A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel
Abstract
:1. Introduction

2. Characteristics, Classification and History
2.1. Basic Characteristics
- (1)
- Structure characteristic: gel-like structure, normally with nanoscale coherent skeletons and pores; hierarchical and fractal microstructure (primary structure coexists and is related with larger-scale structure); able to form macroscopic monolith; randomly crosslinking network, normally composed of non-crystalline matter.
- (2)
- Property characteristic: unique bulk properties different from solid matter, gas matter or normal foam, such as ultralow thermal conductivity, ultralow modulus, ultralow refractive index, ultralow dielectric constant, ultralow sound speed, high specific surface area and ultrawide adjustable ranges of the density and the refractive index (especially for silica aerogel); ultralow relative density and ultrahigh porosity.
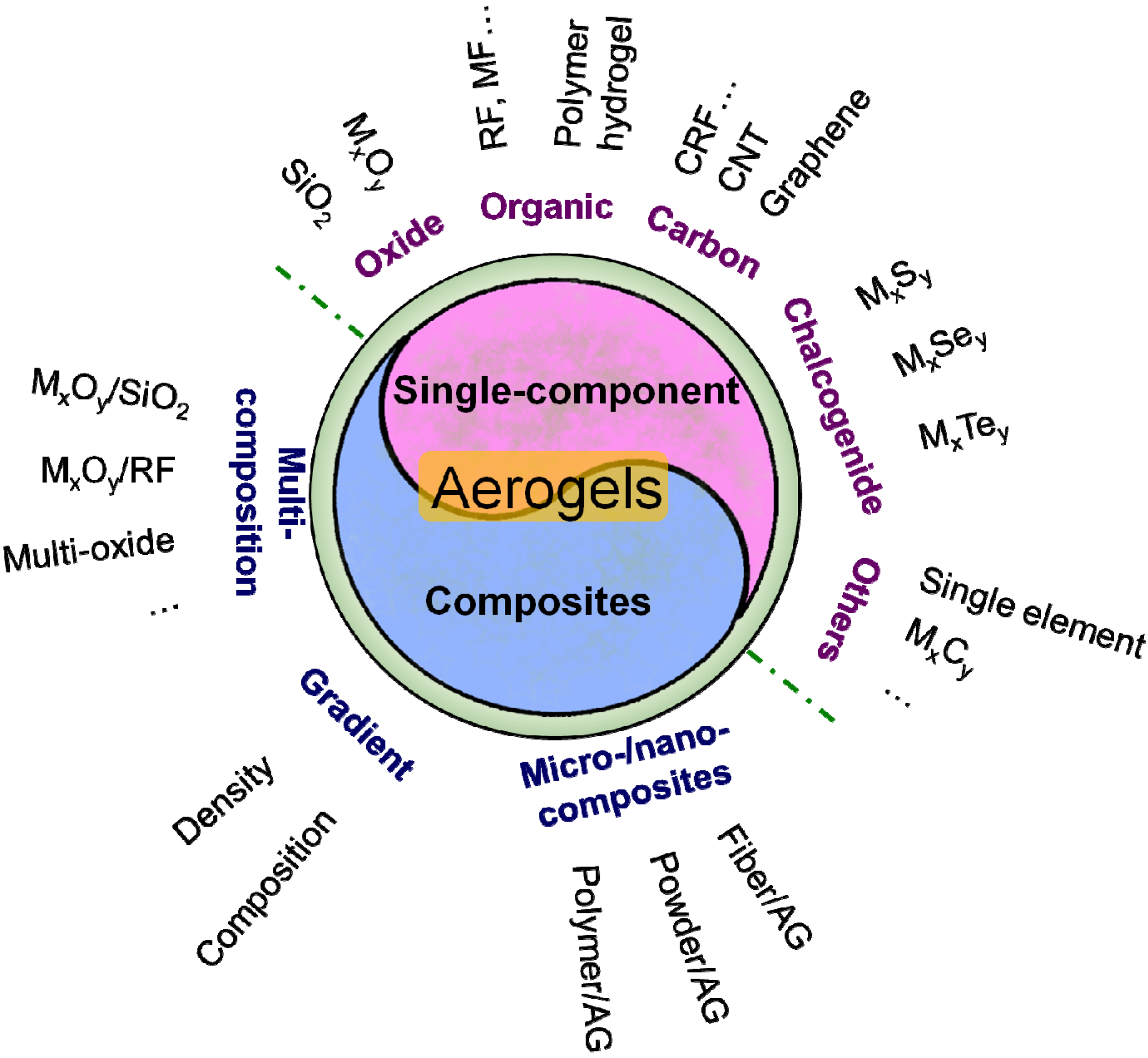
2.2. Classification
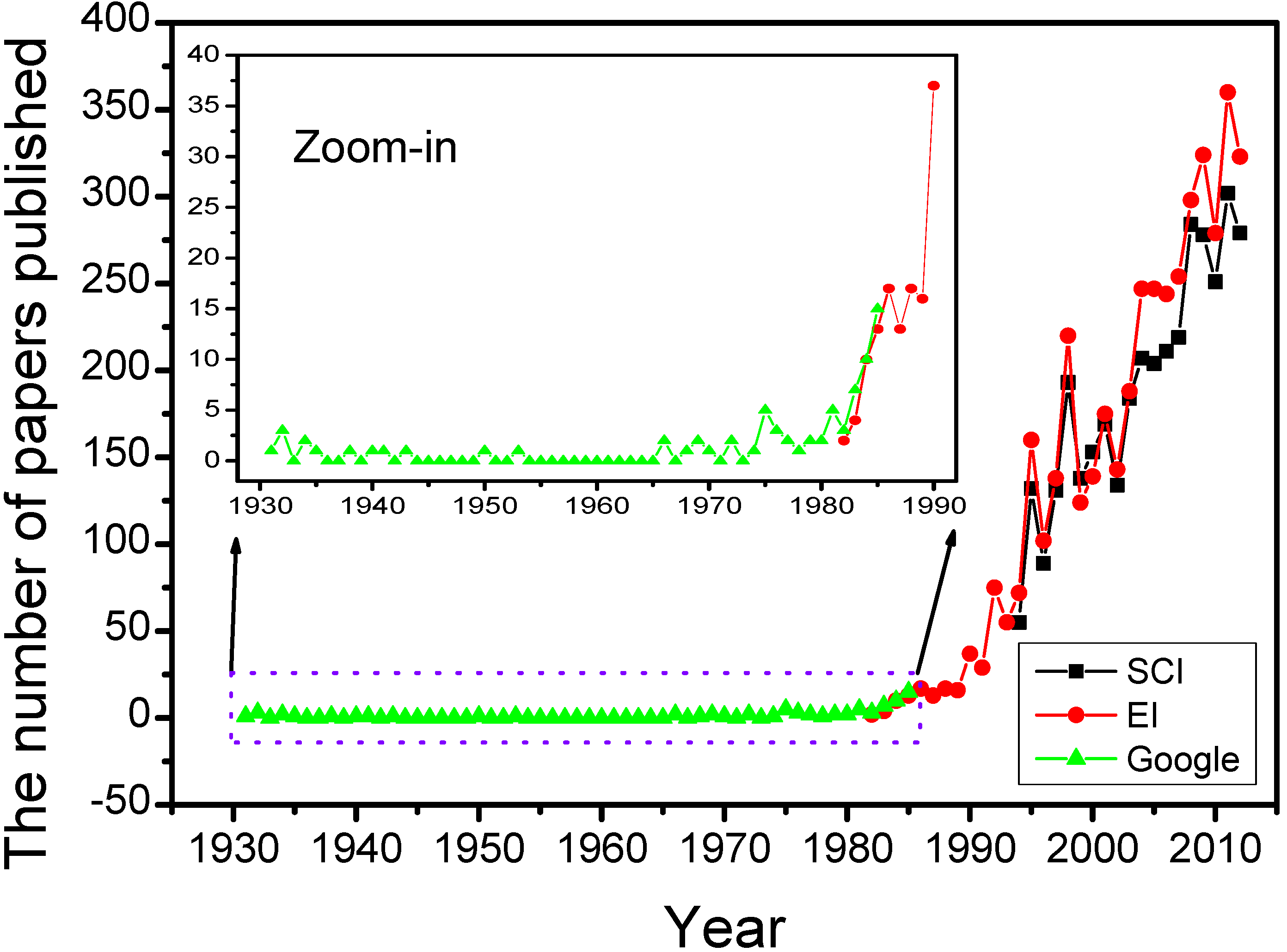
2.3. Brief History
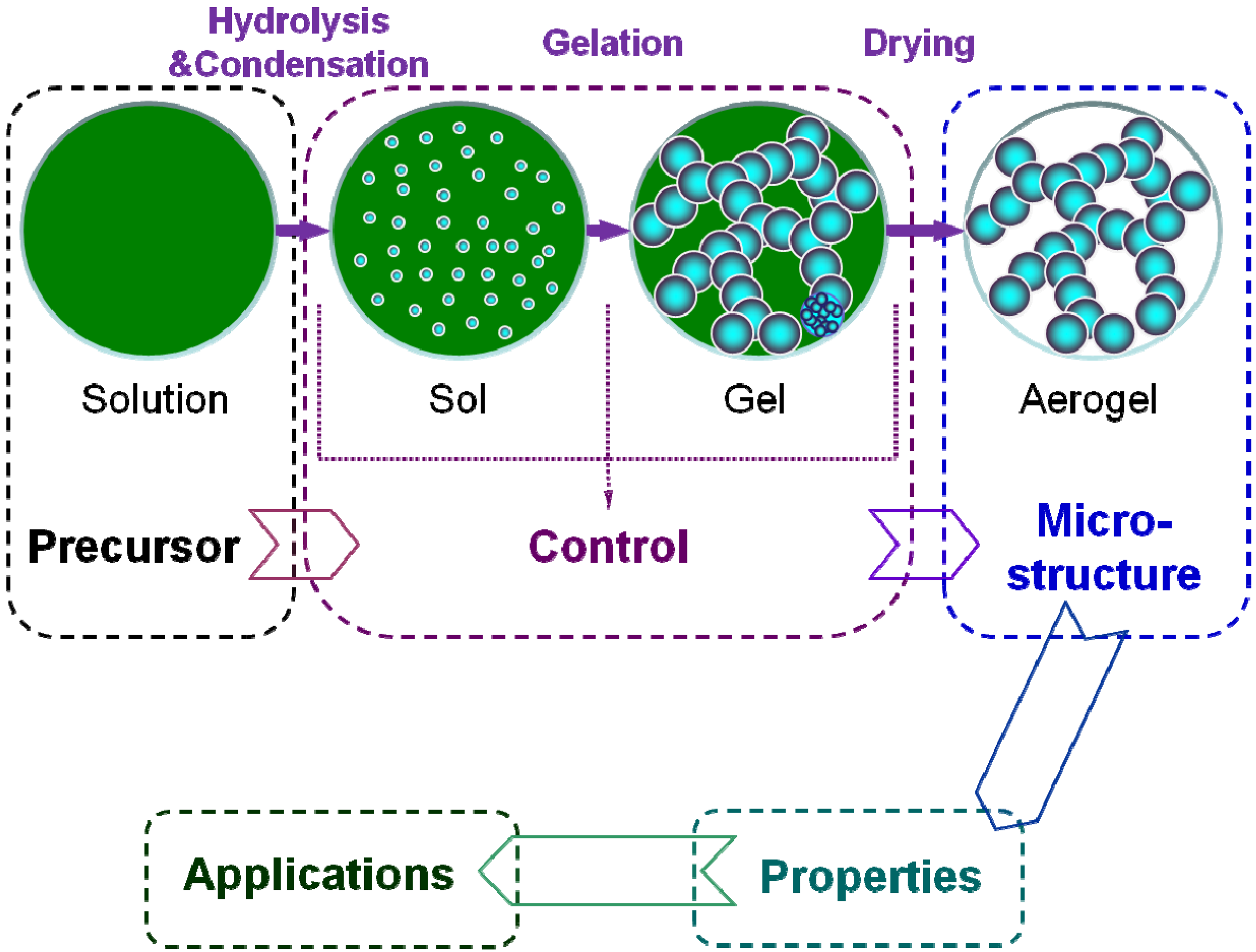
3. Preparation
- (i)
- Solution-sol transition: nanoscale sol particles are formed in the precursor solution spontaneously or catalyzed by the catalysts via hydrolysis and condensation reactions.
- (ii)
- Sol-gel transition (gelation): the sol particles are crosslinked and hierarchically assembled into a wet gel with the coherent network.
- (iii)
- Gel-aerogel transition (drying): the solvent inside the wet gel is replayed by the air without serious microstructure damage.
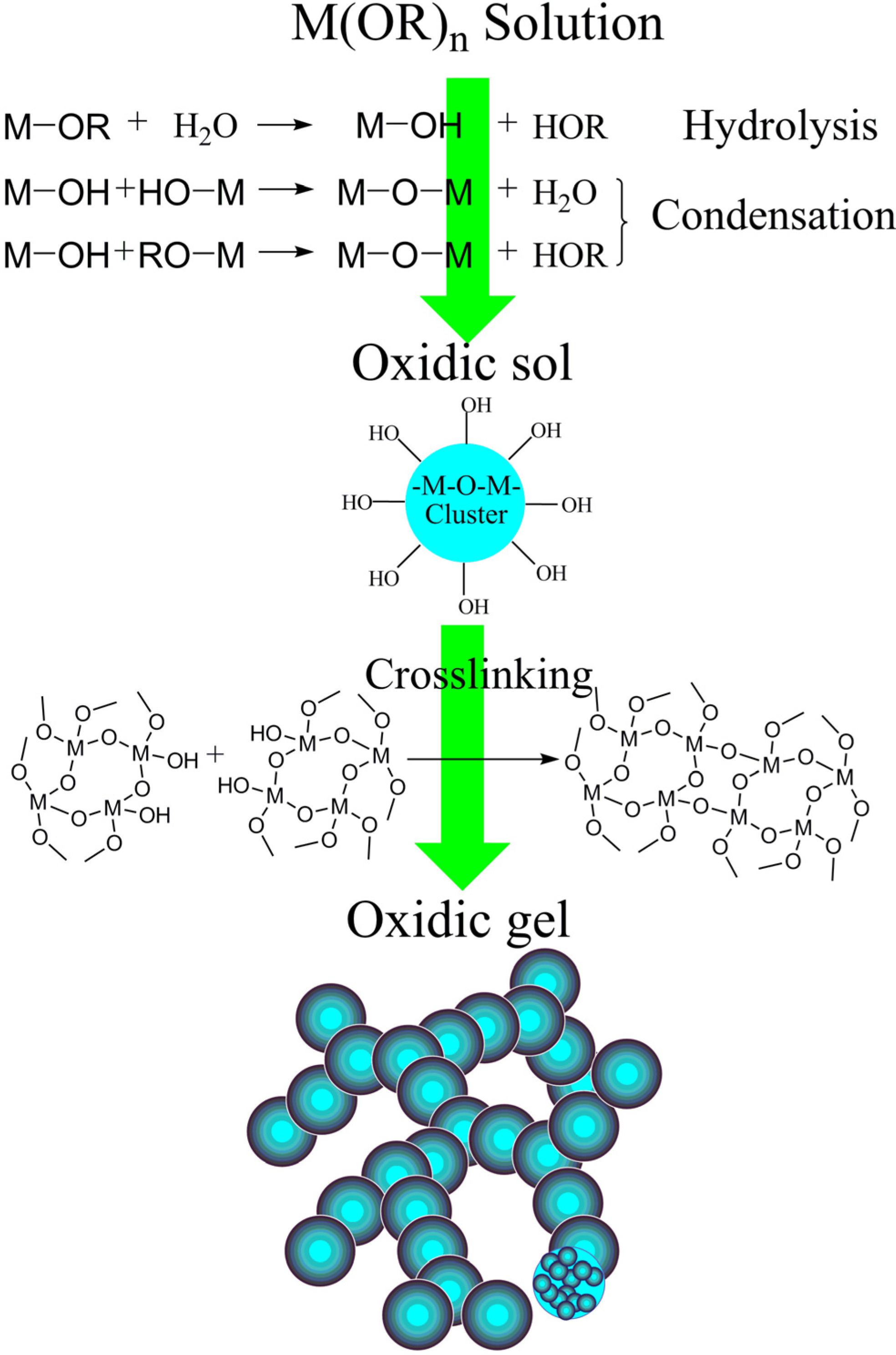
3.1. The Preparation of the Single-Component Aerogel
3.1.1. Oxide-Based Aerogel

3.1.2. Organic Aerogel



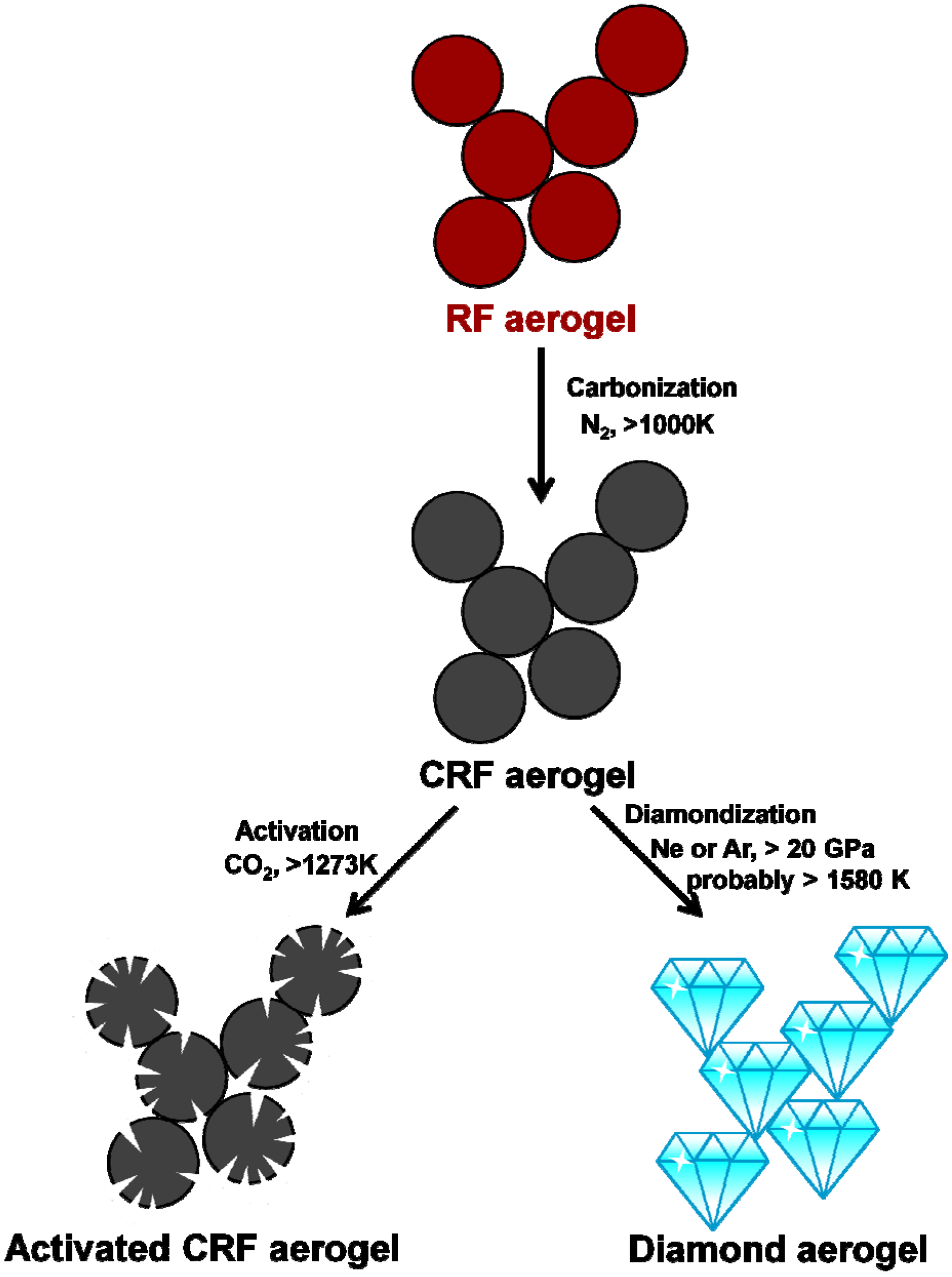
3.1.3. Carbon Aerogel
3.1.4. Chalcogenide Aerogel
3.1.5. Other Single-Component Aerogel
3.2. The Preparation of the Composite Aerogel
3.2.1. Multi-Composition Aerogel
3.2.2. Gradient Aerogel
3.2.3. Micro-/Nano-Composite Aerogel
4. Research Tendency
4.1. The Preparation of Novel Single-Component Aerogels
4.2. Material Design of Composite Aerogels
4.3. Industrial Application of the Aerogels
5. Conclusions
Acknowledgments
References
- Alvarez-Arenas, T.E.G. Acoustic impedance matching of piezoelectric transducers to the air. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Clarisse, J.M.; Boudesocque-Dubois, C.; Leidinger, J.P.; Willien, J.L. A linear perturbation computation method applied to hydrodynamic instability growth predictions in ICF targets. J. Phys. IV 2006, 133, 201–204. [Google Scholar]
- Pant, H.C.; Desai, T. Coherent structures in ablatively compressed ICF targets and Rayleigh-Taylor instability. Phys. Scripta 1996, T63, 158–161. [Google Scholar] [CrossRef]
- Back, C.A.; Davis, J.; Grun, J.; Suter, L.J.; Landen, O.L.; Hsing, W.W.; Miller, M.C. Multi-keV X-ray conversion efficiency in laser-produced plasmas. Phys. Plasmas 2003, 10, 2047–2055. [Google Scholar] [CrossRef]
- Fiedorowicz, H.; Bartnik, A.; Jarocki, R.; Rakowski, R.; Szczurek, M. Enhanced X-ray emission in the 1-keV range from a laser-irradiated gas puff target produced using the double-nozzle setup. Appl. Phys. B 2000, 70, 305–308. [Google Scholar] [CrossRef]
- Hu, G.Y.; Zheng, J.; Shen, B.F.; Lei, A.L.; Liu, S.Y.; Zhang, J.Y.; Yang, J.M.; Ding, Y.K.; Hu, X.; Huang, Y.X.; et al. Characterization of a multi-keV X-ray source produced by nanosecond laser irradiation of a solid target: The influence of laser focus spot and target thickness. Phys. Plasmas 2008, 15, 023103:1–023103:7. [Google Scholar]
- Tompkins, R.J.; Mercer, I.P.; Fettweis, M.; Barnett, C.J.; Klug, D.R.; Porter, L.G.; Clark, I.; Jackson, S.; Matousek, P.; Parker, A.W.; et al. 5–20 keV laser-induced X-ray generation at 1 kHz from a liquid-jet target. Rev. Sci. Instrum. 1998, 69, 3113–3117. [Google Scholar] [CrossRef]
- Adachi, I.; Ishii, Y.; Kawai, H.; Kuratani, A.; Tabata, M. Study of a silica aerogel for a Cherenkov radiator. Nucl. Instrum. Methods Phys. Res. A 2008, 595, 180–182. [Google Scholar] [CrossRef]
- Bellunato, T.; Calvi, M.; Matteuzzi, C.; Musy, M.; Negri, P.; Braem, A.; Chesi, E.; Hansen, C.; Liko, D.; Joram, C.; et al. Performance of aerogel as Cherenkov radiator. Nucl. Instrum. Methods Phys. Res. A 2004, 519, 493–507. [Google Scholar] [CrossRef]
- Fricke, J. Aerogels-highly tenuous solids with fascinating properties. J. Non-Cryst. Solids 1988, 100, 169–173. [Google Scholar] [CrossRef]
