Synthetic Aspects and Electro-Optical Properties of Fluorinated Arylenevinylenes for Luminescence and Photovoltaics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorinated Oligo(arylenevinylene)s
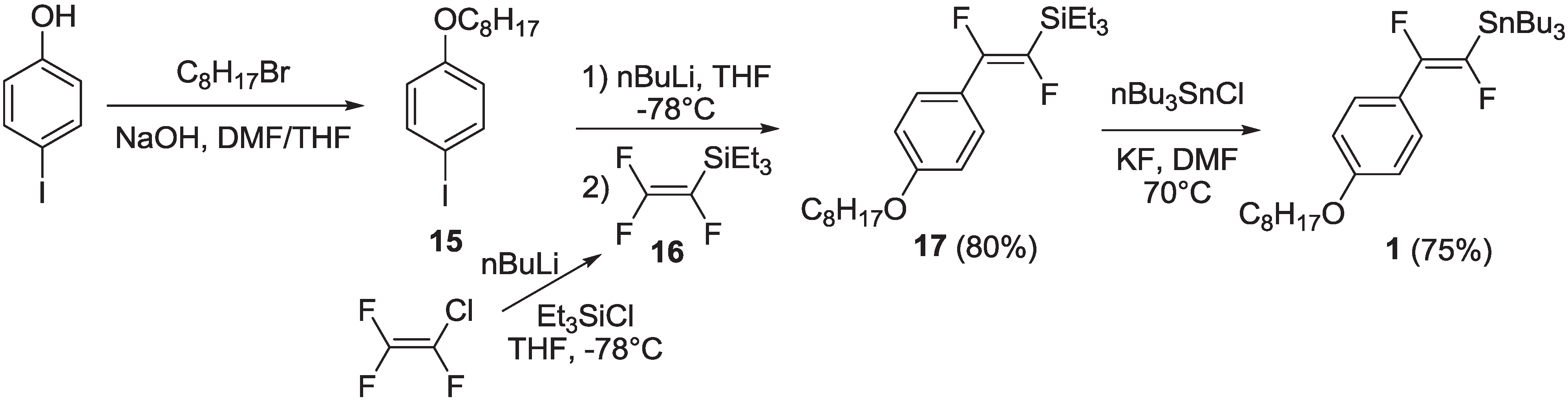
2.1.1. Synthesis
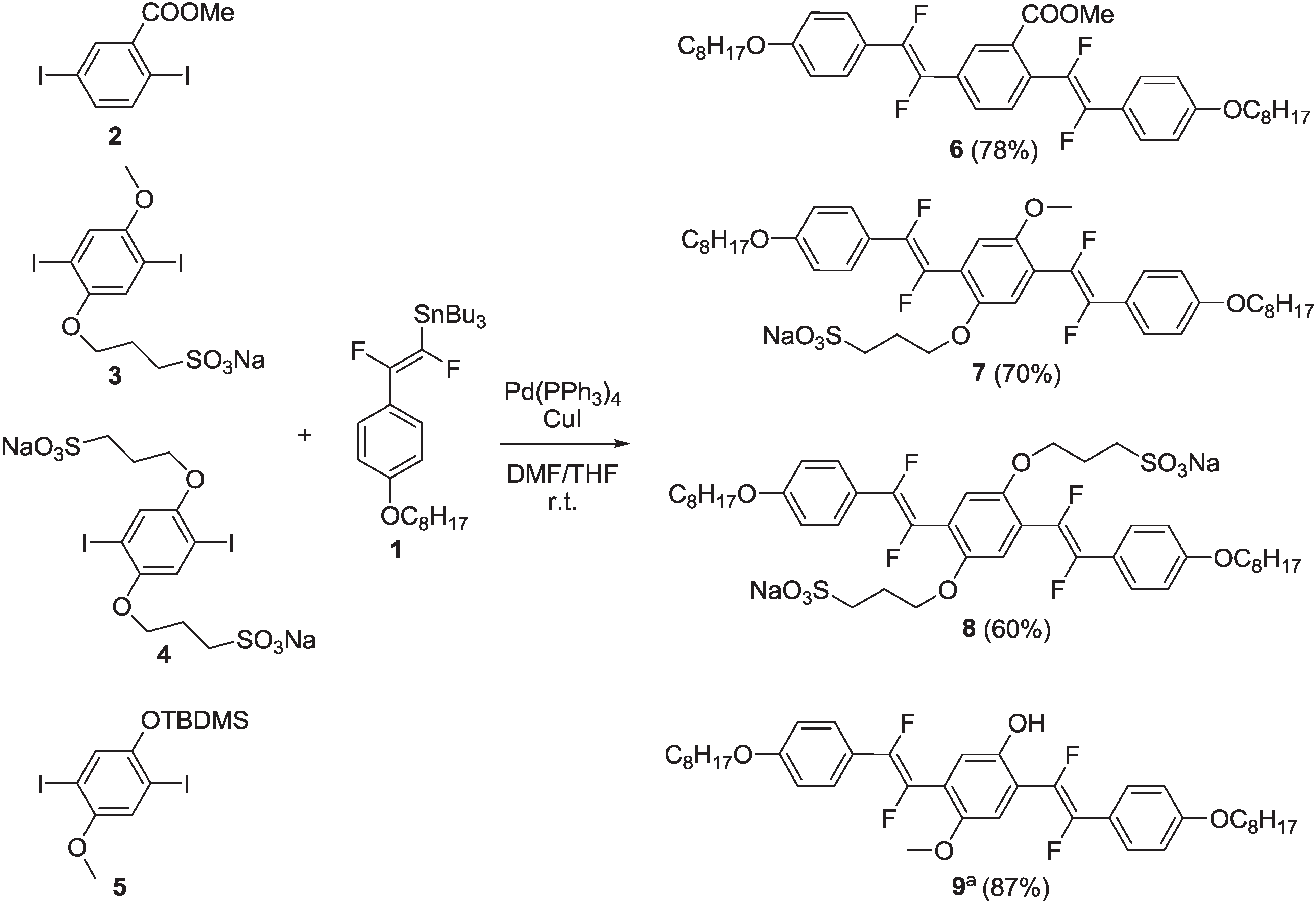
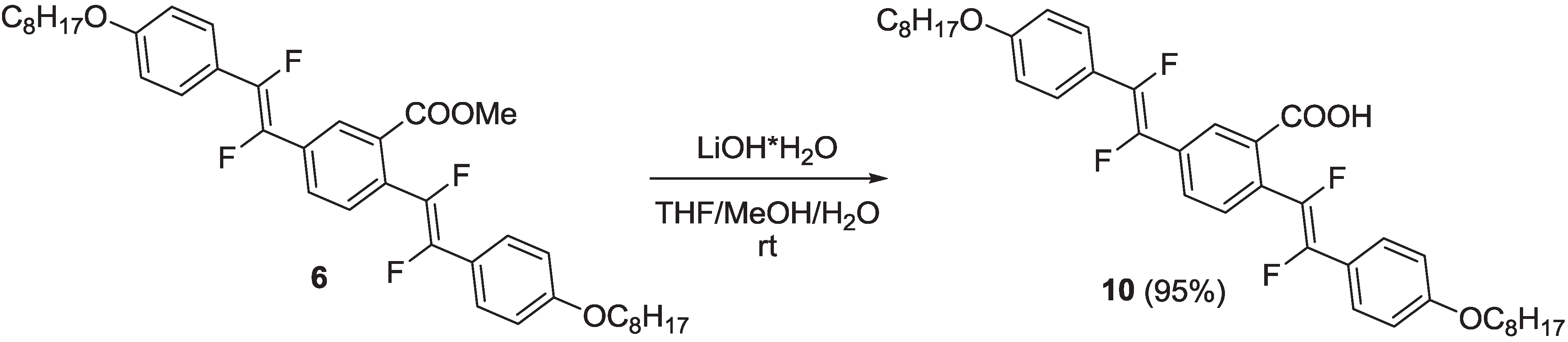

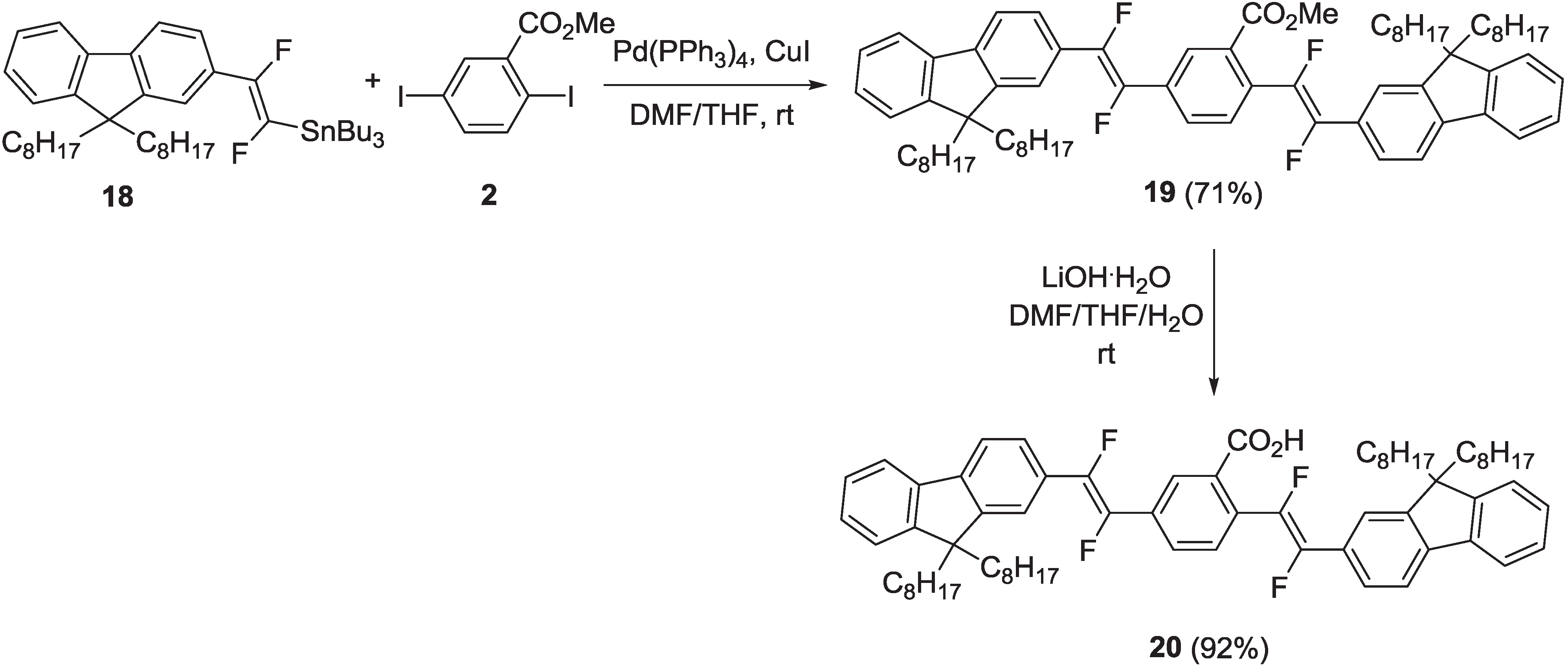
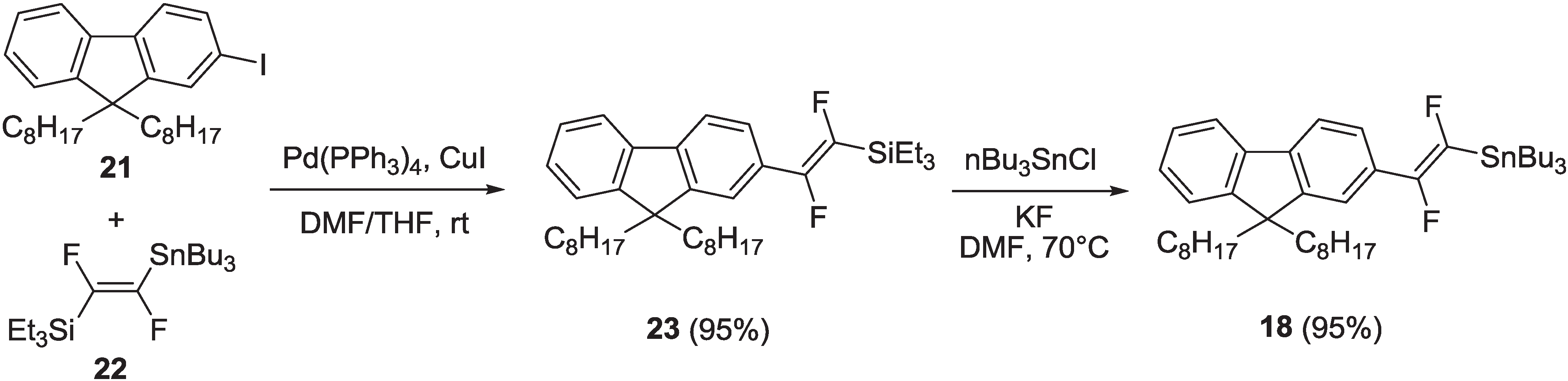
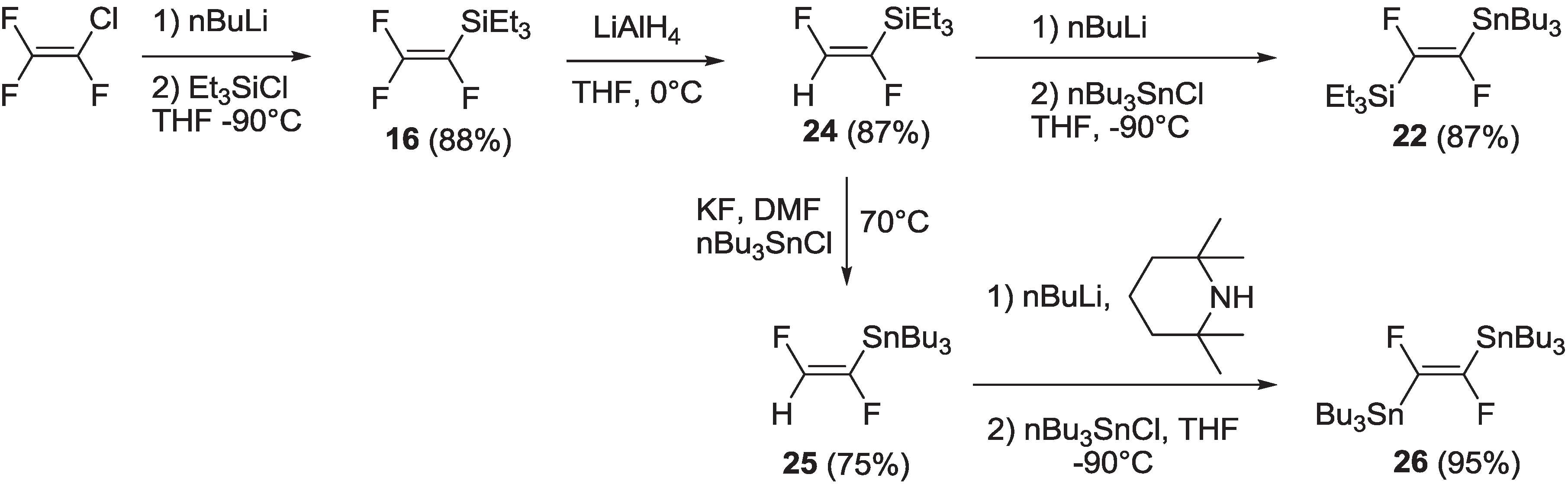
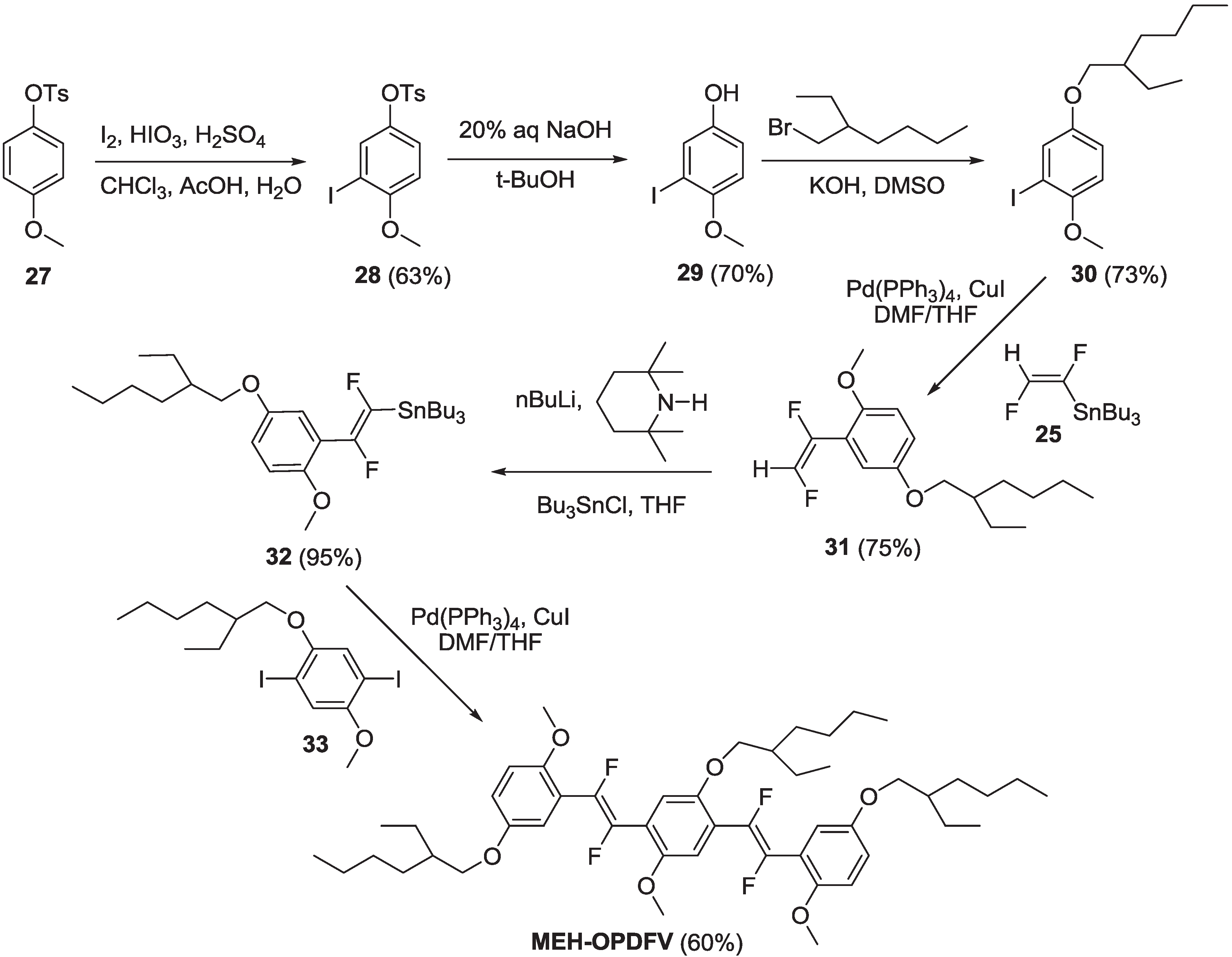
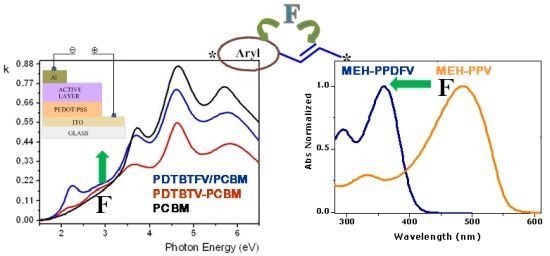
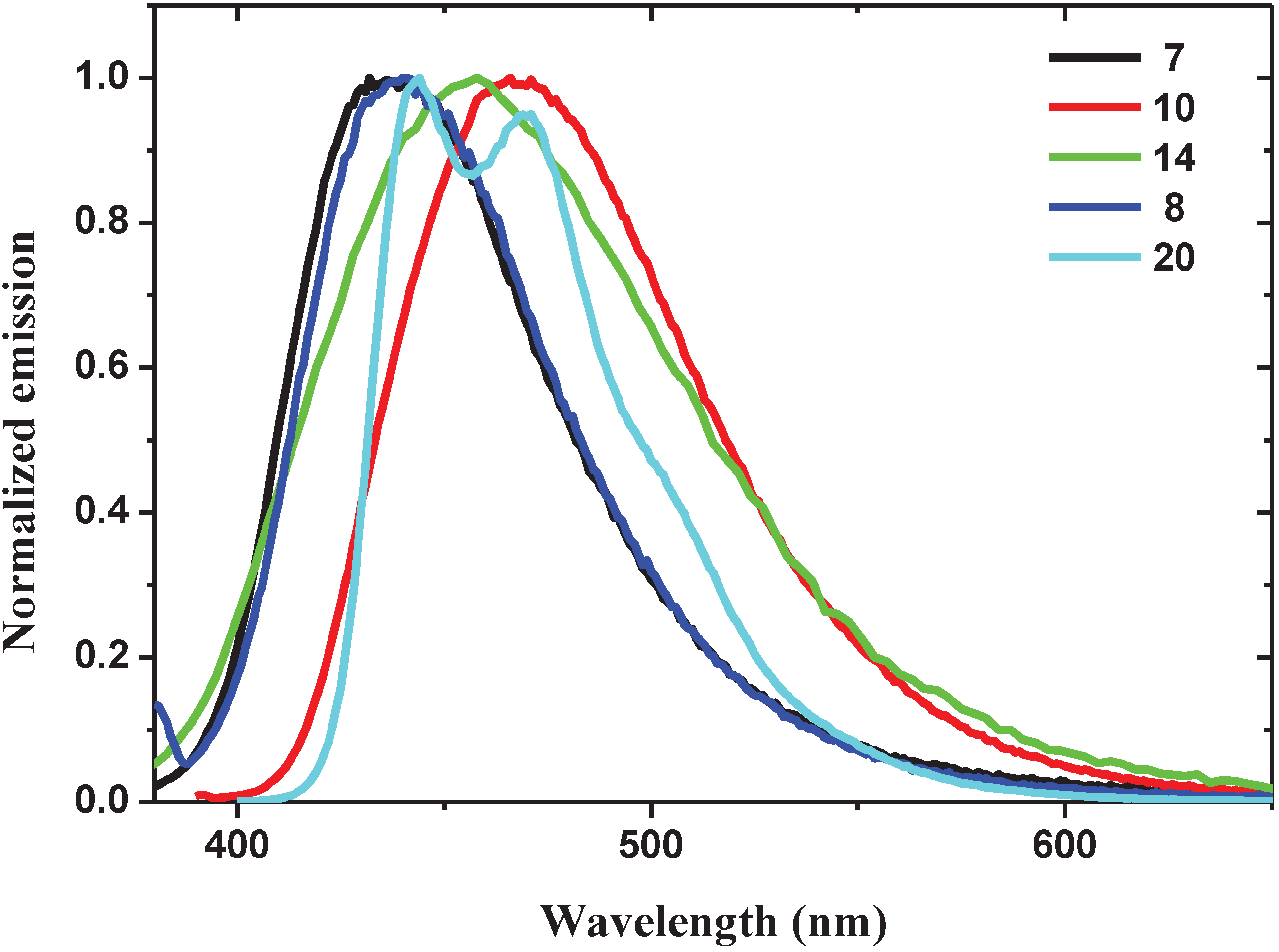
2.1.2. Spectroscopic Properties of Oligo(arylenedifluorovinylene)s
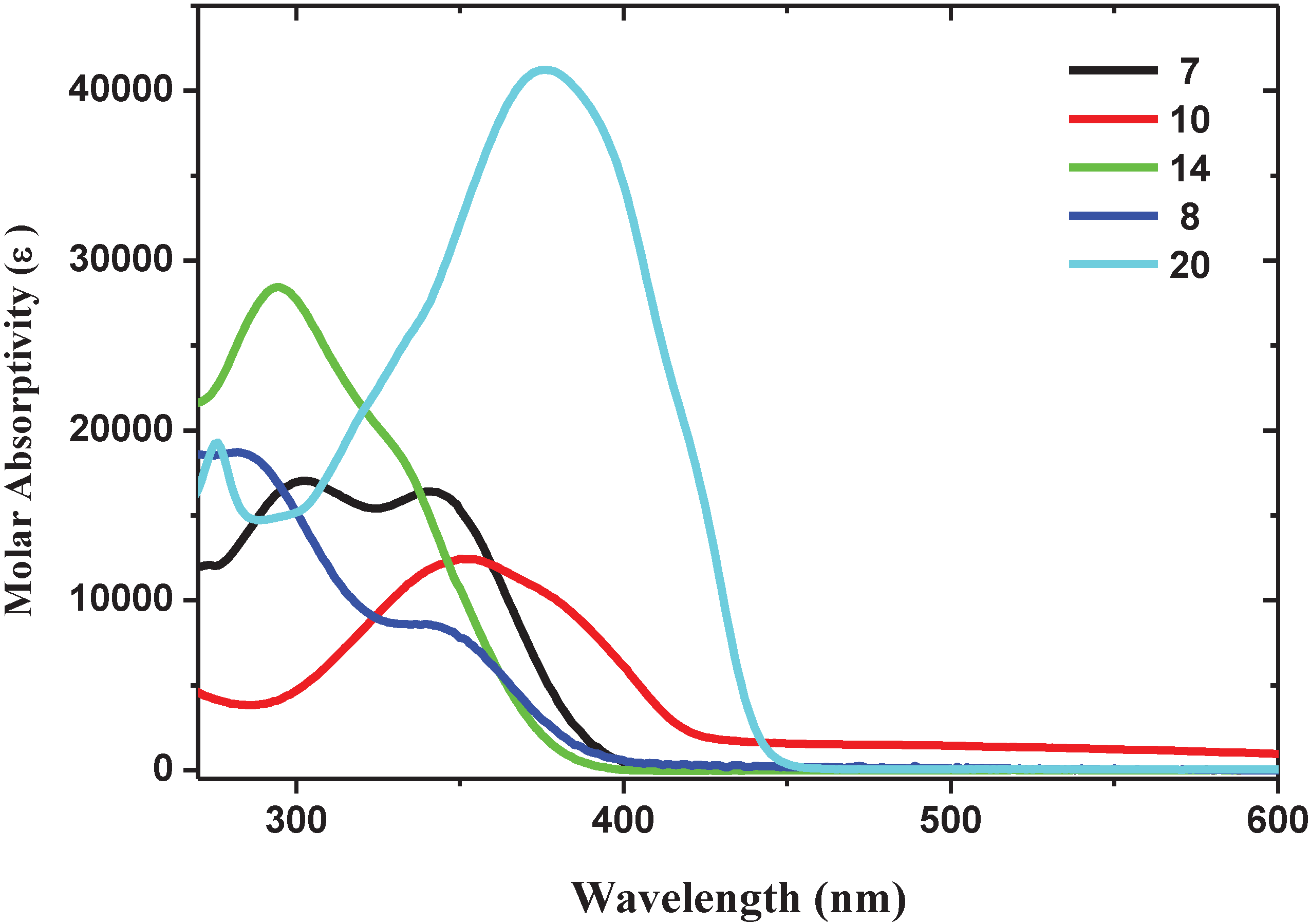
| Oligomer | Molar Absorptivity ε (×103 M−1 cm−1) (λ, nm) | Emission λmax (nm) | Φ (%) (solvent) |
|---|---|---|---|
| 7 | 13 (302), 12 (342) | 436 | 0.87 (CH2Cl2) |
| 8 | 14 (283), 6 (342) | 440 | 0.97 (DMSO) |
| 10 | 14 (352) | 466 | 5.4 (MeOH) |
| 14 | 29 (295) | 458 | 0.43 (CH2Cl2) |
| 20 | 19 (276), 41 (376) | 444, 471 | 8.12 (CH2Cl2) |
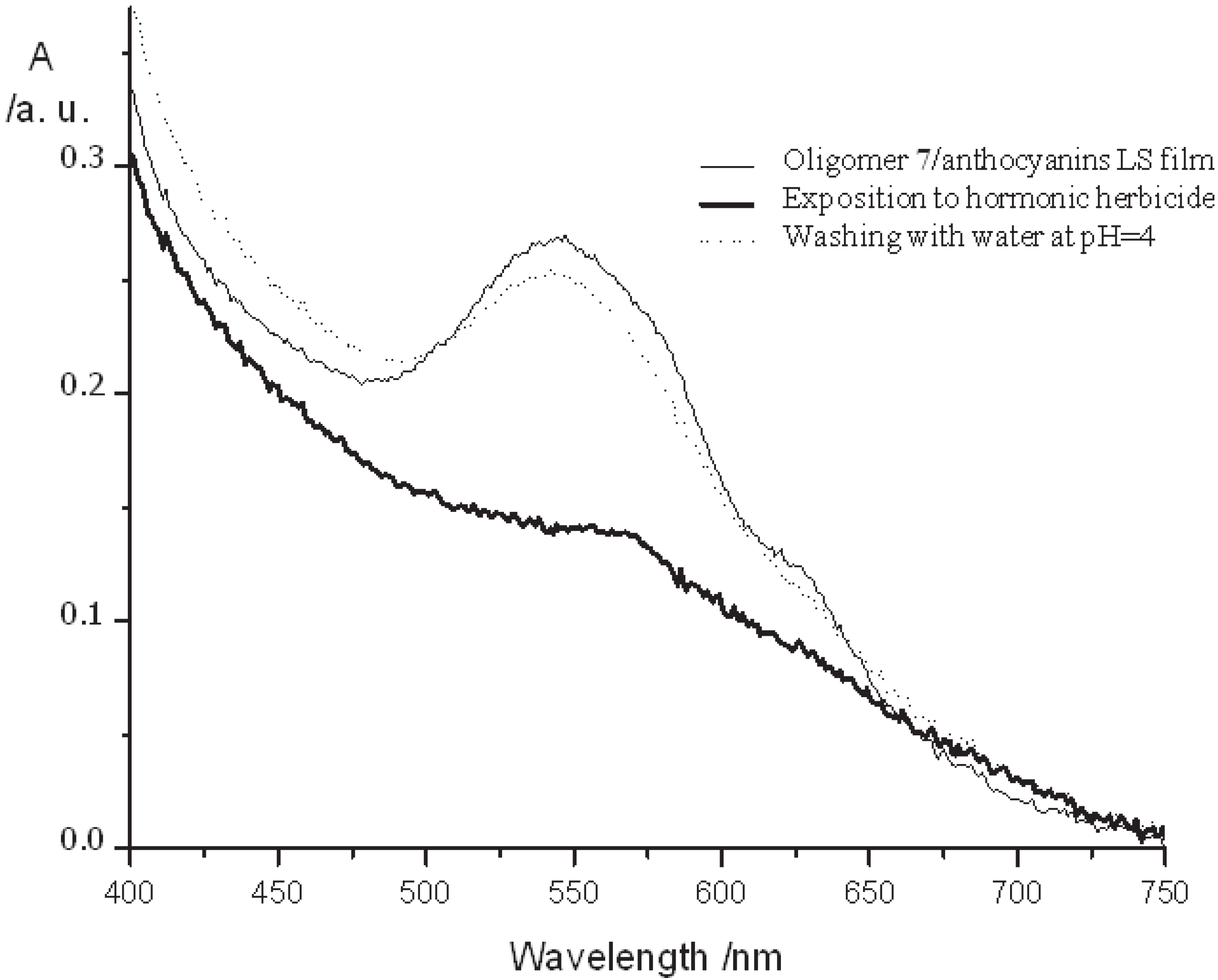
2.1.3. Application of Oligomer 7 in a Chemical Sensor for Herbicide
2.2. Fluorinated Poly(arylenevinylene)s
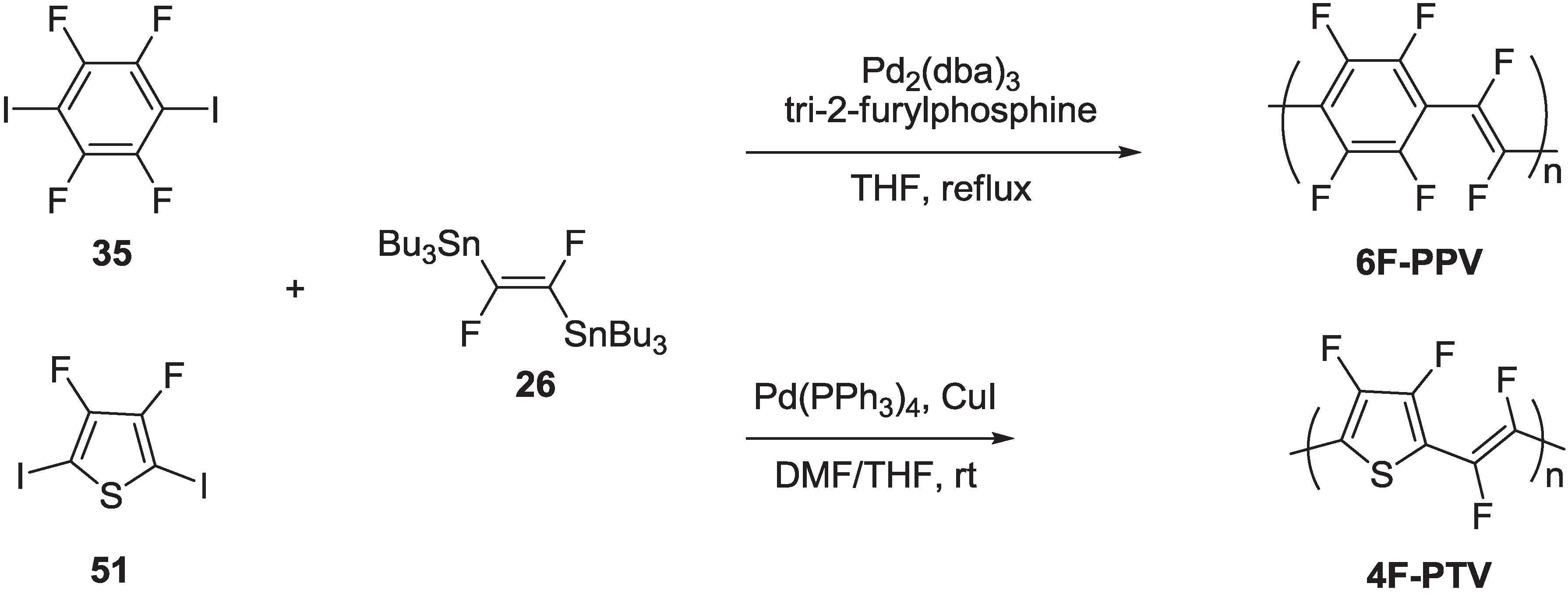
2.2.1. Synthesis of Poly(arylenevinylene)s Fluorinated on the Aromatic Ring

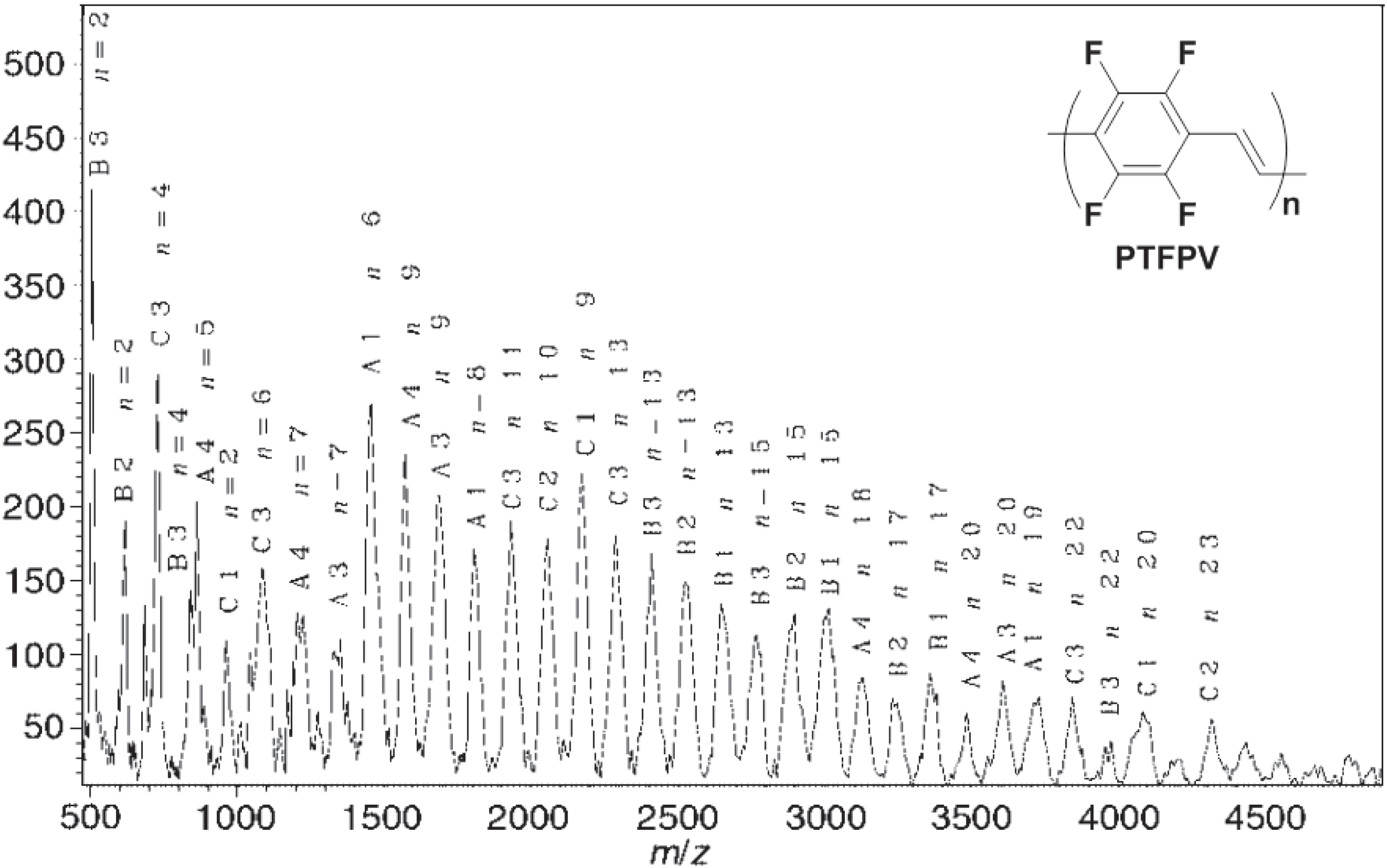
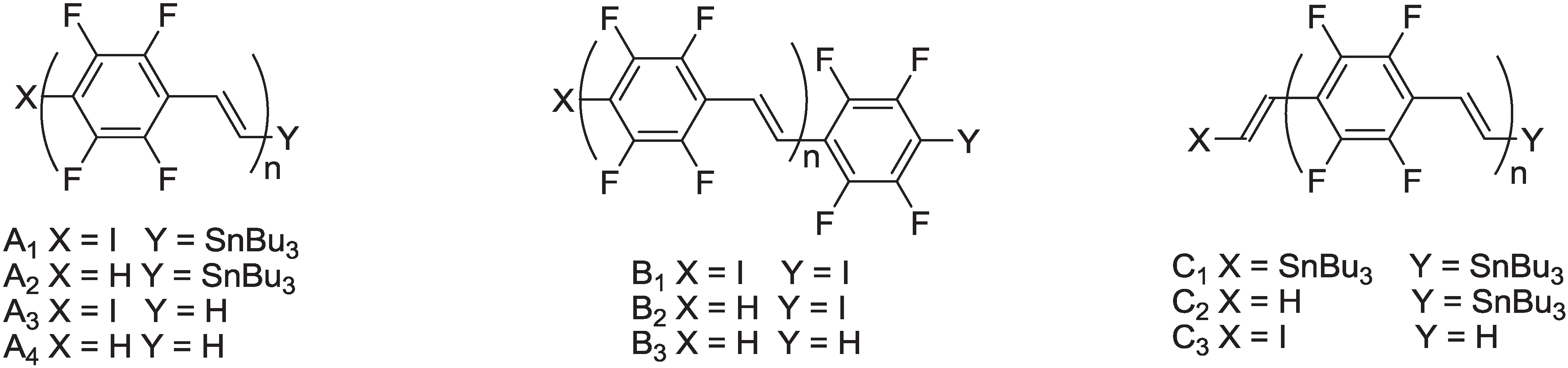
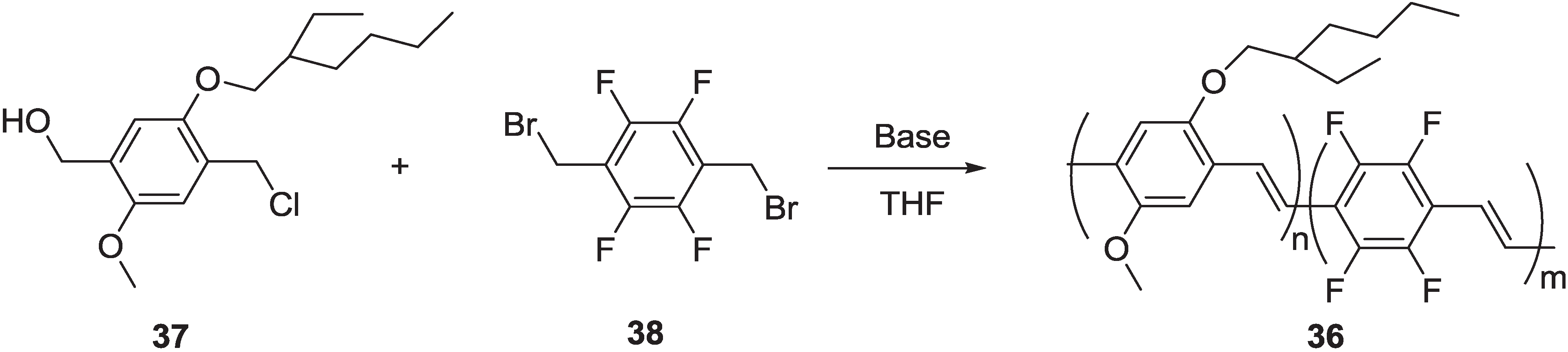
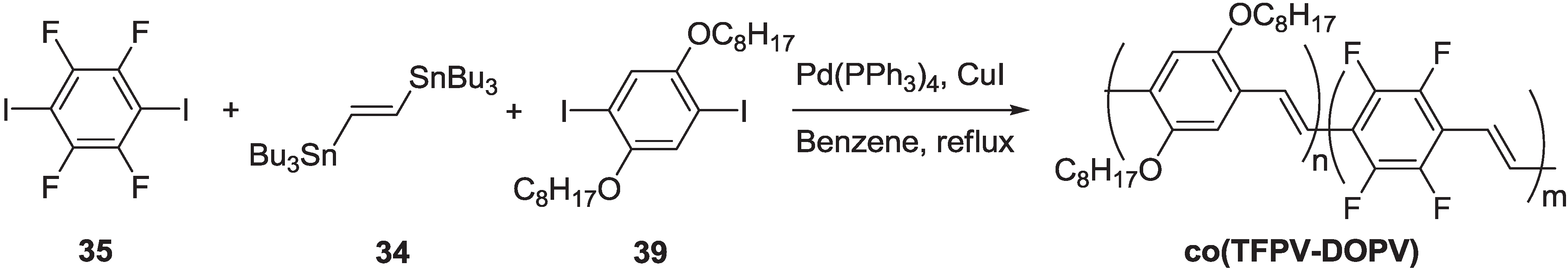
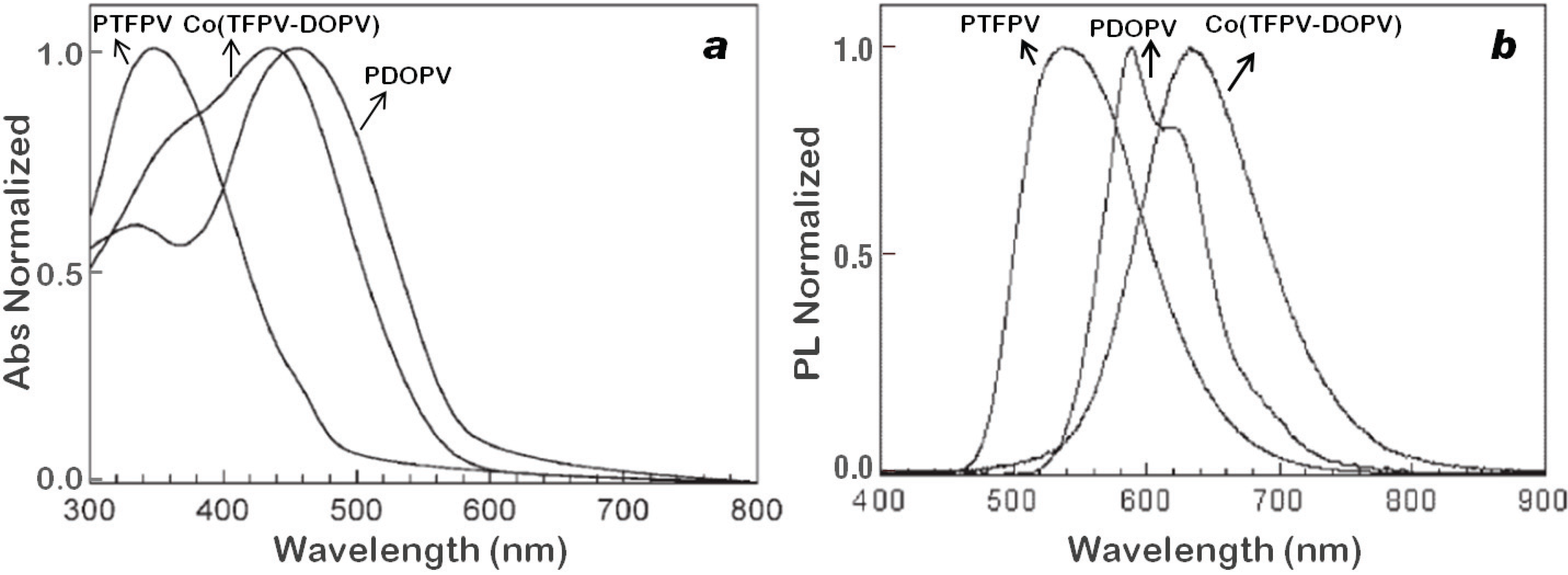
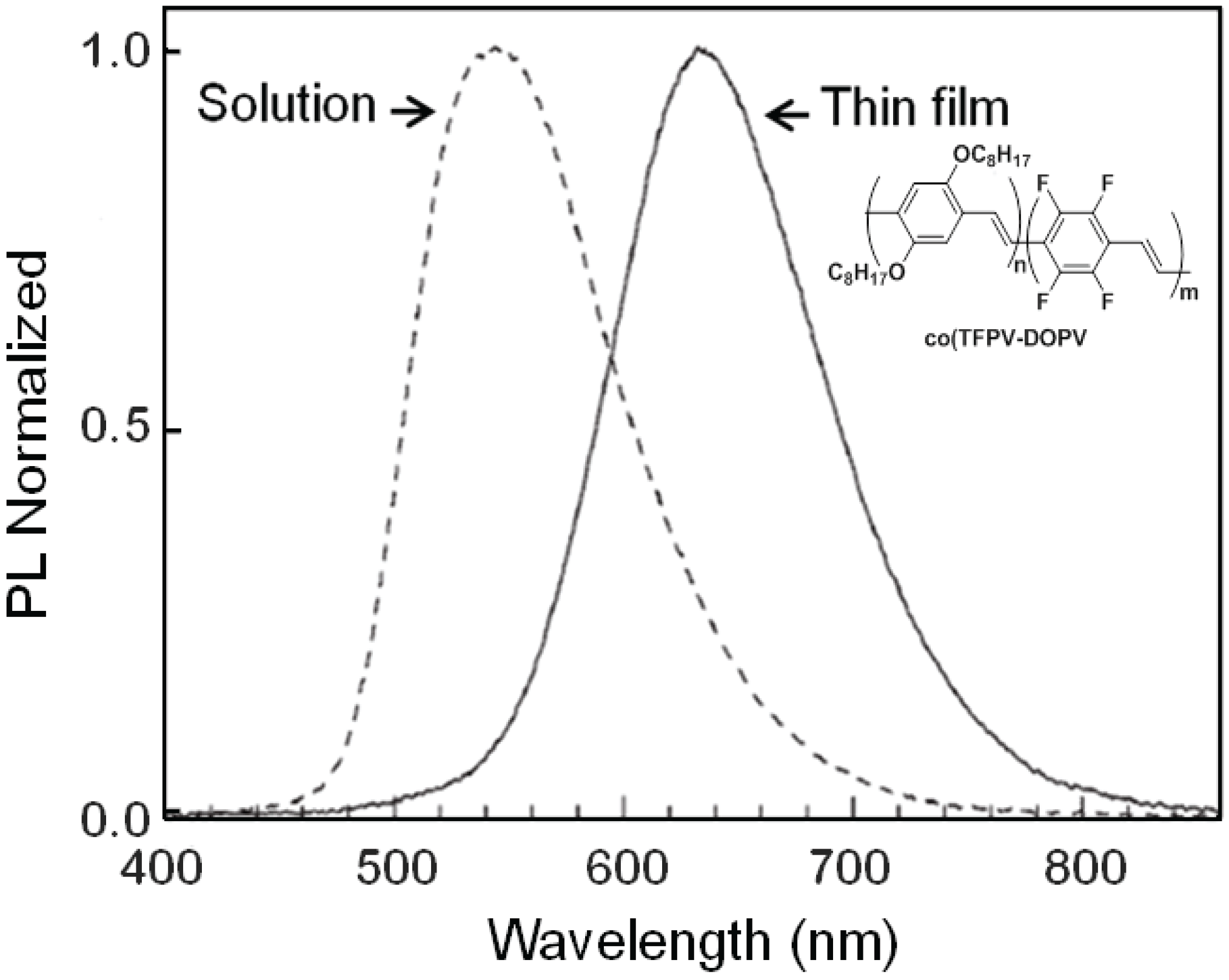
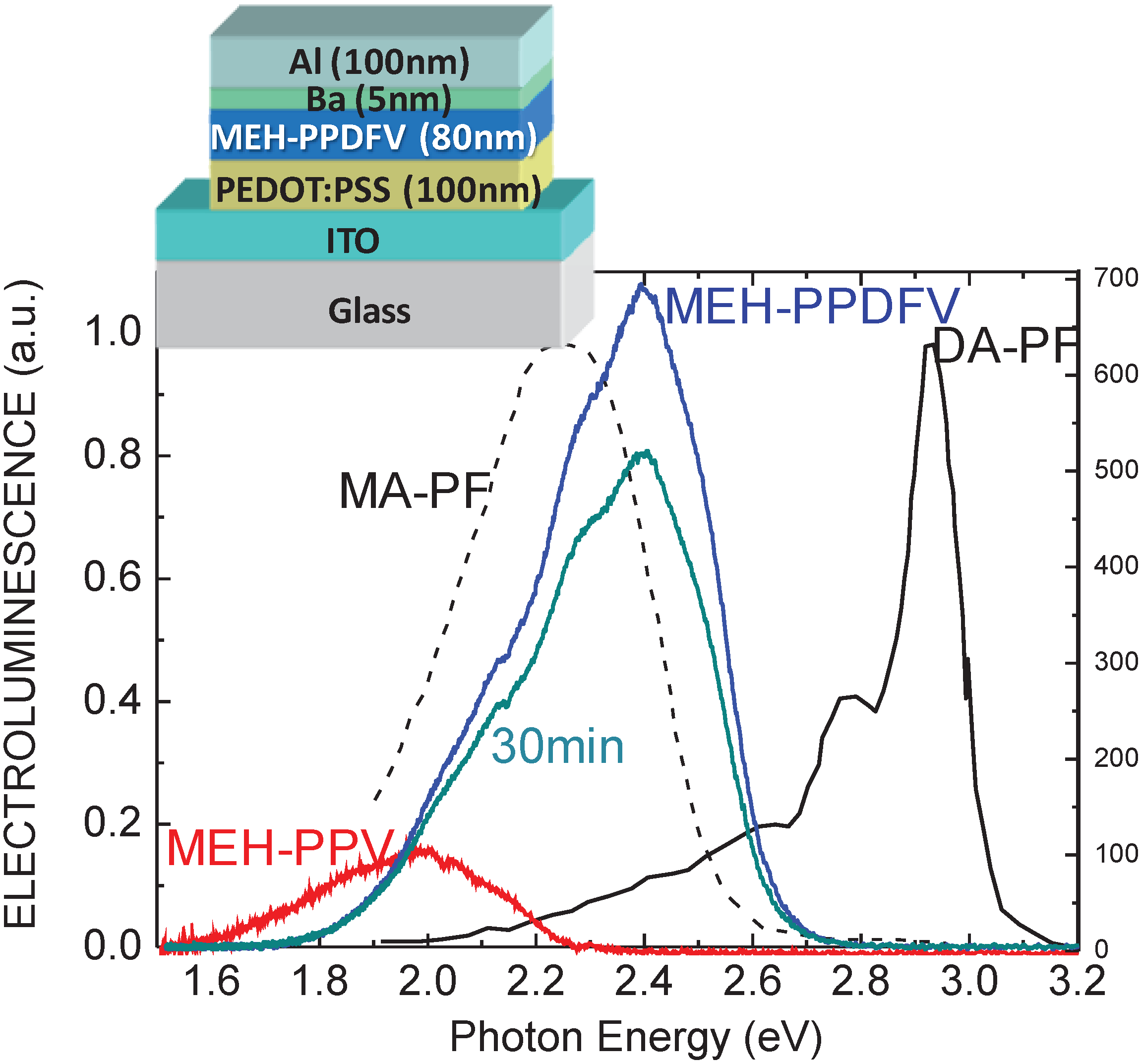
2.2.2. Optical and Electro-Optical Properties of Poly(arylenevinylene)s Fluorinated on the Aromatic Ring
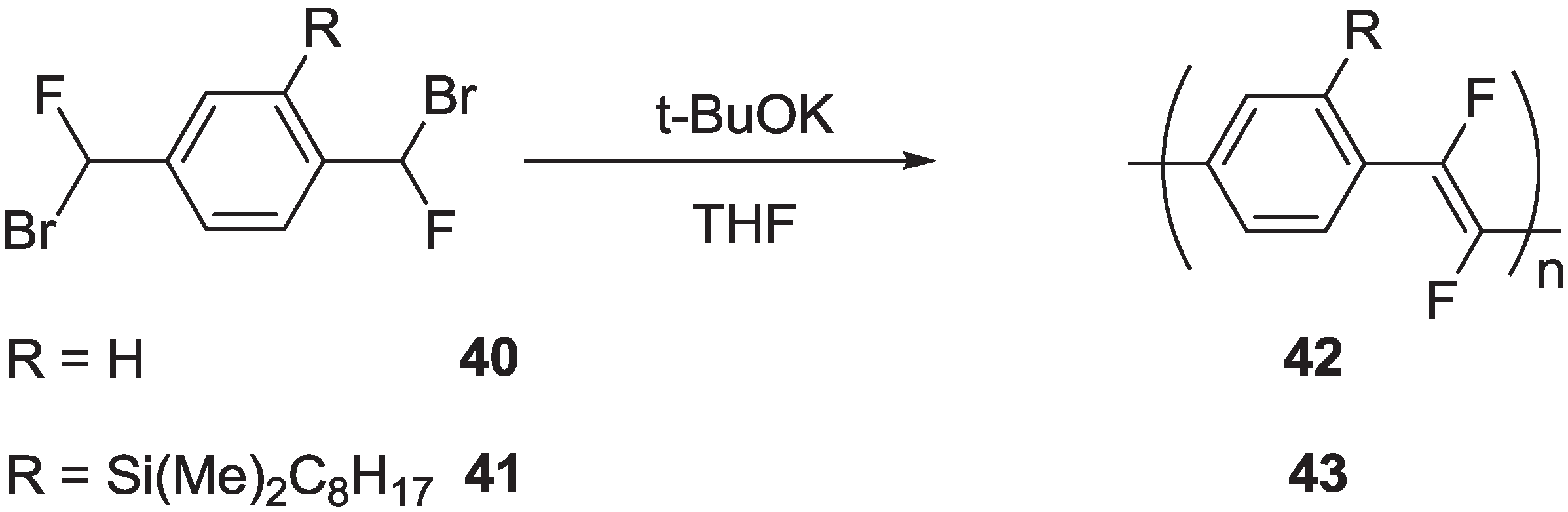
2.2.3. Synthesis of Poly(arylenevinylene)s Fluorinated on the Vinylene Units
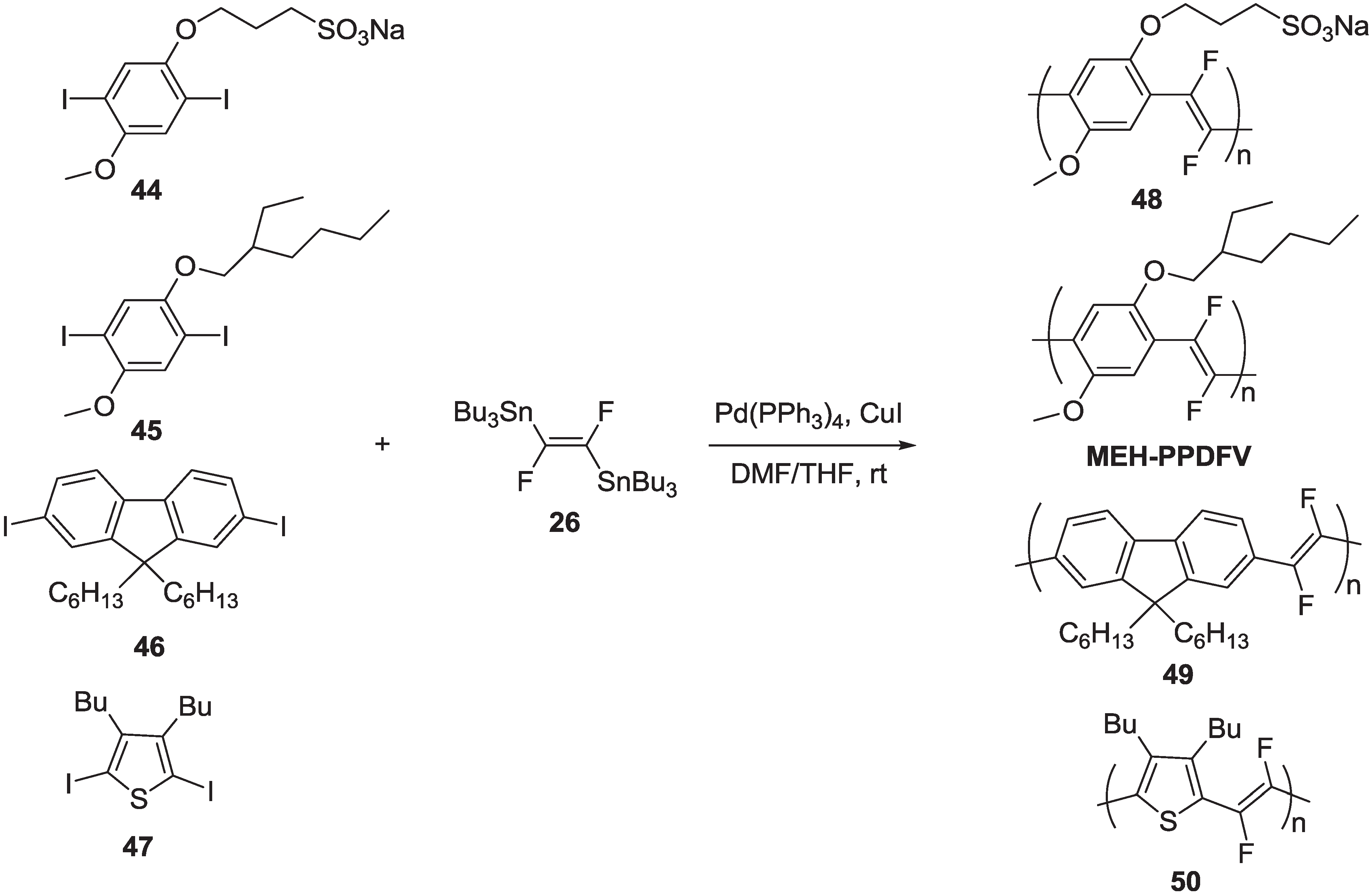
| Polymer | Yield (%) | Mw | Mw/Mn |
|---|---|---|---|
| 48 | 55 | 65150 | 1.6 |
| MEH-PPDFV | 87 | 47000 | 1.7 |
| 49 | 82 | 45600 | 2.0 |
| 50 | 50 | 47100 | 2.9 |
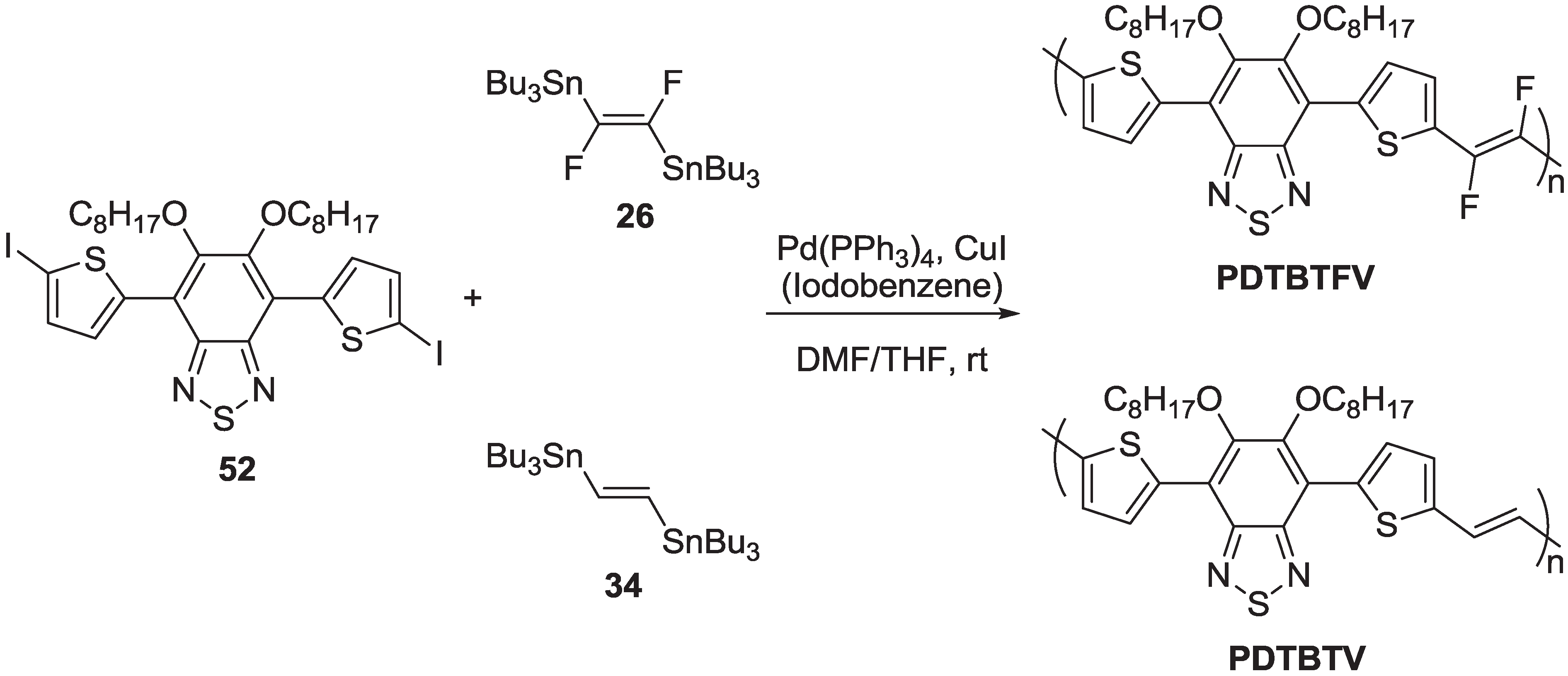
| Polymer | Yield (%) | Mw | Mw/Mn |
|---|---|---|---|
| PDTBTV | 70 | 36,900 | 4.67 |
| PDTBTFV | 75 | 43,700 | 4.46 |
2.2.4. Optical and Electro-Optical Properties of Poly(arylenevinylene)s Fluorinated on the Vinylene Units
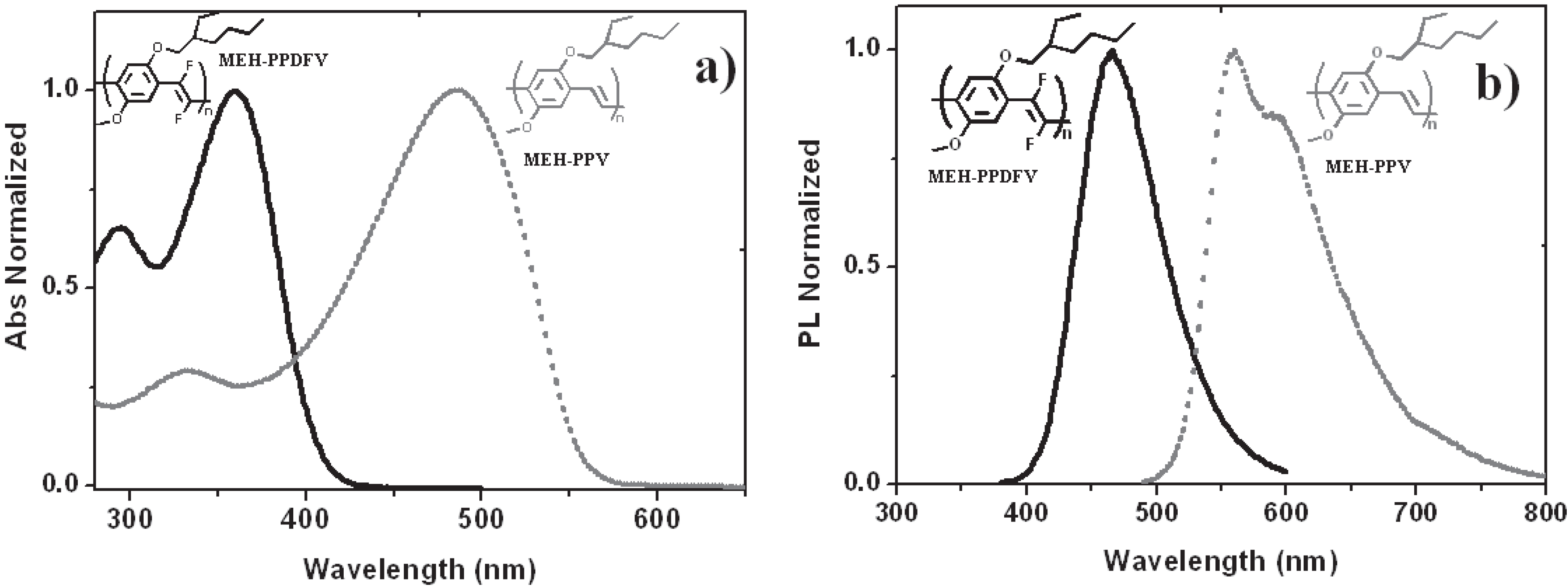

