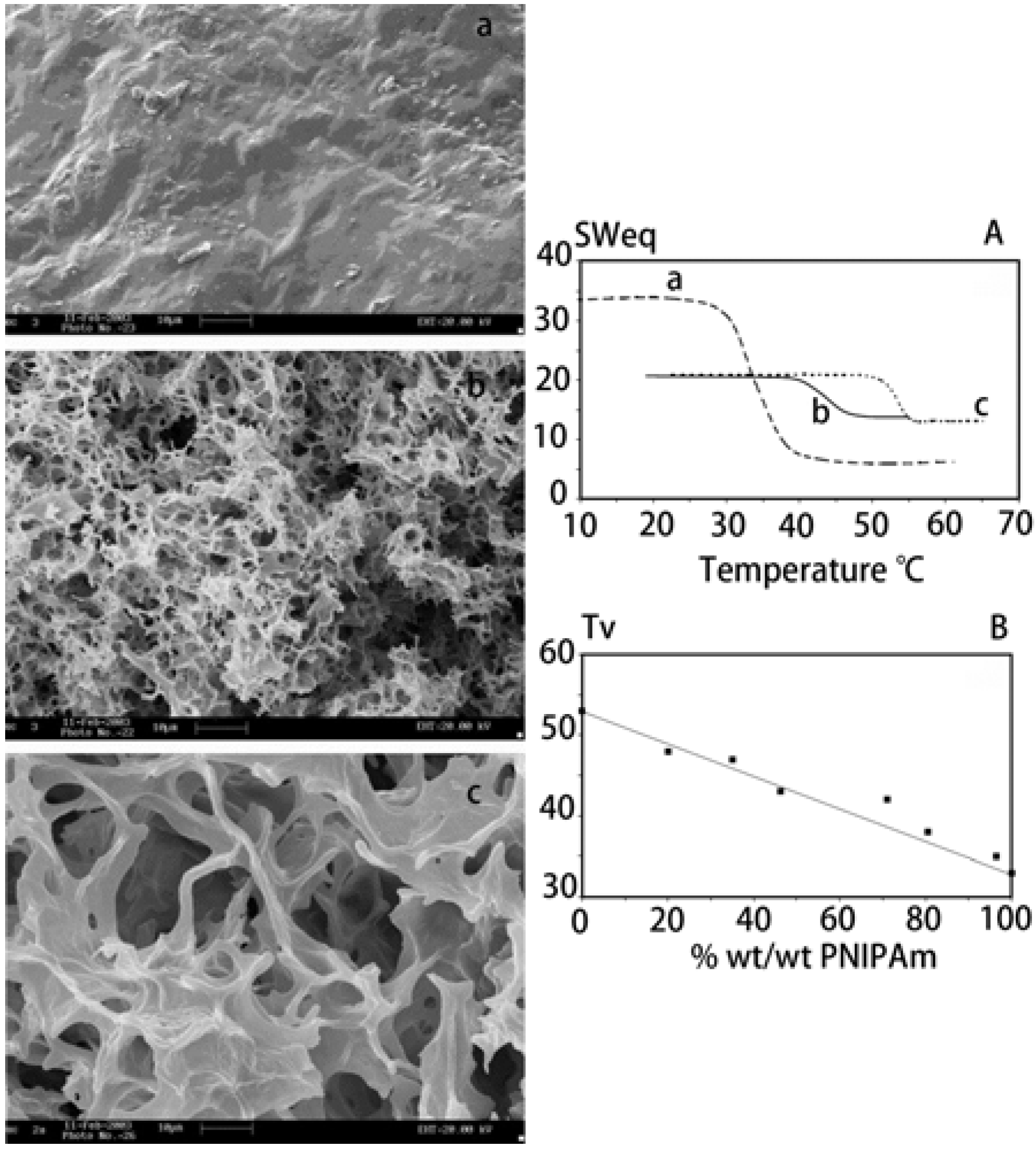
3.1.2. Microcapsules and Nanoparticles
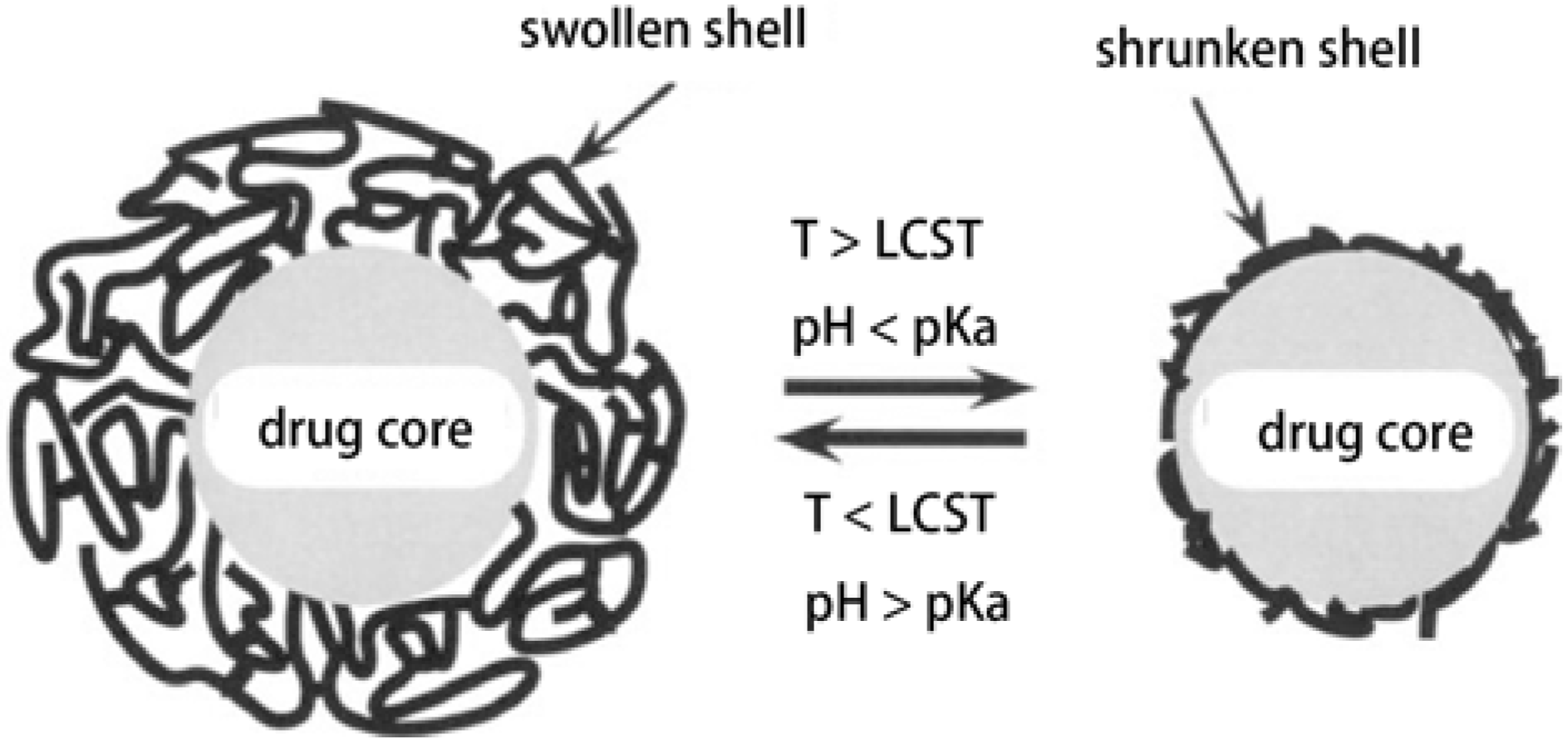
3.1.3. Membranes

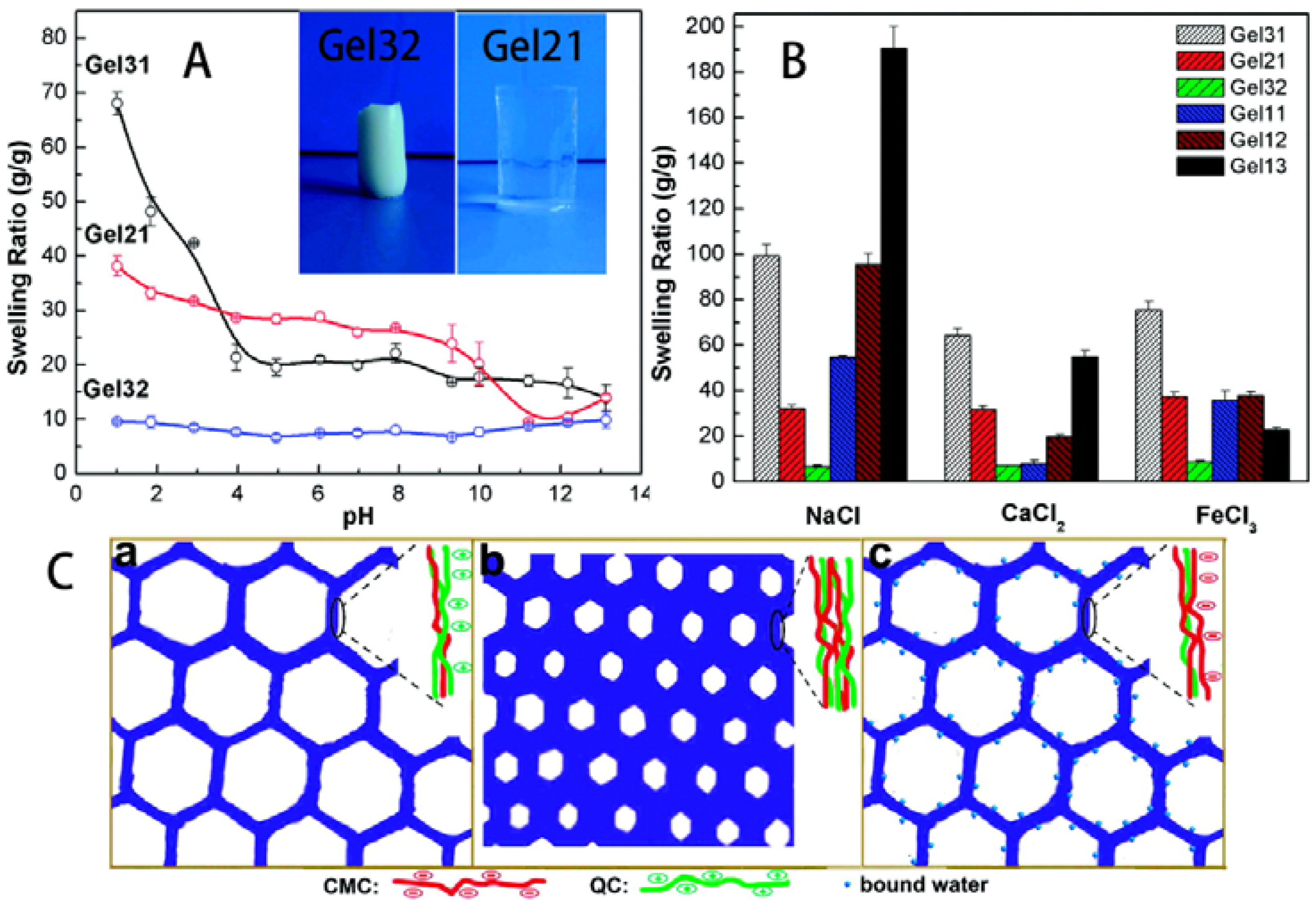
3.2. Hydrogels
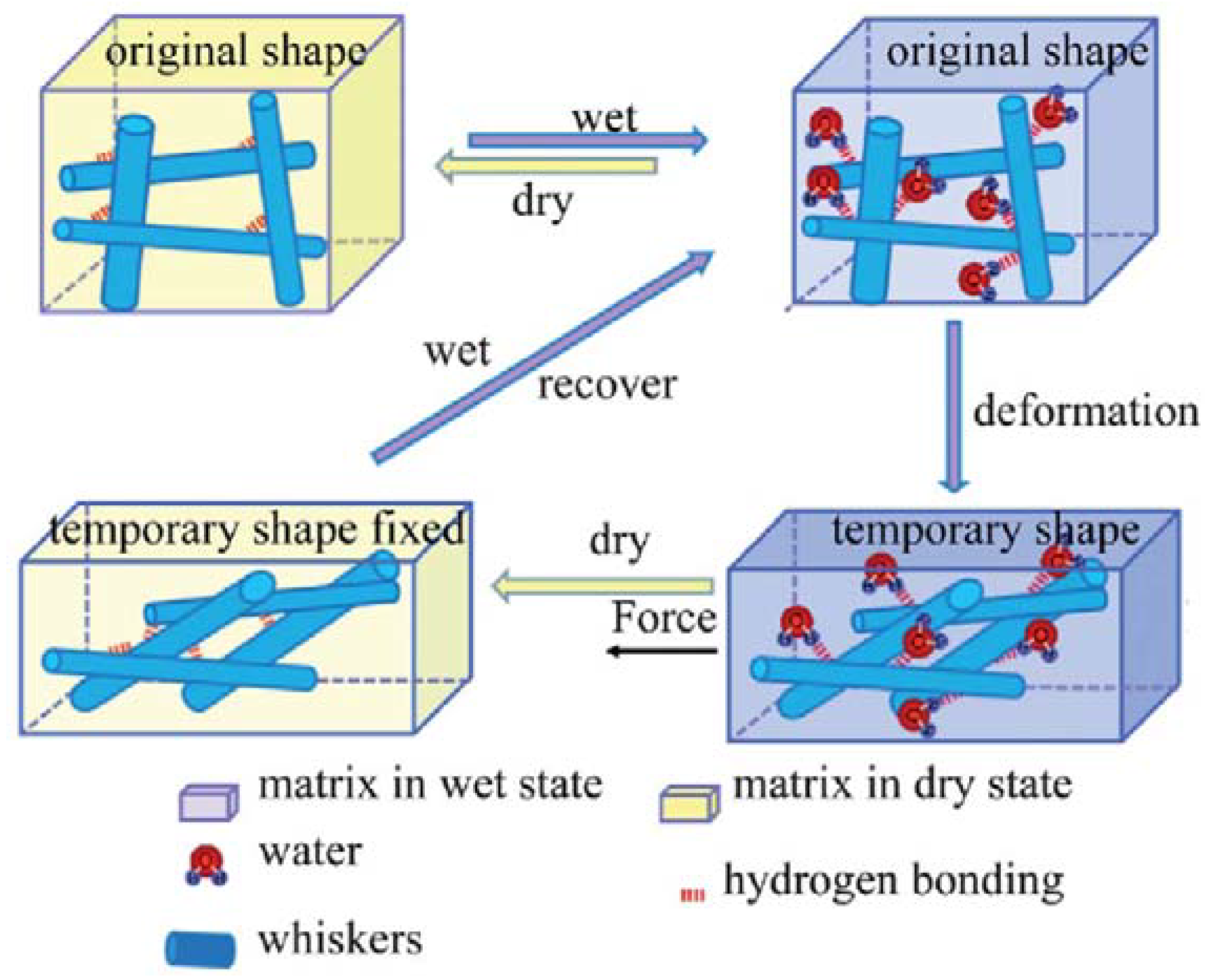
3.3. Mechanical-Adaptive Materials
3.4. Electro Active Materials
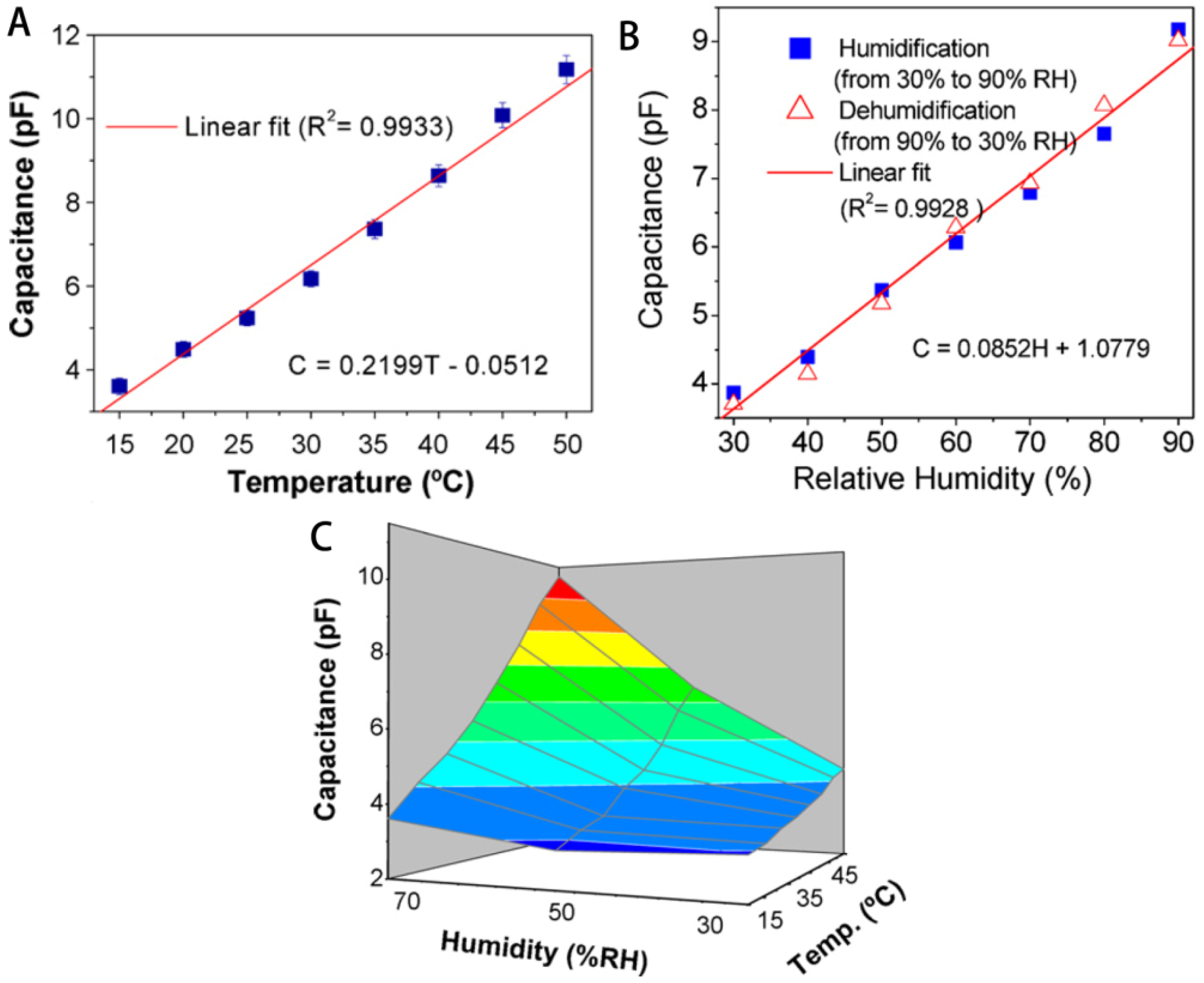
3.5. Sensors
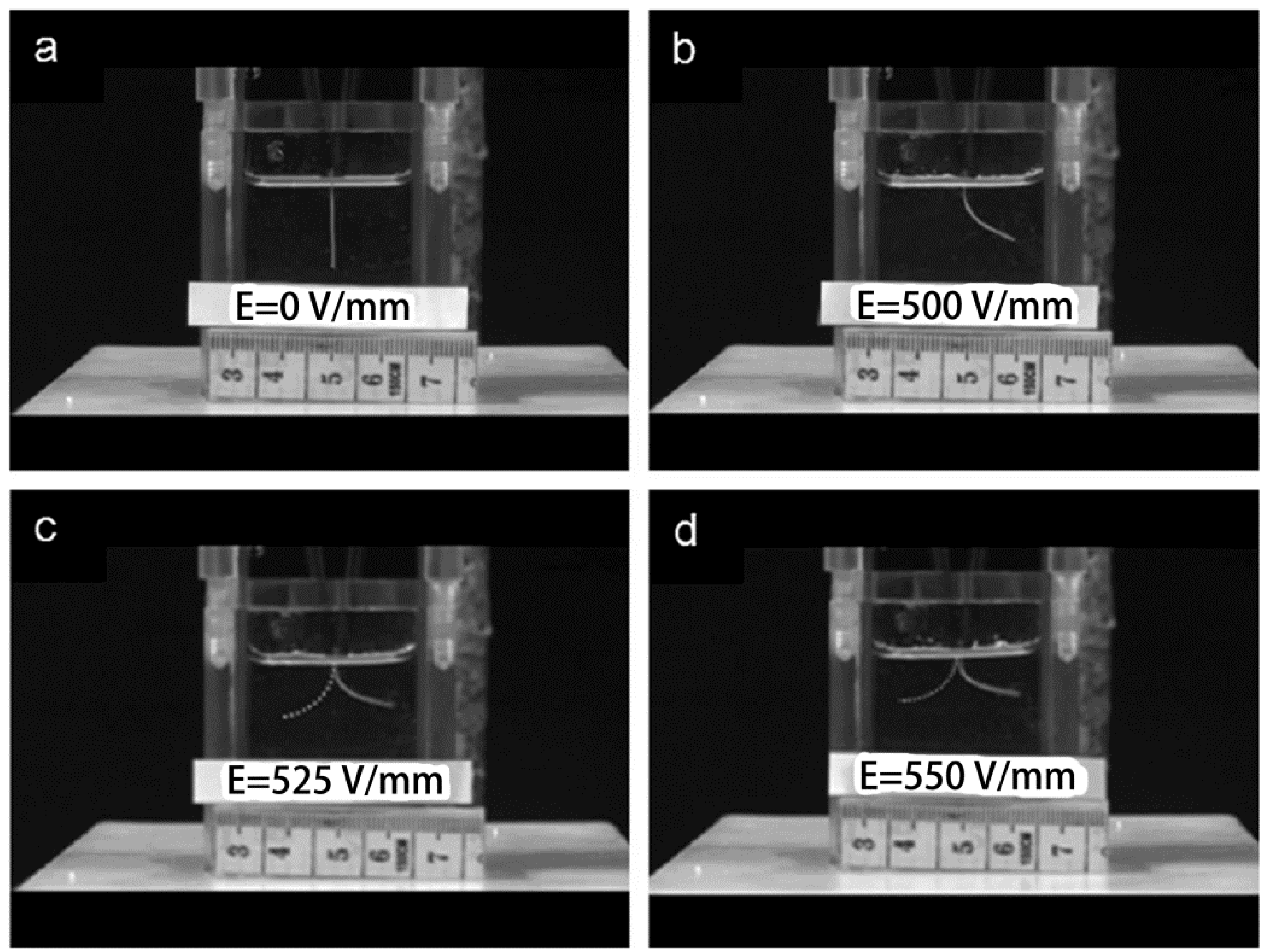
3.6. Other Applications
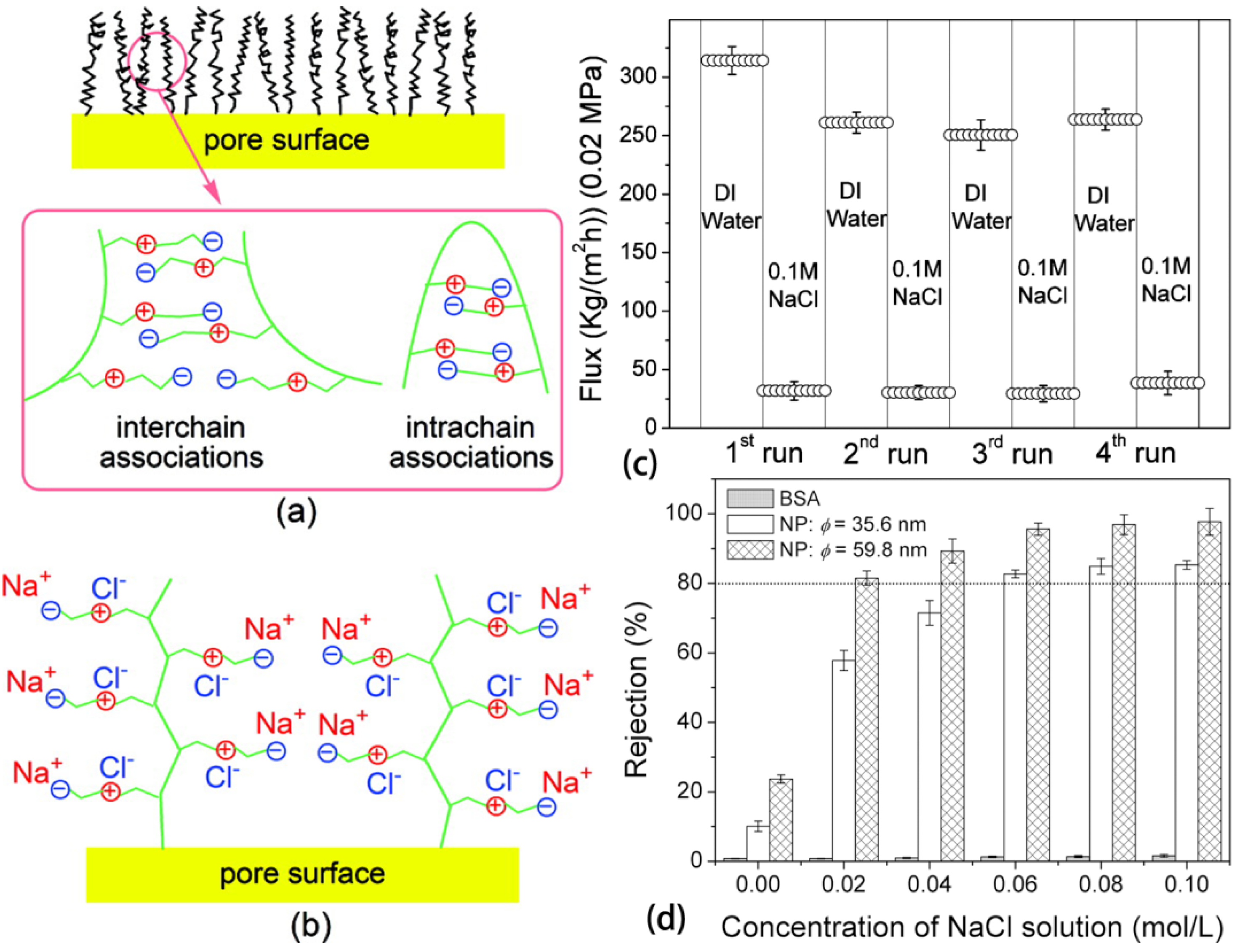
4. Conclusions and Outlooks
Acknowledgements
References
- Doelker, E. Cellulose derivatives. Adv. Polym. Sci. 1993, 107, 199–265. [Google Scholar]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry, Volume 1: Fundamentals and Analytical Methods; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1998. [Google Scholar]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fibers. Progr. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Mashkour, M.; Tajvidi, M.; Kimura, T.; Kimura, F.; Ebrahimi, G. Fabricating unidirectional magnetic papers using permanent magnets to align magnetic nanoparticale coveres natural cellulose fibers. BioResources 2011, 6, 4731–4738. [Google Scholar]
- Belgacem, M.N.; Gandini, A. The surface modification of cellulose fibers for use as reinforcing elements in compostite materials. Compos. Interfaces 2005, 12, 41–75. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibers and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Reid, M.L.; Brown, M.B.; Moss, G.P.; Jones, S.A. An investigation into solvent-membrane interactions when assessing drug release from organic vehicles using regenerated cellulose membranes. J. Pharm. Pharmacol. 2008, 60, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Progr. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Kontturi, E.; Tammelin, T.; Österberg, M. Cellulose—Model films and the fundamental approach. Chem. Soc. Rev. 2006, 35, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Pawlak, J.J.; Hubbe, M.A. Water vapor barrier properties of coated and filled microfibrillated cellulose composite films. BioResources 2011, 6, 4370–4388. [Google Scholar]
- Tizzotti, M.; Charlot, A.; Fleury, E.; Stenzel, M.; Bernard, J. Modification of polysaccharides through controlled/living radical polymerization grafting—Towards the generation of high performance hybrids. Macromol. Rapid Commun. 2010, 31, 1751–1772. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A critical review of all-cellulose composites. J. Mater. Sci. 2012, 47, 1171–1186. [Google Scholar] [CrossRef]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J.; Lucia, L.A.; Sain, M. Cellulosic nanocomposites: A review. BioResources 2008, 3, 929–980. [Google Scholar]
- Khalil, H.P. S.A.; Bhat, A.H.; Yusra, A.F. I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samird, M.A. S.A. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Földváry, C.M.; Takács, E. Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: A review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M. Cellulosic associative thickeners. Carbohydr. Polym. 2001, 45, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.-M. New water-soluble cellulosic polymers: A review. Macromol. Mater. Eng. 2001, 285, 267–275. [Google Scholar] [CrossRef]
- Murphy, E.B.; Wudl, F. The world of smart healable materials. Progr. Polym. Sci. 2010, 35, 223–251. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol. Biosci. 2006, 6, 991–1008. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Prabaharan, M.; Tiwari, A.; Li, S. Polysaccharides/poly(N-isopropylacrylamide)-based stimuli-responsive hydrogels as novel biomaterials. In Smart Polymer Materials for Biomedical Applications; Li, S., Tiwari, A., Prabaharan, M., Aryal, S., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 33–56. [Google Scholar]