- Fricke, J.; Emmerling, A. Aerogels-recent progress in production techniques and novel applications. J. Sol-Gel Sci. Technol. 1998, 13, 299–303. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef] [PubMed]
- Vacher, R.; Woignier, T.; Pelous, J.; Courtens, E. Structure and self-similarity of silica aerogels. Phys. Rev. B 1988, 37, 6500–6503. [Google Scholar] [CrossRef]
- Wagh, P.; Begag, R.; Pajonk, G.; Rao, A.V.; Haranath, D. Comparison of some physical properties of silica aerogel monoliths synthesized by different precursors. Mater. Chem. Phys. 1999, 57, 214–218. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Keefer, K.D. Structure of random porous materials: Silica aerogel. Phys. Rev. Lett. 1986, 56, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Gesser, H.; Goswami, P. Aerogels and related porous materials. Chem. Rev. 1989, 89, 765–788. [Google Scholar] [CrossRef]
- Kistler, S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Baumann, T.F.; Gash, A.E.; Chinn, S.C.; Sawvel, A.M.; Maxwell, R.S.; Satcher, J.H. Synthesis of high-surface-area alumina aerogels without the use of alkoxide precursors. Chem. Mater. 2005, 17, 395–401. [Google Scholar] [CrossRef]
- Baumann, T.F.; Kucheyev, S.O.; Gash, A.E.; Satcher, J.H. Facile synthesis of a crystalline, high-surface-area SnO2 aerogel. Adv. Mater. 2005, 17, 1546–1548. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Strong akaganeite aerogel monoliths using epoxides: Synthesis and characterization. Chem. Mater. 2003, 15, 3268–3275. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Monolithic nickel(II)-based aerogels using an organic epoxide: the importance of the counterion. J. Non-Cryst. Solids 2004, 350, 145–151. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Sadigh, B.; Baumann, T.F.; Wang, Y.M.; Felter, T.E.; van Buuren, T.; Gash, A.E.; Satcher, J.H.; Hamza, A.V. Electronic structure of chromia aerogels from soft X-ray absorption spectroscopy. J. Appl. Phys. 2007, 101, 124315:1–124315:8. [Google Scholar] [CrossRef]
- Gan, L.H.; Yue, T.Y.; Chen, L.W.; Li, G.M.; Zhou, B. Preparation and characterization of beta-FeOOH aerogels. Acta Phys. Chim. Sin. 1997, 13, 48–51. [Google Scholar]
- Bi, Y.T.; Ren, H.B.; Chen, B.W.; Zhang, L. Synthesis and characterization of nickel-based monolithic aerogel via sol-gel method. Adv. Mater. Res. 2011, 335–336, 368–371. [Google Scholar] [CrossRef]
- Ren, H.B.; Zhang, L.; Shang, C.W.; Wang, X.; Bi, Y.T. Synthesis of a low-density tantalum oxide tile-like aerogel monolithic. J. Sol-Gel Sci. Technol. 2010, 53, 307–311. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Shen, J.; Gui, J.Y.; Zhong, Y.H.; Liu, C.Z.; Zhang, Z.H.; Wu, G.M. A versatile sol-gel route to monolithic oxidic gels via polyacrylic acid template. New J. Chem. 2011, 35, 1096–1102. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Shen, J.; Xiao, S.F.; Zhang, Z.H.; Liu, C.Z.; Zhang, M.X. Monolithic copper oxide aerogel via dispersed inorganic sol-gel method. J. Non-Cryst. Solids 2009, 355, 175–181. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhong, Y.H.; Zhu, X.R.; Gao, G.H.; Wu, G.M.; Zhang, Z.H.; Shen, J. Hierarchical microstructure and formative mechanism of low-density molybdena-based aerogel derived from MoCl5. J. Sol-Gel Sci. Technol. 2011, 58, 225–231. [Google Scholar]
- Pala, I.R.; Brock, S.L. ZnS Nanoparticle gels for remediation of Pb2+ and Hg2+ polluted water. ACS Appl. Mater. Interfaces 2012, 4, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Zhou, C.; Morelli, D.; Sakamoto, J.; Brock, S.L. Synthesis and characterization of telluride aerogels: Effect of gelation on thermoelectric performance of Bi2Te3 and Bi2−xSbxTe3 nanostructures. J. Phys. Chem. C 2012, 116, 17431–17439. [Google Scholar] [CrossRef]
- Yu, H.; Bellair, R.; Kannan, R.M.; Brock, S.L. Engineering strength, porosity, and emission intensity of nanostructured CdSe networks by altering the building-block shape. J. Am. Chem. Soc. 2008, 130, 5054–5055. [Google Scholar] [CrossRef] [PubMed]
- Kalebaila, K.K.; Georgiev, D.G.; Brock, S.L. Synthesis and characterization of germanium sulfide aerogels. J. Non-Cryst. Solids 2006, 352, 232–240. [Google Scholar] [CrossRef]
- Arachchige, I.U.; Brock, S.L. Sol-gel assembly of CdSe nanoparticles to form porous aerogel networks. J. Am. Chem. Soc. 2006, 128, 7964–7971. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, J.L.; Arachchige, I.U.; Brock, S.L. Porous semiconductor chalcogenide aerogels. Science 2005, 307, 397–400. [Google Scholar] [PubMed]
- Mohanan, J.L.; Brock, S.L. A new addition to the aerogel community: Unsupported CdS aerogels with tunable optical properties. J. Non-Cryst. Solids 2004, 350, 1–8. [Google Scholar] [CrossRef]