2.2.5. Photovoltaic Performance of PDTBTFV and PDTBTV in BHJ Solar Cells: Effects of Fluorination of Double Bonds
| Polymer | Polymer/PCBM Weight ratio | VOC [V] | ISC [mA cm−2] | FF [%] | η [%] |
|---|---|---|---|---|---|
| PDTBTFV | 2:7 | 0.73 | 2.94 | 38.57 | 0.83 |
| 3:7 | 0.75 | 3.55 | 37.97 | 1.05 | |
| 1:1 | 0.83 | 4.32 | 34.56 | 1.24 | |
| PDTBTV | 2:7 | 0.55 | 3.33 | 28.83 | 0.53 |
| 3:7 | 0.56 | 2.42 | 24.50 | 0.33 | |
| 1:1 | 0.43 | 3.15 | 33.51 | 0.46 |
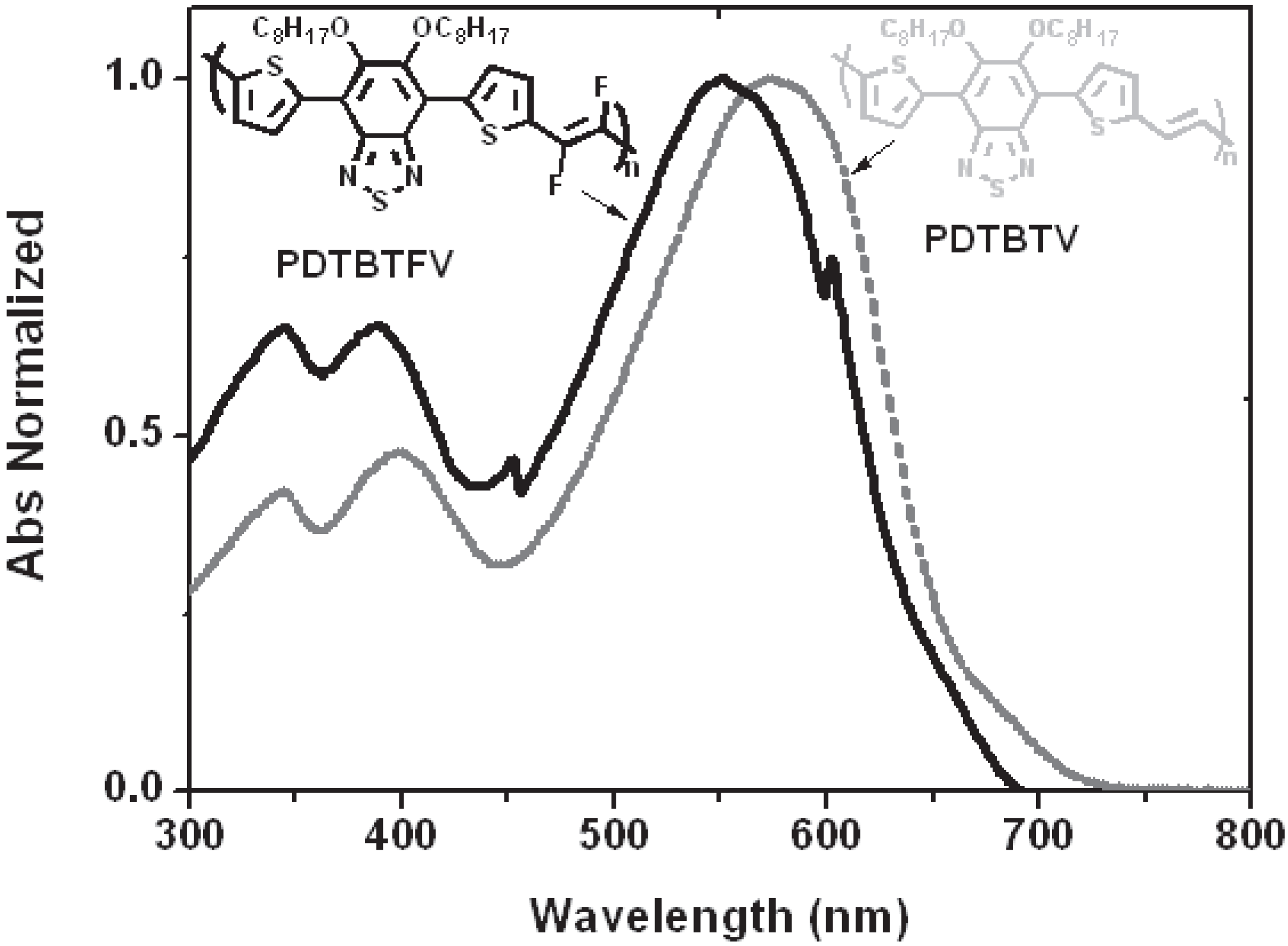
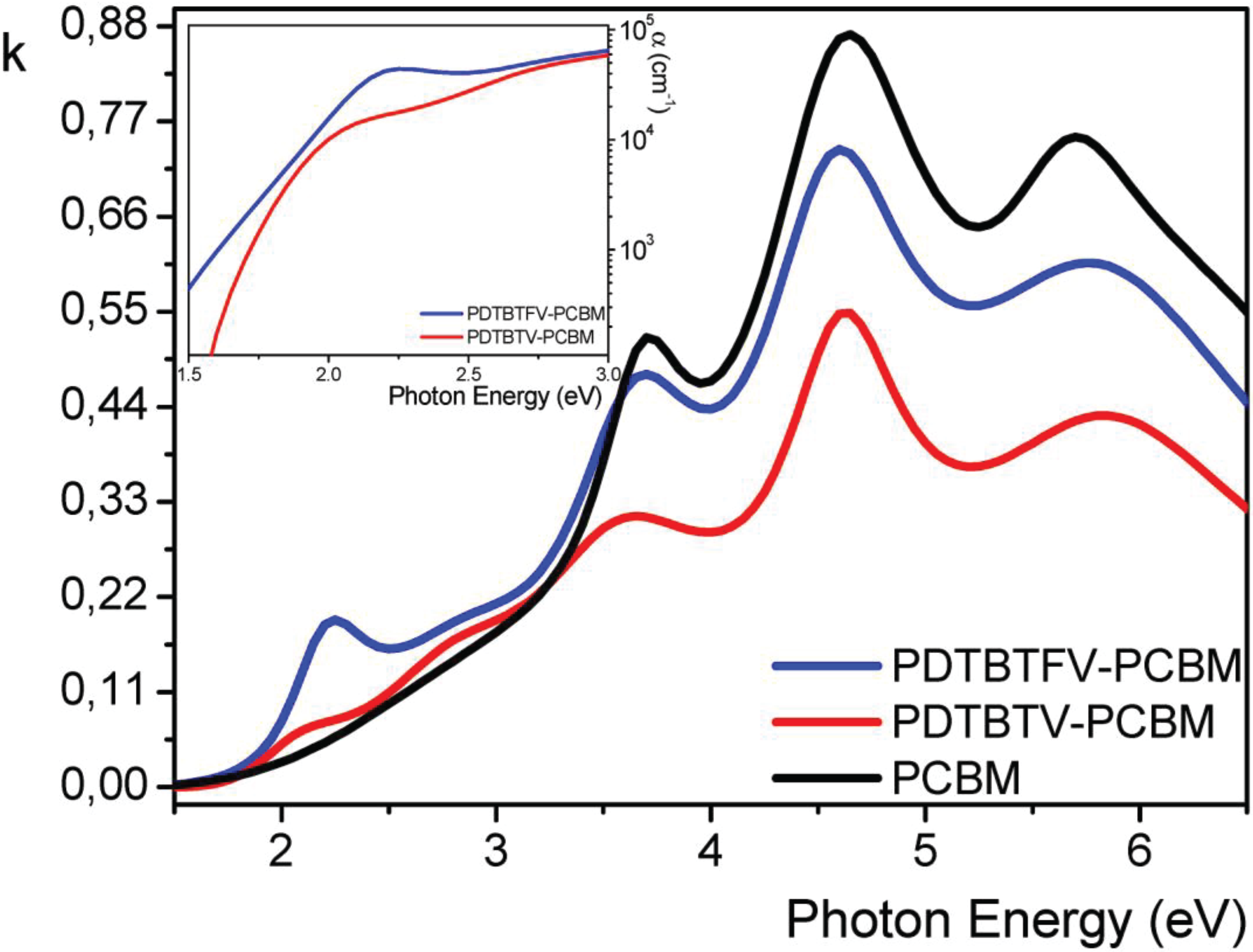
3. Conclusions
Acknowledgments
References and Notes
- Miller, R.D.; Chandross, E.A. 2010 Materials for Electronics. Chem. Rev. 2010, 110, 1–574. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. p-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Nalwa, H.S. Handbook of Organic Electronics and Photonics; American Scientific Publishers: Stevenson Ranch, CA, USA, 2008. [Google Scholar]
- Nguyen, T.P.; Destruel, P. Electroluminescent Devices Based on Organics and Conjugated Polymers. In Handbook of Luminescence, Display Materials and Devices; Nalwa, H.S., Rohwer, L.S., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2003; Volume 1, pp. 1–129. [Google Scholar]
- Kulkarni, A.P.; Tonzola, C.J.; Babel, A.; Jenekhe, S.A. Electron transport materials for organic light-emitting diodes. Chem. Mater. 2004, 16, 4556–4573. [Google Scholar] [CrossRef]
- Müllen, K.; Scherf, U. Organic Light-Emitting Devices: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.B.; Sariciftci, N.S.; Jaiswal, M.; Menon, R. Organic Field-Effect Transistors: From Materials to Device Physics. In Handbook of Organic Electronics and Photonics; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2008. [Google Scholar]
- Dimitrakopoulos, C.D.; Malenfant, P.R.L. Organic Thin Film Transistors for Large Area Electronics. Adv. Mater. 2002, 14, 99–117. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.; Dyakonov, V.; Parisi, J.; Sariciftci, N.S. Organic Photovoltaics: Concepts and Realization; Springer: Berlin, Germany, 2003. [Google Scholar]
- Thompson, B.C.; Fréchet, J.M.J. Polymer-Fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R. Fluorinated organic materials for electronic and optoelectronic applications: The role of fluorine atom. Chem. Commun. 2007, 1003–1022. [Google Scholar]