- Tan, J.; Liu, R.; Wang, W.; Liu, W.; Tian, Y.; Wu, M.; Huang, Y. Controllable aggregation and reversible pH sensitivity of AuNPs regulated by carboxymethyl cellulose. Langmuir 2010, 26, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
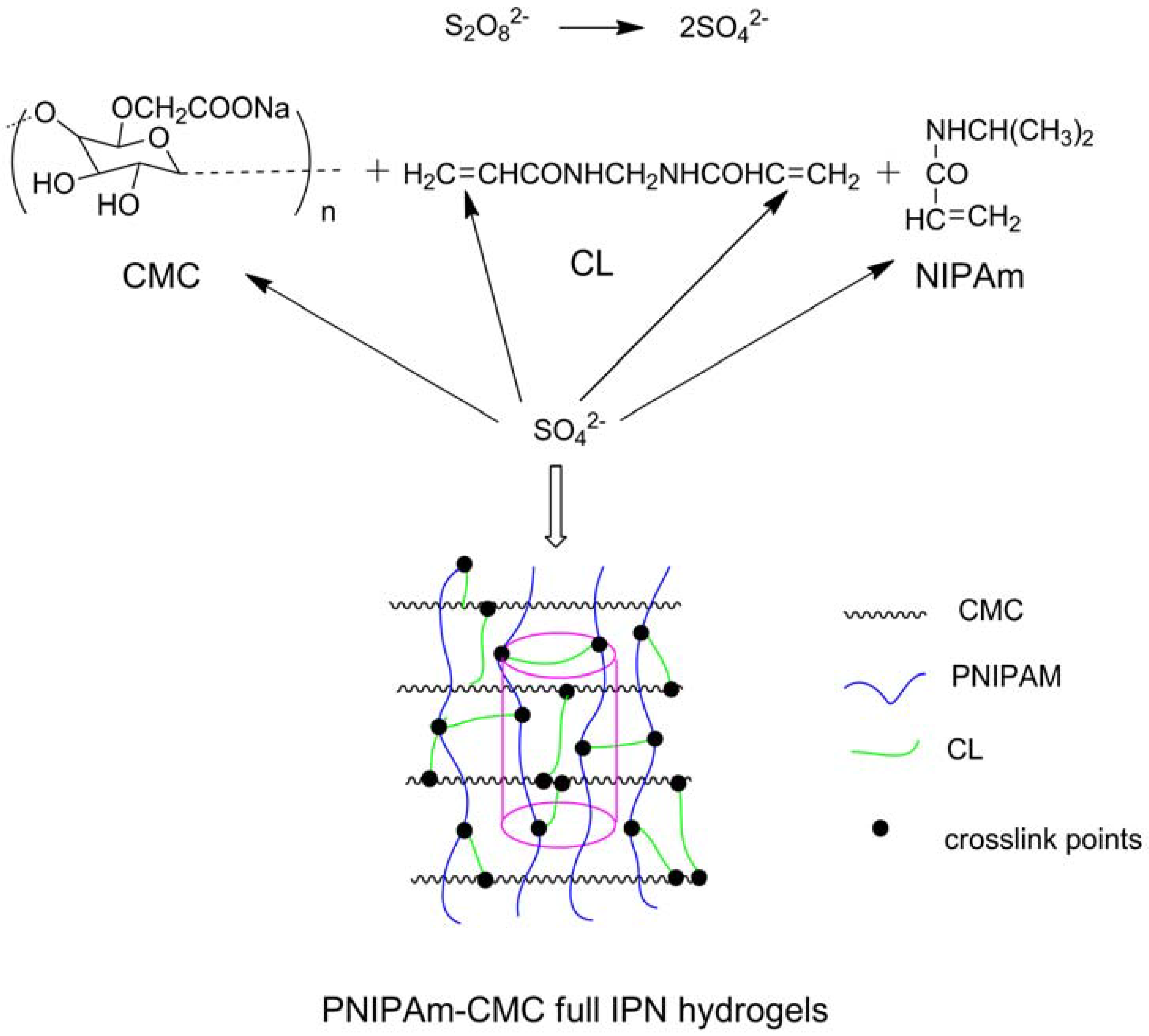
- Ekici, S. Intelligent poly(N-isopropylacrylamide)-carboxymethyl cellulose full interpenetrating polymeric networks for protein adsorption studies. J. Mater. Sci. 2011, 46, 2843–2850. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry, Volume 2: Functionalization of Cellulose; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1998. [Google Scholar]
- Zhang, Z.; Chen, L.; Zhao, C.; Bai, Y.; Deng, M.; Shan, H.; Zhuang, X.; Chen, X.; Jing, X. Thermo- and pH-responsive HPC-g-AA/AA hydrogels for controlled drug delivery applications. Polymer 2011, 52, 676–682. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Z.; Zhang, A.; Chen, L.; He, C.; Zhuang, X.; Chen, X. Novel thermo- and pH-responsive hydroxypropyl cellulose- and poly (L-glutamic acid)-based microgels for oral insulin controlled release. Carbohydr. Polym. 2012, 89, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Progr. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Development of carboxymethyl cellulose acrylate for various biomedical applications. Biomed. Mater. 2006, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, A. Preparation, swelling, and stimuli-responsive characteristics of superabsorbent nanocomposites based on carboxymethyl cellulose and rectorite. Polym. Adv. Technol. 2011, 22, 1602–1611. [Google Scholar] [CrossRef]
- Eldin, M.S. M.; El-Sherif, H.M.; Soliman, E.A.; Elzatahry, A.A.; Omer, A.M. Polyacrylamide-grafted carboxymethyl cellulose: Smart pH-sensitive hydrogel for protein concentration. J. Appl. Polym. Sci. 2011, 122, 469–479. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Zohuriaan-Mehr, M.J.; Ghasempoori, S.N.; Hossienzadeh, H. Modified CMC. V. Synthesis and super-swelling behavior of hydrolyzed CMC-g-PAN hydrogel. J. Appl. Polym. Sci. 2007, 103, 877–883. [Google Scholar] [CrossRef]
- Karakasyan, C.; Lack, S.; Brunel, F.; Maingault, P.; Hourdet, D. Synthesis and rheological properties of responsive thickeners based on polysaccharide architectures. Biomacromolecules 2008, 9, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Rzaev, Z.M.O.; Dinçer, S.; Pis-kin, E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Progr. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Cha, R.; He, Z.; Ni, Y. Preparation and characterization of thermal/pH-sensitive hydrogel from carboxylated nanocrystalline cellulose. Carbohydr. Polym. 2012, 88, 713–718. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Synthesis and application of new temperature-responsive hydrogels based on carboxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohydr. Polym. 2011, 85, 664–673. [Google Scholar] [CrossRef]
- Sannino, A.; Pappadà, S.; Giotta, L.; Valli, L.; Maffezzoli, A. Spin coating cellulose derivatives on quartz crystal microbalance plates to obtain hydrogel-based fast sensors and actuators. J. Appl. Polym. Sci. 2007, 106, 3040–3050. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling behaviors of pH- and salt-responsive cellulose-based hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Mahdavinia, G.R. MBA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohydr. Polym. 2006, 66, 386–395. [Google Scholar] [CrossRef]
- Fang, A.; Cathala, B. Smart swelling biopolymer microparticles by a microfluidic approach: Synthesis, in situ encapsulation and controlled release. Colloids Surf. B Biointerfaces 2011, 82, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Salmawi, K.M.E.; Ibrahim, S.M. Characterization of superabsorbent carboxymethylcellulose/clay hydrogel prepared by electron beam irradiation. Macromol. Res. 2011, 19, 1029–1034. [Google Scholar] [CrossRef]