- Jones, S.M.; Flynn, G. Hypervelocity capture of meteoritic particles in nonsilica aerogels. Meteorit. Planet. Sci. 2011, 46, 1253–1264. [Google Scholar] [CrossRef]
- Jones, S.M. A method for producing gradient density aerogel. J. Sol-Gel Sci. Technol. 2007, 44, 255–258. [Google Scholar] [CrossRef]
- Horz, F.; Bastien, R.; Borg, J.; Bradley, J.P.; Bridges, J.C.; Brownlee, D.E.; Burchell, M.J.; Chi, M.F.; Cintala, M.J.; Dai, Z.R.; et al. Impact features on Stardust: Implications for comet 81P/Wild 2 dust. Science 2006, 314, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P. Silica Aerogel captures cosmic dust intact. J. Non-Cryst. Solids 1995, 186, 415–427. [Google Scholar] [CrossRef]
- Zou, J.H.; Liu, J.H.; Karakoti, A.S.; Kumar, A.; Joung, D.; Li, Q.A.; Khondaker, S.I.; Seal, S.; Zhai, L. Ultralight multiwalled carbon nanotube aerogel. ACS Nano 2010, 4, 7293–7302. [Google Scholar] [CrossRef] [PubMed]
- Skaltsas, T.; Avgouropoulos, G.; Tasis, D. Impact of the fabrication method on the physicochemical properties of carbon nanotube-based aerogels. Microporous Mesoporous Mater. 2011, 143, 451–457. [Google Scholar] [CrossRef]
- Kim, K.H.; Vural, M.; Islam, M.F. Single-walled carbon nanotube aerogel-based elastic conductors. Adv. Mater. 2011, 23, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Aliev, A.E.; Oh, J.Y.; Kozlov, M.E.; Kuznetsov, A.A.; Fang, S.L.; Fonseca, A.F.; Ovalle, R.; Lima, M.D.; Haque, M.H.; Gartstein, Y.N.; et al. Giant-stroke, superelastic carbon nanotube aerogel muscles. Science 2009, 323, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Li, P.G.; Gao, C. Strong, conductive, lightweight, neat graphene aerogel fibers with aligned pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z.; Zhou, J.; Xing, W.; Wang, G.Q.; Cui, H.Y.; Zhuo, S.P.; Xue, Q.Z.; Yan, Z.F.; Qiao, S.Z. High-rate capacitive performance of graphene aerogel with a superhigh C/O molar ratio. J. Mater. Chem. 2012, 22, 23186–23193. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Liu, C.Y. Tri-isocyanate reinforced graphene aerogel and its use for crude oil adsorption. J. Colloid Interface Sci. 2012, 382, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.T.; Sui, Z.Y.; Xu, B.; Yue, S.F.; Luo, Y.J.; Zhan, W.C.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Lin, Y.R.; Ehlert, G.J.; Bukowsky, C.; Sodano, H.A. Superhydrophobic functionalized graphene aerogels. ACS Appl. Mater. Interfaces 2011, 3, 2200–2203. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ellsworth, M.W. Graphene aerogels. ECS Trans. 2009, 19, 241–247. [Google Scholar]
- Chen, K.; Bao, Z.H.; Du, A.; Zhu, X.R.; Shen, J.; Wu, G.M.; Zhang, Z.H.; Zhou, B. One-pot synthesis, characterization and properties of acid-catalyzed resorcinol/formaldehyde cross-linked silica aerogels and their conversion to hierarchical porous carbon monoliths. J. Sol-Gel Sci. Technol. 2012, 62, 294–303. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.H.; Liu, D.; Zhu, X.R.; Zhang, Z.H.; Zhou, B. Confined synthesis and properties of porous silicon from silica aerogel templates by magnesiothermic reduction. Acta Phys. Chim. Sin. 2011, 27, 2719–2725. [Google Scholar]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click synthesis of monolithic silicon carbide aerogels from polyacrylonitrile-coated 3D silica networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Cervantes, O.; Han, T.Y.J.; Gash, A.E.; Satcher, J.H.; Baumann, T.F. Route to high surface area TiO2/C and TiCN/C composites. J. Mater. Chem. 2009, 19, 7146–7150. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Pauzauskie, P.J.; Cervantes, O.; Zaug, J.M.; Gash, A.E.; Satcher, J.H.; Baumann, T.F. High surface area carbon nanotube-supported titanium carbonitride aerogels. J. Mater. Chem. 2009, 19, 5503–5506. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Blackwell Science Oxford: Cambridge, UK, 1997. [Google Scholar]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Husing, N.; Schubert, U. Aerogels airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 23–45. [Google Scholar] [CrossRef]
- Schaedler, T.A.; Jacobsen, A.J.; Torrents, A.; Sorensen, A.E.; Lian, J.; Greer, J.R.; Valdevit, L.; Carter, W.B. Ultralight metallic microlattices. Science 2011, 334, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S. Coherent expanded-aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Nicolaon, G.; Teichner, S. New preparation process for silica xerogels and aerogels, and their textural properties. Bull. Soc. Chim. Fr. 1968, 5, 1900–1906. [Google Scholar]