- Farinola, G.M.; Cardone, A.; Babudri, F.; Martinelli, C.; Naso, F.; Bruno, G.; Losurdo, M. Fluorinated Poly(p-phenylenevinylene)s: Synthesis and optical properties of an intriguing class of luminescent polymers. Materials 2010, 3, 3077–3091. [Google Scholar] [CrossRef]
- Cardone, A.; Martinelli, C.; Babudri, F.; Naso, F.; Pinto, V.; Farinola, G.M. Synthesis of fluorinated (electro)luminescent arylenevinylene polymers and oligomers. Curr. Org. Synth. 2012, 9, 150–162. [Google Scholar] [CrossRef]
- Facchetti, A.; Deng, Y.; Wang, A.; Koide, Y.; Sirringhaus, H.; Marks, T.J.; Friend, R.H. Tuning the semiconducting properties of sexithiophene by α,ω-substitution-α,ω-diperfluorohexylsexithiophene: the first n-type sexithiophene for thin film transistors. Angew. Chem. Int. Ed. 2000, 39, 4547–4551. [Google Scholar] [CrossRef]
- Facchetti, A.; Yoon, M.-H.; Stern, C.L.; Hutchinson, G.R.; Ratner, M.A.; Marks, T.J. Building blocks for n-type molecular and polymeric electronics. Perfluoroalkyl- versus alkyl-functionalized oligothiophenes (nTs; n = 2–6). Systematic synthesis, spectroscopy, electrochemistry, and solid-state organization. J. Am. Chem. Soc. 2004, 126, 13480–13501. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Komatsu, S.; Suzuki, T. Tetradecafluorosexithiophene: The first perfluorinated oligothiophene. J. Am. Chem. Soc. 2001, 123, 4643–4644. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.R.; Frisbie, C.D.; da Silva Filho, D.A.; Brédas, J.-L.; Ewbank, P.C.; Mann, K.R. Introduction to organic thin film transistors and design of n-channel organic semiconductors. Chem. Mater. 2004, 16, 4436–4451. [Google Scholar] [CrossRef]
- Usta, H.; Facchetti, A.; Marks, T.J. n-Channel Semiconductor Materials Design for Organic Complementary Circuits. Acc. Chem. Res. 2011, 44, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y. Recent progress in n-channel organic thin-film transistors. Adv. Mater. 2010, 22, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Kryachko, E.; Scheiner, S. C–H···F Hydrogen bonds. Dimers of fluoromethanes. J. Phys. Chem. A 2004, 108, 2527–2535. [Google Scholar] [CrossRef]
- Müllen, K.; Wegner, G. Electronic Materials: The Oligomer Approach; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- He, F.; Tian, L.; Xie, W.; Li, M.; Gao, Q.; Hanif, M.; Zhang, Y.; Cheng, G.; Yang, B.; Ma, Y.; et al. Highly efficient blue organic light emitting devices based on improved guest/host combination. J. Phys. Chem. C 2008, 112, 12024–12029. [Google Scholar] [CrossRef]