- Liao, Q.; Shao, Q.; Qiu, G.; Lu, X. Methacrylic acid-triggered phase transition behavior of thermosensitive hydroxypropylcellulose. Carbohydr. Polym. 2012, 89, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ding, D.; Mao, Z.; He, Y.; Hu, Y.; Wu, W.; Jiang, X. Synthesis of hydroxypropylcellulose-poly(acrylic acid) particles with semi-interpenetrating polymer network structure. Biomacromolecules 2008, 9, 2609–2614. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.B.; Caykara, T.; Demiray, M.; Guru, M. Effect of pore-forming agent type on swelling properties of macroporous poly(N-[3-(dimethylaminopropyl)]-methacrylamide-co-acrylamide) hydrogels. J. Macromol. Sci. A Pure Appl. Chem. 2009, 46, 58–64. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Mahajan, S. Structural aspects and nature of swelling medium as equilibrium swelling determinants of acrylamide and cellulosicbased smart hydrogels. J. Appl. Polym. Sci. 2002, 85, 1161–1169. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Tan, J.; Wang, D.; Jin, X.; Kang, H.; Wu, M.; Huang, Y. Self-assembly and dual-stimuli sensitivities of hydroxypropylcellulose -graft-poly(N,N-dimethyl aminoethyl methacrylate) copolymers in aqueous solution. Langmuir 2010, 26, 8697–8703. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kang, H.; Liu, R.; Huang, Y. Smart assembly behaviors of hydroxypropylcellulose-graft-poly(4-vinyl pyridine) copolymers in aqueous solution by thermo and pH stimuli. Langmuir 2010, 26, 18519–18525. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Zhu, Y.; Liu, F.S.; Nie, J.; Ma, J.; Yang, W.T. Comb-shaped conjugates comprising hydroxypropyl cellulose backbones and low-molecular-weight poly(N-isopropylacryamide) side chains for smart hydrogels: Synthesis, characterization, and biomedical applications. Bioconjug. Chem. 2010, 21, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Marsano, E.; Bianchi, E.; Viscardi, A. Stimuli responsive gels based on interpenetrating network of hydroxypropylcellulose and poly(N-isopropylacrylamide). Polymer 2004, 45, 157–163. [Google Scholar] [CrossRef]
- Çaykara, T.; Şengül, G.; Birlik, G. Preparation and swelling properties of temperature-sensitive semi-interpenetrating polymer networks composed of poly[(N-tert-butylacrylamide)-co-acrylamide] and hydroxypropyl cellulose. Macromol. Mater. Eng. 2006, 291, 1044–1051. [Google Scholar] [CrossRef]
- Tan, J.; Kang, H.; Liu, R.; Wang, D.; Jin, X.; Li, Q.; Huang, Y. Dual-stimuli sensitive nanogels fabricated by self-association of thiolated hydroxypropyl cellulose. Polym. Chem. 2011, 2, 672–678. [Google Scholar] [CrossRef]
- Wan, S.; Jiang, M.; Zhang, G. Dual temperature- and pH-dependent self-assembly of cellulose-based copolymer with a pair of complementary grafts. Macromolecules 2007, 40, 5552–5558. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, F. Synthesis and properties of temperature-sensitive hydrogel based on hydroxyethyl cellulose. Int. J. Polym. Mater. 2010, 59, 450–461. [Google Scholar] [CrossRef]
- Kim, B.; Kang, H.; Kim, J. Thermo-sensitive microparticles of PNIPAM-grafted ethylcellulose by spray-drying method. J. Microencapsul. 2002, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, J.; Zou, H.; Shen, T.; Ren, J. Amphiphilic ethyl cellulose brush polymers with mono and dual side chains: Facile synthesis, self-assembly, and tunable temperature-pH responsivities. Polymer 2012, 53, 956–966. [Google Scholar] [CrossRef]