- Russo, R.; Hunt, A. Comparison of ethyl versus methyl sol-gels for silica aerogels using polar nephelometry. J. Non-Cryst. Solids 1986, 86, 219–230. [Google Scholar] [CrossRef]
- Tewari, P.H.; Hunt, A.J.; Lofftus, K.D. Ambient-temperature supercritical drying of transparent silica aerogels. Mater. Lett. 1985, 3, 363–367. [Google Scholar] [CrossRef]
- Pekala, R.W. Low Density, Resorcinol-Formaldehyde Aerogels. U.S. Patent 4,873,218, 10 October 1989. [Google Scholar]
- Smith, D.M.; Deshpande, R.; Jeffrey Brinke, C. Preparation of low-density aerogels at ambient pressure. Mater. Res. Soc. Symp. Proc. 1992, 271, 567–572. [Google Scholar] [CrossRef]
- Ni, X.Y.; Li, Y.; Zhang, Z.H.; Shen, J.; Zhou, B.; Wu, G.M. Surface modification and properties of SiO2 nano porous aerogels. Key Eng. Mater. 2008, 373, 702–705. [Google Scholar] [CrossRef]
- Pekala, R. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H., Jr.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of epoxides in the sol-gel synthesis of porous iron (III) oxide monoliths from Fe (III) salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H., Jr.; Hrubesh, L.W.; Simpson, R.L. New sol-gel synthetic route to transition and main-group metal oxide aerogels using inorganic salt precursors. J. Non-Cryst. Solids 2001, 285, 22–28. [Google Scholar] [CrossRef]
- Flynn, G.J.; Bleuet, P.; Borg, J.; Bradley, J.P.; Brenker, F.E.; Brennan, S.; Bridges, J.; Brownlee, D.E.; Bullock, E.S.; Burghammer, M.; et al. Elemental compositions of comet 81P/Wild 2 samples collected by Stardust. Science 2006, 314, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Menaa, B.; Menaa, F.; Aiolfi-Guimaraes, C.; Sharts, O. Silica-based nanoporous sol-gel glasses: From bioencapsulation to protein folding studies. Int. J. Nanotechnol. 2010, 7, 1–45. [Google Scholar] [CrossRef]
- Hrubesah, L.W.; Tillotson, T.M.; Poco, J.F. Characterization of ultralow-density silica aerogels made from a condensed silica precursor. Mater. Res. Soc. Symp. Proc. 1990, 180, 315–319. [Google Scholar] [CrossRef]
- Liu, G.W.; Ni, X.Y.; Zhou, B.; Yu, Q.J. Preparation and characterization of ultralow density silica aerogels by acetonitrile supercritical drying. Key Eng. Mater. 2012, 519, 83–86. [Google Scholar] [CrossRef]
- Kugland, N.L.; Moody, J.D.; Kozioziemski, B.J.; Rubenchik, A.M.; Niemann, C. Reduction in helium thermal conductivity by 1 mg/cc silica aerogel foam. Appl. Phys. Lett. 2008, 92, 221913. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Stadermann, M.; Shin, S.J.; Satcher, J.H.; Gammon, S.A.; Letts, S.A.; van Buuren, T.; Hamza, A.V. Super-compressibility of ultralow-density nanoporous silica. Adv. Mater. 2012, 24, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Frederick, C.; Forsman, A.; Hund, J.; Eddinger, S. Fabrication of Ta2O5 aerogel targets for radiation transport experiments using thin film fabrication and laser processing. Fusion Sci. Technol. 2009, 55, 499–504. [Google Scholar]
- Zhang, L.; Ren, H.B.; Wang, X.; Bi, Y.T.; Fan, Y.H. Characterization of monolithic tantalum oxide aerogels using epichlorohydrin as gel initiator. Rare Met. Mater. Eng. 2010, 39, 154–156. [Google Scholar]
- Dong, W.; Dunn, B. Sol-gel synthesis and characterization of molybdenum oxide gels. J. Non-Cryst. Solids 1998, 225, 135–140. [Google Scholar] [CrossRef]
- Dong, W.; Dunn, B. Sol-gel synthesis of monolithic molybdenum oxide aerogels and xerogels. J. Mater. Chem. 1998, 8, 665–670. [Google Scholar] [CrossRef]
- Dong, W.; Mansour, A.N.; Dunn, B. Structural and electrochemical properties of amorphous and crystalline molybdenum oxide aerogels. Solid State Ionics 2001, 144, 31–40. [Google Scholar] [CrossRef]
- Bi, Y.T.; Ren, H.B.; Chen, B.W.; Chen, G.; Mei, Y.; Zhang, L. Synthesis monolithic copper-based aerogel with polyacrylic acid as template. J. Sol-Gel Sci. Technol. 2012, 63, 140–145. [Google Scholar] [CrossRef]
- Kido, Y.; Nakanishi, K.; Miyasaka, A.; Kanamori, K. Synthesis of monolithic hierarchically porous iron-based xerogels from iron(III) salts via an epoxide-mediated sol-gel process. Chem. Mater. 2012, 24, 2071–2077. [Google Scholar] [CrossRef]
- Pekala, R.; Alviso, C.; Kong, F.; Hulsey, S. Aerogels derived from multifunctional organic monomers. J. Non-Cryst. Solids 1992, 145, 90–98. [Google Scholar] [CrossRef]
- Pekala, R.; Alviso, C.; Lu, X.; Gross, J.; Fricke, J. New organic aerogels based upon a phenolic-furfural reaction. J. Non-Cryst. Solids 1995, 188, 34–40. [Google Scholar] [CrossRef]