- Sander, R.; Stümpflen, V.; Wendorff, J.H.; Greiner, A. Synthesis, properties, and guest-host systems of triphenylamine-based oligo(arylene-vinylene)s: Advanced materials for led applications. Macromolecules 1996, 29, 7705–7708. [Google Scholar] [CrossRef]
- Peeters, E.; van Hal, P.A.; Knol, J.; Bramec, C.J.; Sariciftci, N.S.; Hummelen, J.C.; Janssen, R.A.J. Synthesis, photophysical properties, and photovoltaic devices of oligo(p-phenylene vinylene)-fullerene dyads. J. Phys. Chem. B 2000, 104, 10174–10190. [Google Scholar] [CrossRef]
- Neuteboom, E.E.; Meskers, S.C.J.; van Hal, P.A.; van Duren, J.K.J.; Meijer, E.W.; Janssen, R.A.J.; Dupin, H.; Pourtois, G.; Cornil, J.; Lazzaroni, R.; et al. Alternating oligo(p-phenylene vinylene)-perylene bisimide copolymers: Synthesis, photophysics, and photovoltaic properties of a new class of donor-acceptor materials. J. Am. Chem. Soc. 2003, 125, 8625–8638. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Krebs, F.C. Stepwise unidirectional synthesis of oligo phenylene vinylenes with a series of monomers: Use in plastic solar cells. J. Org. Chem. 2005, 70, 6004–6017. [Google Scholar] [CrossRef] [PubMed]
- Babudri, F.; Cardone, A.; de Cola, L.; Farinola, G.M.; Kottas, G.S.; Martinelli, C.; Naso, F. Synthesis of oligoarylenevinylenes with fluorinated double bonds. Synthesis 2008, 1580–1588. [Google Scholar]
- Xue, L.; Lu, L.; Pedersen, S.D.; Liu, Q.; Narske, R.M.; Burton, D.J. A novel stereospecific route to (E)- and (Z)-(2-substituted-1,2-difluoroethenyl) stannanes. J. Org. Chem. 1997, 62, 1064–1071. [Google Scholar] [CrossRef]
- Burton, D.J.; Liu, Q. Stereoselective preparation of (E)-(1,2-difluoro-1,2-ethenediyl) bis[tributylstannane] and stereospecific synthesis of (E)-1,2-difluorostilbenes. Org. Lett. 2002, 4, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.F. Reference materials for fluorescence measurements. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Piacenza, M.; Della Sala, F.; Farinola, G.M.; Martinelli, C.; Gigli, G. Large blue-shift in the optical spectra of fluorinated polyphenylenevinylenes. A combined theoretical and experimental study. J. Phys. Chem. B 2008, 112, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Valli, L.; Santino, A.; Martinelli, C.; Farinola, G.M.; Cardone, A.; Sgobba, V.; Giancane, G. Spectroscopic investigations, characterization and chemical sensor application of composite Langmuir-Schäfer films of anthocyanins and oligophenylenevinylene derivatives. Dyes Pigment. 2012, 94, 156–162. [Google Scholar] [CrossRef]