- Estrada, R.; Rodríguez, R.; Castaño, V.M. Smart polymeric membranes: pH-induced non-linear changes in pore size. Appl. Phys. A Mater. Sci. Process. 2010, 99, 723–728. [Google Scholar] [CrossRef]
- Cao, S.; Hu, B.; Liu, H. Synthesis of pH-responsive crosslinked poly[styrene-co-(maleic sodium anhydride)] and cellulose composite hydrogel nanofibers by electrospinning. Polym. Int. 2009, 58, 545–551. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose solvents—Remarkable history, bright future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T.F., Heinze, T.J., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar]
- Sui, X.; Yuan, J.; Zhou, M.; Zhang, J.; Yang, H.; Yuan, W.; Wei, Y.; Pan, C. Synthesis of cellulose-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) copolymers via homogeneous ATRP and their aggregates in aqueous media. Biomacromolecules 2008, 9, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, H.; Pfeifer, A.; Heinze, T. Water soluble photoactive cellulose derivatives: Synthesis and characterization of mixed 2-[(4-methyl-2-oxo-2H-chromen-7-yl) oxy] acetic acid-(3-carboxypropyl) trimethylammonium chloride esters of cellulose. Cellulose 2012, 19, 1327–1335. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, J. Characteristics and performance of electroactive paper actuator made with cellulose/polyurethane semi-interpenetrating polymer networks. J. Appl. Polym. Sci. 2008, 109, 3689–3695. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Habibi, Y.; Rojas, O.J.; Venditti, R.A.; Johansson, L.-S.; Efimenko, K.; Österberg, M.; Laine, J. Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 2010, 11, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Azzam, F.; Heux, L.; Putaux, J.-L.; Jean, B. Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. pH-Responsive cellulose nanocrystal gels and nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Morandi, G.; Thielemans, W. Synthesis of cellulose nanocrystals bearing photocleavable grafts by ATRP. Polym. Chem. 2012, 3, 1402–1407. [Google Scholar] [CrossRef]
- Pan, K.; Zhang, X.; Ren, R.; Cao, B. Double stimuli-responsive membranes grafted with block copolymer by ATRP method. J. Membr. Sci. 2010, 356, 133–137. [Google Scholar] [CrossRef]
- Qiu, X.; Ren, X.; Hu, S. Fabrication of dual-responsive cellulose-based membrane via simplified surface-initiated ATRP. Carbohydr. Polym. 2012, 92, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Gorey, C.; Escobar, I.C. N-isopropylacrylamide (NIPAAM) modified cellulose acetate ultrafiltration membranes. J. Membr. Sci. 2011, 383, 272–279. [Google Scholar] [CrossRef]
- Kubota, H.; Suka, I.G.; Kuroda, S.-I.; Kondo, T. Introduction of stimuli-responsive polymers into regenerated cellulose film by means of photo-grafting. Eur. Polym. J. 2001, 37, 1367–1372. [Google Scholar] [CrossRef]
- Gorey, C.; Escobar, I.C.; Gruden, C.; Coleman, M.; Mileyeva-Biebesheimer, O. Development of smart membrane filters for microbial sensing. Sep. Sci. Technol. 2008, 43, 4056–4074. [Google Scholar] [CrossRef]
- Isaad, J.; Achari, A.E. Colorimetric sensing of cyanide anions in aqueous media based on functional surface modification of natural cellulose materials. Tetrahedron 2011, 67, 4939–4947. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Andersson, M.; Berntsson, P.; Chihani, T.; Gatenholm, P. Swelling behavior of stimuli-responsive cellulose fibers. Polymer 1998, 39, 3589–3596. [Google Scholar] [CrossRef]