- Li, W.; Reichenauer, G.; Fricke, J. Carbon aerogels derived from cresol-resorcinol-formaldehyde for supercapacitors. Carbon 2002, 40, 2955–2959. [Google Scholar] [CrossRef]
- Li, W.; Guo, S. Preparation of low-density carbon aerogels from a cresol/formaldehyde mixture. Carbon 2000, 38, 1499–1524. [Google Scholar] [CrossRef]
- Brandt, R.; Petricevic, R.; Pröbstle, H.; Fricke, J. Acetic acid catalyzed carbon aerogels. J. Porous Mater. 2003, 10, 171–178. [Google Scholar] [CrossRef]
- Brandt, R.; Fricke, J. Acetic-acid-catalyzed and subcritically dried carbon aerogels with a nanometer-sized structure and a wide density range. J. Non-Cryst. Solids 2004, 350, 131–135. [Google Scholar] [CrossRef]
- Reuß, M.; Ratke, L. Subcritically dried RF-aerogels catalysed by hydrochloric acid. J. Sol-Gel Sci. Technol. 2008, 47, 74–80. [Google Scholar] [CrossRef]
- Wu, D.; Fu, R.; Yu, Z. Organic and carbon aerogels from the NaOH-catalyzed polycondensation of resorcinol-furfural and supercritical drying in ethanol. J. Appl. Polym. Sci. 2005, 96, 1429–1435. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-efficient acid-catalyzed synthesis of resorcinol-formaldehyde aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Tan, C.B.; Fung, B.M.; Newman, J.K.; Vu, C. Organic aerogels with very high impact strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Jin, H.; Kettunen, M.; Laiho, A.; Pynnonen, H.; Paltakari, J.; Marmur, A.; Ikkala, O.; Ras, R.H.A. Superhydrophobic and superoleophobic nanocellulose aerogel membranes as bioinspired cargo carriers on water and oil. Langmuir 2011, 27, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, M.; Silvennoinen, R.J.; Houbenov, N.; Nykanen, A.; Ruokolainen, J.; Sainio, J.; Pore, V.; Kemell, M.; Ankerfors, M.; Lindstrom, T.; et al. Photoswitchable superabsorbency based on nanocellulose aerogels. Adv. Funct. Mater. 2011, 21, 510–517. [Google Scholar] [CrossRef]
- Ding, B.B.; Cai, J.; Huang, J.C.; Zhang, L.N.; Chen, Y.; Shi, X.W.; Du, Y.M.; Kuga, S. Facile preparation of robust and biocompatible chitin aerogels. J. Mater. Chem. 2012, 22, 5801–5809. [Google Scholar] [CrossRef]
- Tingaut, P.; Zimmermann, T.; Sebe, G. Cellulose nanocrystals and microfibrillated cellulose as building blocks for the design of hierarchical functional materials. J. Mater. Chem. 2012, 22, 20105–20111. [Google Scholar] [CrossRef]
- Hanzawa, Y.; Kaneko, K.; Pekala, R.; Dresselhaus, M. Activated carbon aerogels. Langmuir 1996, 12, 6167–6169. [Google Scholar] [CrossRef]
- Liu, D.; Shen, J.; Li, Y.J.; Liu, N.P.; Liu, B. Pore structures of carbon aerogels and their effects on electrochemical supercapacitor performance. Acta Phys. Chim. Sin. 2012, 28, 843–849. [Google Scholar]
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes. J. Electrochem. Soc. 1996, 143, 159–169. [Google Scholar] [CrossRef]
- Halama, A.; Szubzda, B.; Pasciak, G. Carbon aerogels as electrode material for electrical double layer supercapacitors-Synthesis and properties. Electrochim. Acta 2010, 55, 7501–7505. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Pekala, R.; Farmer, J.; Alviso, C.; Tran, T.; Mayer, S.; Miller, J.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non-Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Carrasco-Marin, F.; Fairen-Jimenez, D.; Moreno-Castilla, C. Carbon aerogels from gallic acid-resorcinol mixtures as adsorbents of benzene, toluene and xylenes from dry and wet air under dynamic conditions. Carbon 2009, 47, 463–469. [Google Scholar] [CrossRef]
- Zhang, W.F.; Huang, Z.H.; Zhou, C.J.; Cao, G.P.; Kang, F.Y.; Yang, Y.S. Porous carbon for electrochemical capacitors prepared from a resorcinol/formaldehyde-based organic aquagel with nano-sized particles. J. Mater. Chem. 2012, 22, 7158–7163. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Carrasco-Marin, F.; Moreno-Castilla, C. Preparation, surface characteristics, and electrochemical double-layer capacitance of KOH-activated carbon aerogels and their O- and N-doped derivatives. J. Power Source 2012, 219, 80–88. [Google Scholar] [CrossRef]
- Haji, S.; Erkey, C. Removal of dibenzothiophene from model diesel by adsorption on carbon aerogels for fuel cell applications. Ind. Eng. Chem. Res. 2003, 42, 6933–6937. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Dawidziuk, M.B.; Carrasco-Marin, F.; Zapata-Benabithe, Z. Surface characteristics and electrochemical capacitances of carbon aerogels obtained from resorcinol and pyrocatechol using boric and oxalic acids as polymerization catalysts. Carbon 2011, 49, 3808–3819. [Google Scholar] [CrossRef]