- Torskangerpoll, K.; Andersen, O.M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burn, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Gilch, H.G.; Wheelwright, W.L. Polymerization of α-halogenated p-xylenes with base. J. Polym. Sci. A Polym. Chem. 1966, 4, 1337–1349. [Google Scholar] [CrossRef]
- Wessling, R.A. The polymerization of xylylene bisdialkyl sulfonium salts. J. Polym. Sci. Polym. Symp. 1985, 72, 55–66. [Google Scholar] [CrossRef]
- Gourley, K.D.; Lillya, C.P.; Reynolds, J.R.; Chien, J.C.W. Electrically conducting polymers: Arsenic pentafluoride-doped poly(phenylenevinylene) and its analogs. Macromolecules 1984, 17, 1025–1033. [Google Scholar] [CrossRef]
- Chen, Z.-K.; Meng, H.; Lai, Y.-H.; Huang, W. Photoluminescent Poly(p-phenylenevinylene)s with an aromatic oxadiazole moiety as the side chain: Synthesis, electrochemistry and spectroscopy study. Macromolecules 1999, 32, 4351–4358. [Google Scholar] [CrossRef]
- Ahn, T.; Song, S.-Y.; Shim, H.-K. Highly photoluminescent and blue-green electroluminescent polymers: New silyl- and alkoxy-substituted Poly(p-phenylenevinylene) related copolymers containing carbazole or fluorene groups. Macromolecules 2000, 33, 6764–6771. [Google Scholar] [CrossRef]
- Greenham, N.C.; Moratti, S.C.; Bradley, D.D.C.; Friend, R.H.; Holmes, A.B. Efficient light-emitting diodes based on polymers with high electron affinities. Nature 1993, 365, 628–630. [Google Scholar]
- Moratti, S.C.; Cervini, R.; Holmes, A.B.; Baigent, D.R.; Friend, R.H.; Greenham, N.C.; Grüner, J.; Hamer, P.J. High electron affinity polymers for LEDs. Synth. Met. 1995, 71, 2117–2120. [Google Scholar] [CrossRef]
- Chen, S.-A.; Chang, E.-C. Structure and Properties of Cyano-Substituted Poly(2,5-dialkoxy-p-phenylene vinylene)s. Macromolecules 1998, 31, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ju, J.; Kim, J.; Lee, S.; Kim, J.Y.; Park, S.H.; Son, S.-M.; Jin, S.-H.; Lee, K.; Suh, H. Design, synthesis and electroluminescent property of CN-Poly(dihexylfluorenevinylene) for LEDs. Macromolecules 2003, 36, 6970–6975. [Google Scholar] [CrossRef]