- Peng, J.; Liu, Q.; Xu, Z.; Masliyah, J. Synthesis of interfacially active and magnetically responsive nanoparticles for multiphase separation applications. Adv. Funct. Mater. 2012, 22, 1732–1740. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Wong, J.E.; Müller-Schulte, D.; Bahadur, D.; Richtering, W. Magnetic nanoparticles encapsulated within a thermoresponsive polymer. J. Nanosci. Nanotechnol. 2009, 9, 5355–5361. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Progr. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.Y. Temperature and pH-responsive polymeric composite membranes for controlled delivery of proteins and peptides. Biomaterials 2004, 25, 5281–5291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, X.Y. Modulated insulin permeation across a glucose-sensitive polymeric composite membrane. J. Control. Release 2002, 80, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Regmi, B.P.; Monk, J.; El-Zahab, B.; Das, S.; Hung, F.R.; Hayes, D.J.; Warner, I.M. A novel composite film for detection and molecular weight determination of organic vapors. J. Mater. Chem. 2012, 22, 13732–13741. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Chen, K.-S.; Lin, S.-Y. Development and investigation of a thermo-responsive cholesteryl oleyl carbonate-embedded membrane. J. Control. Release 1996, 41, 163–170. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Lin, H.-L.; Li, M.-J. Reproducibility of temperature response and long-term stability of thermo-responsive membrane prepared by adsorption of binary liquid crystals. J. Membr. Sci. 2003, 225, 135–143. [Google Scholar] [CrossRef]
- Atyabi, F.; Khodaverdi, E.; Dinarvand, R. Temperature modulated drug permeation through liquid crystal embedded cellulose membranes. Int. J. Pharm. 2007, 339, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Suedee, R.; Jantarat, C.; Lindner, W.; Viernstein, H.; Songkro, S.; Srichana, T. Development of a pH-responsive drug delivery system for enantioselective-controlled delivery of racemic drugs. J. Control. Release 2010, 142, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Waich, K.; Mayr, T.; Klimant, I. Fluorescence sensors for trace monitoring of dissolved ammonia. Talanta 2008, 77, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, S.K.; Yun, S.; Kim, J. Flexible humidity and temperature sensor based on cellulose-polypyrrole nanocomposite. Sens. Actuators A Phys. 2011, 166, 194–199. [Google Scholar] [CrossRef]
- Ichinose, I.; Kunitake, T. Polymerization-induced adsorption: A preparative method of ultrathin polymer films. Adv. Mater. 1999, 11, 413–415. [Google Scholar] [CrossRef]
- Csoka, L.; Hoeger, I.C.; Rojas, O.J.; Peszlen, I.; Pawlak, J.J.; Peralta, P.N. Piezoelectric effect of cellulose nanocrystals thin films. ACS Macro Lett. 2012, 1, 867–870. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Mahadeva, S.K.; Yun, K.; Yang, S.Y.; Maniruzzaman, M. Paper actuators made with cellulose and hybrid materials. Sensors 2010, 10, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.K.; Takagi, H.; Nakagaito, A.N.; Saini, D.R.; Ahn, S.-H. An overview on the cellulose based conducting composites. Compos. B Eng. 2012, 43, 2822–2826. [Google Scholar] [CrossRef]
- Kim, J.; Wang, N.; Chen, Y.; Lee, S.-K.; Yun, G.-Y. Electroactive-paper actuator made with cellulose/NaOH/urea and sodium alginate. Cellulose 2007, 14, 217–223. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Li, J.; Vadahanambi, S.; Kee, C.-D.; Oh, I.-K. Electrospun fullerenol-cellulose biocompatible actuators. Biomacromolecules 2011, 12, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Kunchornsup, W.; Sirivat, A. Physically cross-linked cellulosic gel via 1-butyl-3-methylimidazolium chloride ionic liquid and its electromechanical responses. Sens. Actuators A Phys. 2012, 175, 155–164. [Google Scholar] [CrossRef]
- Kacmaz, S.; Ertekin, K.; Suslu, A.; Ergun, Y.; Celik, E.; Cocen, U. Sub-nanomolar sensing of ionic mercury with polymeric electrospun nanofibers. Mater. Chem. Phys. 2012, 133, 547–552. [Google Scholar] [CrossRef]