- Pauzauskie, P.J.; Crowhurst, J.C.; Worsley, M.A.; Laurence, T.A.; Kilcoyne, A.L.D.; Wang, Y.M.; Willey, T.M.; Visbeck, K.S.; Fakra, S.C.; Evans, W.J.; et al. Synthesis and characterization of a nanocrystalline diamond aerogel. PNAS 2011, 108, 8550–8553. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Kucheyev, S.O.; Kuntz, J.D.; Olson, T.Y.; Han, T.Y.J.; Hamza, A.V.; Satcher, J.H.; Baumann, T.F. Carbon scaffolds for stiff and highly conductive monolithic oxide-carbon nanotube composites. Chem. Mater. 2011, 23, 3054–3061. [Google Scholar] [CrossRef]
- Bryning, M.B.; Milkie, D.E.; Islam, M.F.; Hough, L.A.; Kikkawa, J.M.; Yodh, A.G. Carbon nanotube aerogels. Adv. Mater. 2007, 19, 661–664. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, Y.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra lightweight, flexible nanowall, carbon microtube material with outstanding mechanical performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Gacoin, T.; Malier, L.; Boilot, J.P. New transparent chalcogenide materials using a sol-gel process. Chem. Mater. 1997, 9, 1502–1504. [Google Scholar] [CrossRef]
- Bag, S.; Trikalitis, P.N.; Chupas, P.J.; Armatas, G.S.; Kanatzidis, M.G. Porous semiconducting gels and aerogels from chalcogenide clusters. Science 2007, 317, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, I.U.; Brock, S.L. Sol-gel methods for the assembly of metal chalcogenide quantum dots. Acc. Chem. Res. 2007, 40, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.H.; Brock, S.L. Optical sensing of triethylamine using CdSe aerogels. Nanotechnology 2010, 21, 115502:1–115502:10. [Google Scholar] [CrossRef]
- Yu, H.T.; Brock, S.L. Effects of nanoparticle shape on the morphology and properties of porous CdSe assemblies (aerogels). ACS Nano 2008, 2, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Liu, Y.; Brock, S.L. Tuning the optical band gap of quantum dot assemblies by varying network density. ACS Nano 2009, 3, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Hector, A.L.; Hyde, J.R.; Kalaji, A.; Smith, D.C. A non-oxide sol-gel route to synthesise silicon imidonitride monolithic gels and high surface area aerogels. Chem. Commun. 2008, 5304–5306. [Google Scholar]
- Worsley, M.A.; Kuntz, J.D.; Satcher, J.H.; Baumann, T.F. Synthesis and characterization of monolithic, high surface area SiO2/C and SiC/C composites. J. Mater. Chem. 2010, 20, 4840–4844. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Mulik, S.; Sotiriou-Leventis, C. The effect of compactness on the carbothermal conversion of interpenetrating metal oxide/resorcinol-formaldehyde nanoparticle networks to porous metals and carbides. J. Mater. Chem. 2010, 20, 7456–7471. [Google Scholar] [CrossRef]
- Babic, B.; Bucevac, D.; Radosavljevic-Mihajlovic, A.; Dosen, A.; Zagorac, J.; Pantic, J.; Matovic, B. New manufacturing process for nanometric SiC. J. Eur. Ceram. Soc. 2012, 32, 1901–1906. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.D.; Cui, S.; Zhong, Y. Preparation of mesoporous alpha-SiC from RF/SiO2 composite aerogels. Chin. J. Inorg. Chem. 2012, 28, 2071–2076. [Google Scholar]
- Xu, J.; Liu, Y.M.; Xue, B.; Li, Y.X.; Cao, Y.; Fan, K.N. A hybrid sol-gel synthesis of mesostructured SiC with tunable porosity and its application as a support for propane oxidative dehydrogenation. Phys. Chem. Chem. Phys. 2011, 13, 10111–10118. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liang, S.Q.; Wang, K.; Wang, H.T.; Williams, T.; Huang, H.; Cheng, Y.B. Synthesis of mesoporous carbon-bonded TiC/SiC composites by direct carbothermal reduction of sol-gel derived monolithic precursor. J. Am. Ceram. Soc. 2011, 94, 4025–4031. [Google Scholar] [CrossRef]
- Jung, S.M.; Jung, H.Y.; Dresselhaus, M.S.; Jung, Y.J.; Kong, J. A facile route for 3D aerogels from nanostructured 1D and 2D materials. Sci. Rep. 2012, 2, 849. [Google Scholar] [PubMed]
- Clapsaddle, B.J.; Gash, A.E.; Satcher, J.H.; Simpson, R.L. Silicon oxide in an iron(III) oxide matrix: The sol-gel synthesis and characterization of Fe-Si mixed oxide nanocomposites that contain iron oxide as the major phase. J. Non-Cryst. Solids 2003, 331, 190–201. [Google Scholar] [CrossRef]
- Clapsaddle, B.J.; Sprehn, D.W.; Gash, A.E.; Satcher, J.H.; Simpson, R.L. A versatile sol-gel synthesis route to metal-silicon mixed oxide nanocomposites that contain metal oxides as the major phase. J. Non-Cryst. Solids 2004, 350, 173–181. [Google Scholar] [CrossRef]