- Babudri, F.; Farinola, G.M.; Naso, F. Organometallic chemistry directed towards the synthesis of electroactive materials: Stereoselective routes to extended polyconjugated systems. Pure Appl. Chem. 1999, 71, 1485–1492. [Google Scholar]
- Babudri, F.; Farinola, G.M.; Naso, F. Synthesis of conjugated oligomers and polymers: The organometallic way. J. Mater. Chem. 2004, 14, 11–34. [Google Scholar] [CrossRef]
- Farinola, G.M.; Babudri, F.; Cardone, A.; Hassan Omar, O.; Naso, F. Sinthesis of substituted conjugated polymers: Tuning properties by functionalization. Pure Appl. Chem. 2008, 80, 1735–1746. [Google Scholar] [CrossRef]
- Babudri, F.; Cicco, S.R.; Chiavarone, L.; Farinola, G.M.; Lopez, L.C.; Naso, F.; Scamarcio, G. Synthesis and optical investigations of low molecular weight alkoxy-substituted poly(p-phenylenevinylene)s. J. Mater. Chem. 2000, 10, 1573–1579. [Google Scholar] [CrossRef]
- Brooke, G.M.; Mawson, S.D. The attempted synthesis of polyparatetrafluorophenylene vinylene via water-soluble and organic solvent-soluble precursor polymers. J. Fluor. Chem. 1990, 50, 101–109. [Google Scholar] [CrossRef]
- Brooke, G.M.; Mawson, S.D. Oligomeric polyparatetrafluorophenylene vinylenes: A new synthesis based on the nucleophilic substitution of the para fluorine during the reaction of (E)-2-(pentafluorophenyl)ethenyl lithium with hexafluorobenzene. J. Fluor. Chem. 1990, 50, 101–109. [Google Scholar] [CrossRef]
- Babudri, F.; Cardone, A.; Chiavarone, L.; Ciccarella, G.; Farinola, G.M.; Naso, F.; Scamarcio, G. Synthesis and characterization of poly(2,3,5,6-tetrafluoro-1,4-phenylenevinylene). Chem. Commun. 2001, 1940–1941. [Google Scholar]
- Farina, V. New perspective in the cross-coupling reactions of organostannanes. Pure Appl. Chem. 1996, 68, 73–78. [Google Scholar] [CrossRef]
- Skelton, R.; Dubois, F.; Zenobi, R.A. MALDI sample preparation method suitable for insoluble polymers. Anal. Chem. 2000, 72, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.R.; Hu, B.; Karasz, F.E.; Akcelrud, L. Emitting polymers containing cyano groups. Synthesis and photophysical properties of a fully conjugated polymer obtained by Wittig reaction. Polymer 2000, 41, 8095–8102. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, W.