- Ongun, M.Z.; Ertekin, K.; Gocmenturk, M.; Ergun, Y.; Suslu, A. Copper ion sensing with fluorescent electrospun nanofibers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 90, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Schueren, L.V.D.; Clerck, K.D.; Brancatelli, G.; Rosace, G.; Damme, E.V.; Vos, W.D. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol-gel matrix. Sens. Actuators B Chem. 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Posey-Dowty, J.D.; Watterson, T.L.; Wilson, A.K.; Edgar, K.J.; Shelton, M.C.; Lingerfelt, L.R., Jr. Zero-order release formulations using a novel cellulose ester. Cellulose 2007, 14, 73–83. [Google Scholar] [CrossRef]
- Karewicz, A.; Zasada, K.; Szczubiałka, K.; Zapotoczny, S.; Lach, R.; Nowakowska, M. “Smart” alginate–hydroxypropylcellulose microbeads for controlled release of heparin. Int. J. Pharm. 2010, 385, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.K.; Singh, S. Formulation and in vitro evaluation of pH trigger polymeric blended buoyant beads of clarithromycin. Int. J. PharmTech Res. 2012, 4, 5–14. [Google Scholar]
- Tripathi, G.; Singh, S. Formulation and in vitro evaluation of pH sensitive oil entrapped polymeric blended gellan gum buoyant beads of clarithromycin. DARU J. Pharm. Sci. 2010, 18, 247–253. [Google Scholar]
- Ichikawa, H.; Fukumori, Y. A novel positively thermosensitive controlled-release microcapsule with membrane of nano-sized poly(Nisopropylacrylamide) gel dispersed in ethylcellulose matrix. J. Control. Release 2000, 63, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, M.; Silvennoinen, R.J.; Houbenov, N.; Nykänen, A.; Ruokolainen, J.; Sainio, J.; Pore, V.; Kemell, M.; Ankerfors, M.; Lindström, T.; et al. Photoswitchable superabsorbency based on nanocellulose aerogels. Adv. Funct. Mater. 2011, 21, 510–517. [Google Scholar] [CrossRef]
- Pääkk, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Pääkkö, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindström, T.; Berglund, L.A.; Ikkala, O. Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 4, 2492–2499. [Google Scholar] [CrossRef]
- Katepetch, C.; Rujiravanit, R. Synthesis of magnetic nanoparticle into bacterial cellulose matrix by ammonia gas-enhancing in situ co-precipitation method. Carbohydr. Polym. 2011, 86, 162–170. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Biomimetic mechanically adaptive nanocomposites. Progr. Polym. Sci. 2010, 35, 212–222. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Multifunctional shape-memory polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Yang, B.; Zhao, Y.; Ding, Z. Thermo-moisture responsive polyurethane shape-memory polymer and composites: A review. J. Mater. Chem. 2010, 20, 3367–3381. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Luo, H.; Young, R.J.; Deng, L.; Zhang, S.; Fan, Y.; Ye, G. Rapidly switchable water-sensitive shape-memory cellulose/elastomer nano-composites. Soft Matter 2012, 8, 2509–2517. [Google Scholar] [CrossRef]
- Han, J.; Zhu, Y.; Hu, J.; Luo, H.; Yeung, L.-Y.; Li, W.; Meng, Q.; Ye, G.; Zhang, S.; Fan, Y. Morphology, reversible phase crystallization, and thermal sensitive shape memory effect of cellulose whisker/SMPU nanocomposites. J. Appl. Polym. Sci. 2012, 123, 749–762. [Google Scholar] [CrossRef]
- Luo, H.; Hu, J.; Zhu, Y. Polymeric shape memory nanocomposites with heterogeneous twin switches. Macromol. Chem. Phys. 2011, 212, 1981–1986. [Google Scholar] [CrossRef]
- Luo, H.; Hu, J.; Zhu, Y. Tunable shape recovery of polymeric nano-composites. Mater. Lett. 2011, 65, 3583–3585. [Google Scholar] [CrossRef]
- Auad, M.L.; Contos, V.S.; Nutt, S.; Aranguren, M.I.; Marcovich, N.E. Characterization of nanocellulose-reinforced shape memory polyurethanes. Polym. Int. 2008, 57, 651–659. [Google Scholar] [CrossRef]