- Xu, W.; Du, A.; Tang, J.; Chen, K.; Zou, L.; Zhang, Z.; Shen, J.; Zhou, B. Rapid preparation of highly doped CuO/SiO2 composite aerogels. Acta Phys. Chim. Sin. 2012, 28, 2958–2964. [Google Scholar]
- Shobe, A.M.; Gill, S.K.; Hope-Weeks, L.J. Monolithic CuO-NiO aerogels via an epoxide addition route. J. Non-Cryst. Solids 2010, 356, 1337–1343. [Google Scholar] [CrossRef]
- Davis, M.; Zhang, K.; Wang, S.R.; Hope-Weeks, L.J. Enhanced electrical conductivity in mesoporous 3D indium-tin oxide materials. J. Mater. Chem. 2012, 22, 20163–20165. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Sotiriou-Leventis, C.; Lu, H.B. One-pot synthesis of interpenetrating inorganic/organic networks of CuO/resorcinol-formaldehyde aerogels: Nanostructured energetic materials. J. Am. Chem. Soc. 2009, 131, 4576–4577. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, R.; Kraus, O.; Fricke, J.; Eccardt, P.C.; Kroemer, N.; Magori, V. Modified SiO2 aerogels as acoustic impedance matching layers in ultrasonic devices. J. Non-Cryst. Solids 1992, 145, 227–232. [Google Scholar] [CrossRef]
- Gui, J.Y.; Zhou, B.; Zhong, Y.H.; Du, A.; Shen, J. Fabrication of gradient density SiO2 aerogel. J. Sol-Gel Sci. Technol. 2011, 58, 470–475. [Google Scholar] [CrossRef]
- Zhong, Y.H.; Zhou, B.; Gui, J.Y.; Du, A.; Zhang, Z.H.; Shen, J. Fabrication of multilayer graded density peeled-carbon-aerogel target. Fusion Eng. Des. 2011, 86, 238–243. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Gui, J.Y.; Liu, G.W.; Li, Y.N.; Wu, G.M.; Shen, J.; Zhang, Z.H. Thermal and mechanical properties of density-gradient aerogels for outer-space hypervelocity particle capture. Acta Phys. Chim. Sin. 2012, 28, 1189–1196. [Google Scholar]
- Morris, C.A.; Anderson, M.L.; Stroud, R.M.; Merzbacher, C.I.; Rolison, D.R. Silica sol as a nanoglue: Flexible synthesis of composite aerogels. Science 1999, 284, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Shen, J.; Ni, X.Y.; Wu, G.M.; Zhou, B.; Yang, M.X.; Gu, X.H.; Qian, M.J.; Wu, Y.H. Hydrophobic silica aerogels strengthened with nonwoven fibers. J. Macromol. Sci. A Pure Appl. Chem. 2006, 43, 1663–1670. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Ni, X.Y.; Li, Y.; Wang, B.; Wu, G.M.; Zhou, B. Preparation and characterization of hydrophobic silica aerogels doped with fibers. Rare Met. Mater. Eng. 2008, 37, 16–19. [Google Scholar]
- Deng, Z.S.; Wang, J.; Wu, A.M.; Shen, J.; Zhou, B. High strength SiO2 aerogel insulation. J. Non-Cryst. Solids 1998, 225, 101–104. [Google Scholar] [CrossRef]
- Tillotson, T.M.; Gash, A.E.; Simpson, R.L.; Hrubesh, L.W.; Satcher, J.H.; Poco, J.F. Nanostructured energetic materials using sol-gel methodologies. J. Non-Cryst. Solids 2001, 285, 338–345. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L.; Clapsaddle, B.J. Nanostructured energetic materials with sol-gel methods. Mater. Res. Soc. Symp. Proc. 2004, 800, 55–66. [Google Scholar]
- Prentice, D.; Pantoya, M.L.; Gash, A.E. Combustion wave speeds of sol-gel-synthesized tungsten trioxide and nano-aluminum: The effect of impurities on flame propagation. Energy Fuels 2006, 20, 2370–2376. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.H.; Rawashdeh, A.M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Zhang, G.H.; Dass, A.; Rawashdeh, A.M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Leventis, N.; Palczer, A.; McCorkle, L.; Zhang, G.H.; Sotiriou-Leventis, C. Nanoengineered silica-polymer composite aerogels with no need for supercritical fluid drying. J. Sol-Gel Sci. Technol. 2005, 35, 99–105. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Fabrizio, E.F.; Ilhan, F.; Dass, A.; Zhang, G.H.; Vassilaras, P.; Johnston, J.C.; Leventis, N. Cross-linking amine-modified silica aerogels with epoxies: Mechanically strong lightweight porous materials. Chem. Mater. 2005, 17, 1085–1098. [Google Scholar] [CrossRef]
- Ilhan, U.F.; Fabrizio, E.F.; McCorkle, L.; Scheiman, D.A.; Dass, A.; Palczer, A.; Meador, M.B.; Johnston, J.C.; Leventis, N. Hydrophobic monolithic aerogels by nanocasting polystyrene on amine-modified silica. J. Mater. Chem. 2006, 16, 3046–3054. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Capadona, L.A.; McCorkle, L.; Papadopoulos, D.S.; Leventis, N. Structure-property relationships in porous 3D nanostructures as a function of preparation conditions: Isocyanate cross-linked silica aerogels. Chem. Mater. 2007, 19, 2247–2260. [Google Scholar] [CrossRef]