-L.; Pei, J.; Chen, Z.; Huang, W.; Heeger, A.J. A Novel Series of Copolymers Containing 2,5-Dicyano-1,4-phenylenevinylene-synthetic tuning of the HOMO and LUMO energy levels of conjugated polymers. Chem. Eur. J. 2000, 6, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Riehn, R.; Morgado, J.; Iqbal, R.; Moratti, S.C.; Holmes, A.B.; Volta, S.; Cacialli, F. Electrochemical and electroluminescent properties of random copolymers of fluorine- and alkoxysubstituted poly(p-phenylene vinylene)s. Macromolecules 2000, 33, 3337–3341. [Google Scholar] [CrossRef]
- Farinola, G.M.; Cassano, T.; Tommasi, R.; Babudri, F.; Cardone, A.; Naso, F. Third-order nonlinear optical properties of copoly(2,3,5,6-tetrafluoro-1,4-phenylenevinylene-2,5-dialkoxy-1,4-phenylenevinylene)s, a novel class of push-pull substituted PPVs. Proc. SPIE Int. Soc. Opt. Eng. 2001, 4461, 296–303. [Google Scholar]
- Cassano, T.; Tommasi, R.; Babudri, F.; Cardone, A.; Farinola, G.M.; Naso, F. High third-order nonlinear optical susceptibility in new fluorinated poly(p-phenylenevinylene) copolymers measured with the Z-scan technique. Opt. Lett. 2002, 27, 2176–2178. [Google Scholar] [CrossRef] [PubMed]
- Babudri, F.; Cardone, A.; Farinola, G.M.; Naso, F.; Cassano, T.; Chiavarone, L.; Tommasi, R. Synthesis and optical properties of a copolymer of tetrafluoro- and dialkoxy-substituted poly(p-phenylenevinylene) with a high percentage of fluorinated units. Macromol. Chem. Phys. 2003, 204, 1621–1627. [Google Scholar] [CrossRef]
- Benjamin, I.; Faraggi, E.Z.; Avny, Y.; Davidov, D.; Neumann, R. Fluorinated poly(pphenylenevinylene) copolymers: Preparation and use in light-emitting diodes. Chem. Mater. 1996, 8, 352–355. [Google Scholar] [CrossRef]
- Tommasi, R.; Cassano, T.; Farinola, G.M.; Babudri, F.; Cardone, A.; Naso, F. Nonlinear Optical Measurements in Copoly(2,3,5,6-tetrafluoro-1,4-phenylenevinylene-2,5-dialkoxy-1,4-phenylenevinylene)s using the Z-scan Technique. In Nonlinear Optics: Materials, Fundamentals and Applications; Sawchuk, A., Ed.; Optical Society of America: Wangshington, DC, USA, 2002; pp. 269–271. [Google Scholar]
- Yohannes, T.; Neugebauer, H.; Farinola, G.M.; Winder, C.; Babudri, F.; Cardone, A.; Naso, F.; Sariciftci, N.S. Vibrational spectroscopic study of a push-pull substituted fluorinated poly(p-phenylenevinylene) copolymer. Synth. Met. 2005, 152, 149–152. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, J.; Lee, S.; Kim, J.Y.; Park, S.H.; Lee, K.; Suh, H. Novel electroluminescent polymers with fluoro groups in vinylene units. Macromolecules 2004, 37, 6711–6715. [Google Scholar] [CrossRef]