- Auad, M.L.; Richardson, T.; Orts, W.J.; Medeiros, E.S.; Mattoso, L.H. C.; Mosiewicki, M.A.; Marcovich, N.E.; Aranguren, M.I. Polyaniline-modified cellulose nanofibrils as reinforcement of a smart polyurethane. Polym. Int. 2011, 60, 743–750. [Google Scholar] [CrossRef]
- Mendez, J.; Annamalai, P.K.; Eichhorn, S.J.; Rusli, R.; Rowan, S.J.; Foster, E.J.; Weder, C. Bioinspired mechanically adaptive polymer nanocomposites with water-activated shape-memory effect. Macromolecules 2011, 44, 6827–6835. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Bio-inspired mechanically-adaptive nanocomposites derived from cotton cellulose whiskers. J. Mater. Chem. 2010, 20, 180–186. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Stimuli-responsive mechanically adaptive polymer nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Dagnon, K.L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. Water-triggered modulus changes of cellulose nanofiber nanocomposites with hydrophobic polymer matrices. Macromolecules 2012, 45, 4707–4715. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H. A.; Pereira, A.G. B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Edgar, K.J. Cellulose esters in drug delivery. Cellulose 2007, 14, 49–64. [Google Scholar] [CrossRef]
- Murtaza, G. Ethylcellulose microparticles: A review. Acta Poloniae Pharm. 2012, 69, 11–22. [Google Scholar] [PubMed]
- Rogers, T.L.; Wallick, D. Reviewing the use of ethylcellulose, methylcellulose and hypromellose in microencapsulation. Part 1: Materials used to formulate microcapsules. Drug Dev. Ind. Pharm. 2012, 38, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L.; Wallick, D. Reviewing the use of ethylcellulose, methylcellulose and hypromellose in microencapsulation. Part 2: Techniques used to make microcapsules Drug. Dev. Ind. Pharm. 2011, 37, 1259–1271. [Google Scholar] [CrossRef]
- Rogers, T.L.; Wallick, D. Reviewing the use of ethylcellulose, methylcellulose and hypromellose in microencapsulation. Part 3: Applications for microcapsules. Drug Dev. Ind. Pharm. 2012, 38, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. pH-Sensitive polymer blends used as coating materials to control drug release from spherical beads: Importance of the type of core. Biomacromolecules 2005, 6, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Josephine, L.J.J.; Yathish, M.; Wilson, B.; Premakumari, K.B. Formulation and evaluation of microparticles containing curcumin for colorectal cancer. J. Drug Deliv. Ther. 2012, 2, 125–128. [Google Scholar]
- Wang, J.; Wu, F.-Q.; Shi, K.-H.; Wang, X.-H.; Sun, P.-P. Humidity sensitivity of composite material of lanthanum ferrite/polymer quaternary acrylic resin. Sens. Actuators B Chem. 2004, 99, 586–591. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Li, D.; Chen, H.; Sun, R.-C. Fluorescent amphiphilic cellulose nanoaggregates for sensing trace explosives in aqueous solution. Chem. Commun. 2012, 48, 5569–5571. [Google Scholar] [CrossRef]
- Arias, J.L.; López-Viota, M.; Delgado, Á.V.; Ruiz, M.A. Iron/ethylcellulose (core/shell) nanoplatform loaded with 5-fluorouracil for cancer targeting. Colloids Surf. B Biointerfaces 2010, 77, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Moehwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Wohl, B.M.; Engbersen, J.F.J. Responsive layer-by-layer materials for drug delivery. J. Control. Release 2012, 158, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Sanna, R.; Sanna, D.; Alzari, V.; Nuvoli, D.; Scognamillo, S.; Piccinini, M.; Lazzari, M.; Gioffredi, E.; Malucelli, G.; Mariani, A. Synthesis and characterization of graphene-containing thermoresponsive nanocomposite hydrogels of poly(N-vinlcaprolactam) prepared by frontal polymerization. J. Polym. Sci. A Polym. Chem. 2012, 50, 4110–4118. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Li, X. Stimuli-triggered structural engineering of synthetic and biological polymeric assemblies. Progr. Polym. Sci. 2012, 37, 1130–1176. [Google Scholar] [CrossRef]