- Yin, W.; Venkitachalam, S.M.; Jarrett, E.; Staggs, S.; Leventis, N.; Lu, H.B.; Rubenstein, D.A. Biocompatibility of surfactant-templated polyurea-nanoencapsulated macroporous silica aerogels with plasma platelets and endothelial cells. J. Biomed. Mater. Res. 2010, 92A, 1431–1439. [Google Scholar]
- Mohite, D.P.; Larimore, Z.J.; Lu, H.; Mang, J.T.; Sotiriou-Leventis, C.; Leventis, N. Monolithic hierarchical fractal assemblies of silica nanoparticles cross-linked with polynorbornene via ROMP: A structure-property correlation from molecular to bulk through nano. Chem. Mater. 2012, 24, 3434–3448. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Li, Y.N.; Li, X.Y.; Ye, J.J.; Li, L.X.; Zhang, Z.H.; Gao, G.H.; Shen, J. Aerogel: A potential three-dimensional nanoporous filler for resins. J. Reinf. Plast. Compos. 2011, 30, 912–921. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E.; Satcher, J.H.; Kauzlarich, S.M. Aerogel synthesis of yttria-stabilized zirconia by a non-alkoxide sol-gel route. Chem. Mater. 2005, 17, 3345–3351. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E.; Satcher, J.H.; Kauzlarich, S.M. Role of cyclic ether and solvent in a non-alkoxide sol-gel synthesis of yttria-stabilized zirconia nanoparticles. Chem. Mater. 2006, 18, 4865–4874. [Google Scholar] [CrossRef]
- Chien, H.C.; Cheng, W.Y.; Wang, Y.H.; Lu, S.Y. Ultrahigh specific capacitances for supercapacitors achieved by nickel cobaltite/carbon aerogel composites. Adv. Funct. Mater. 2012, 22, 5038–5043. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wei, T.Y.; Chien, H.C.; Lu, S.Y. Manganese oxide/carbon aerogel composite: An outstanding supercapacitor electrode material. Adv. Energy Mater. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol-gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Back, C.A.; Bauer, J.D.; Hammer, J.H.; Lasinski, B.F.; Turner, R.E.; Rambo, P.W.; Landen, O.L.; Suter, L.J.; Rosen, M.D.; Hsing, W.W. Diffusive, supersonic X-ray transport in radiatively heated foam cylinders. Phys. Plasmas 2000, 7, 2126–2134. [Google Scholar] [CrossRef]
- Back, C.A.; Seely, J.F.; Weaver, J.L.; Feldman, U.; Tommasini, R.; Glendinning, S.G.; Chung, H.K.; Rosen, M.; Lee, R.W.; Scott, H.A.; et al. Underdense radiation sources: Moving towards longer wavelengths. J. Phys. IV 2006, 133, 1173–1175. [Google Scholar]
- Fournier, K.B.; Satcher, J.H.; May, M.J.; Poco, J.F.; Sorce, C.M.; Colvin, J.D.; Hansen, S.B.; MacLaren, S.A.; Moon, S.J.; Davis, J.F.; et al. Absolute X-ray yields from laser-irradiated germanium-doped low-density aerogels. Phys. Plasmas 2009, 16, 052703:1–052703:13. [Google Scholar] [CrossRef]
- Colvin, J.D.; Fournier, K.B.; May, M.J.; Scott, H.A. A computational study of X-ray emission from laser-irradiated Ge-doped foams. Phys. Plasmas 2010, 17, 073111:1–073111:8. [Google Scholar] [CrossRef]
- Tanabe, M.; Nishimura, H.; Ohnishi, N.; Fournier, K.B.; Fujioka, S.; Iwamae, A.; Hansen, S.B.; Nagai, K.; Girard, F.; Primout, M.; Villette, B.; Brebion, D.; Mima, K. Characterization of heat-wave propagation through laser-driven Ti-doped underdense plasma. High Energy Density Phys. 2010, 6, 89–94. [Google Scholar] [CrossRef]
- Girard, F.; Primout, M.; Villette, B.; Brebion, D.; Nishimura, H.; Fournier, K.B. Experimental X-ray characterization of Gekko-XII laser propagation through very low-density aerogels (2–5 mg/cc) creating multi-keV photons from a titanium solid foil. High Energy Density Phys. 2011, 7, 285–287. [Google Scholar] [CrossRef]
- Ganeev, R.A. Generation of harmonics of laser radiation in plasmas. Laser Phys. Lett. 2012, 9, 175–194. [Google Scholar] [CrossRef]
- Perez, F.; Kay, J.J.; Patterson, J.R.; Kane, J.; Villette, B.; Girard, F.; Reverdin, C.; May, M.; Emig, J.; Sorce, C.; et al. Efficient laser-induced 6–8 keV X-ray production from iron oxide aerogel and foil-lined cavity targets. Phys. Plasmas 2012, 19, 083101:1–083101:10. [Google Scholar]
- Zhu, X.R.; Zhou, B.; Du, A.; Chen, K.; Li, Y.N.; Zhang, Z.H.; Shen, J.; Wu, G.M.; Ni, X.Y. Potential SiO2/CRF bilayer perturbation aerogel target for ICF hydrodynamic instability experiment. Fusion Eng. Des. 2012, 87, 92–97. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941-968. https://doi.org/10.3390/ma6030941
Du A, Zhou B, Zhang Z, Shen J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials. 2013; 6(3):941-968. https://doi.org/10.3390/ma6030941
Chicago/Turabian StyleDu, Ai, Bin Zhou, Zhihua Zhang, and Jun Shen. 2013. "A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel" Materials 6, no. 3: 941-968. https://doi.org/10.3390/ma6030941