- Felber, A.E.; Dufresne, M.-H.; Leroux, J.-C. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Sa, B. Evaluation of pH-sensitivity and drug release characteristics of (polyacrylamide-grafted-xanthan)-carboxymethyl cellulose-based pH-sensitive interpenetrating network hydrogel beads. Drug Dev. Ind. Pharm. 2008, 34, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.F.; Rodríguez, R.; Castaño, V.M. Smart polymeric membranes with adjustable pore size. Int. J. Polym. Mater. 2003, 52, 833–843. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Cabrera, R.Q.; Meersman, F.; McMillan, P.F.; Dmitriev, V. Nanomechanical and structural properties of native cellulose under compressive stress. Biomacromolecules 2011, 12, 2178–2183. [Google Scholar] [CrossRef] [PubMed]
- de Susa Lima, M.M.; Borsali, R. Rodlike cellulose microcrystals: Structure, properties, and applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- Kim, J.; Seo, Y.B. Electro-active paper actuators. Smart Mater. Struct. 2002, 11, 355–360. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Yun, K.; Kim, J.; Kim, J.-H. Highly durable, biomimetic electro-active paper actuator based on cellulose polypyrrole-ionic liquid (CPIL) nanocomposite. J. Nanosci. Nanotechnol. 2011, 11, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.-Y.; Kim, J.; Kim, J.-H.; Kim, S.-Y. Fabrication and testing of cellulose EAPap actuators for haptic application. Sens. Actuators A Phys. 2010, A164, 68–73. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, J. Dry and durable electro-active paper actuator based on natural biodegradable polymer. J. Appl. Polym. Sci. 2010, 115, 2044–2049. [Google Scholar] [CrossRef]
- Kim, J.; Wang, N.; Chen, Y. Effect of chitosan and ions on actuation behavior of cellulose-chitosan laminated films as electro-active paper acuators. Cellulose 2007, 14, 439–445. [Google Scholar] [CrossRef]
- Wang, N.-G.; Kim, J.; Chen, Y.; Yun, S.-R.; Lee, S.-K. Electro-active-paper actuator made with LiCl/cellulose films: Effect of LiCl content. Macromol. Res. 2006, 14, 624–629. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, F.; Chen, T. Phenolphthalein immobilized membrane for an optical pH sensor. Anal. Chim. Acta 2004, 510, 189–194. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Wee, K.-H.; Bai, R. A novel electrolyte-responsive membrane with tunable permeation selectivity for protein purification. ACS Appl. Mater. Interf. 2010, 2, 203–211. [Google Scholar] [CrossRef]
- Heitfeld, K.A.; Schaefer, D.W. Structure-property relationships in flavour-barrier membranes with reduced high-temperature diffusivity. Soft Matter 2009, 5, 156–163. [Google Scholar] [CrossRef]
- Yao, J.; Yan, C.-W. Development and analysis of a novel kind of smart thermotropic material. Funct. Mater. Lett. 2010, 3, 135–139. [Google Scholar] [CrossRef]
- Kurioz, Y.; Reznikov, Y.; Tereshchenko, O.; Gerus, I.; Buluy, O.; Ha, K.R.; Kim, D.H.; Kwon, S.B.; Park, S.K. Highly sensitive photoaligning materials on a base of cellulose-cinnamates. Mol. Cryst. Liquid Cryst. 2008, 480, 81–90. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738-781. https://doi.org/10.3390/ma6030738
Qiu X, Hu S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials. 2013; 6(3):738-781. https://doi.org/10.3390/ma6030738
Chicago/Turabian StyleQiu, Xiaoyun, and Shuwen Hu. 2013. "“Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications" Materials 6, no. 3: 738-781. https://doi.org/10.3390/ma6030738