- Jin, Y.; Jee, J.; Kim, K.; Kim, J.; Song, S.; Park, S.H.; Lee, K.; Suh, H. Synthesis and electroluminescent properties of copolymers based on PPV with fluoro groups in vinylene units. Polymer 2007, 48, 1541–1549. [Google Scholar]
- Becker, H.; Spreitzer, H.; Ibrom, K.; Kreuder, W. New insights into the microstructure of Gilch-polymerized PPVs. Macromolecules 1999, 32, 4925–4932. [Google Scholar] [CrossRef]
- Babudri, F.; Cardone, A.; Farinola, G.M.; Martinelli, C.; Mendichi, R.; Naso, F.; Striccoli, M. Synthesis of poly(arylenevinylene)s with fluorinated vinylene units. Eur. J. Org. Chem. 2008, 1977–1982. [Google Scholar]
- Cardone, A.; Martinelli, C.; Pinto, V.; Babudri, F.; Losurdo, M.; Bruno, G.; Pinalysa, C.; Naso, F.; Farinola, G.M. Synthesis and characterization of perfluorinated arylenevinylene polymers. J. Poly. Sci. A Polym. Chem. 2010, 48, 285–291. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L. A New class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 2010, 43, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Huang, Z.G.; Ashraf, R.S.; Smith, J.; D’Angelo, P.; Watkins, S.E.; Anthopoulos, T.D.; Durrant, J.R.; McCulloch, I. Silaindacenodithiophene-based low band gap polymers—The effect of fluorine substitution on device performances and film morphologies. Adv. Funct. Mater. 2012, 22, 1663–1670. [Google Scholar] [CrossRef]
- Price, S.C.; Steuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient photodiodes from interpenetrating polymer networks. Nature 2002, 376, 498–500. [Google Scholar] [CrossRef]
- Thompson, B.C.; Kim, Y.G.; Reynolds, J.R. Spectral broadening in MEH-PPV:PCBM-based photovoltaic devices via blending with a narrow band gap cyanovinylene-dioxythiophene polymer. Macromolecules 2005, 38, 5359–5362. [Google Scholar] [CrossRef]
- Tajima, K.; Suzuki, Y.; Hashimoto, K. Polymer photovoltaic devices using fully regioregular poly[(2-methoxy-5-(3′,7′-dimethyloctyloxy))-1,4-phenylenevinylene]. J. Phys. Chem. C 2008, 112, 8507–8510. [Google Scholar] [CrossRef]
- Shaheen, S.E.; Brabec, C.J.; Sariciftci, N.S.; Padinger, F.; Fromherz, T.; Hunnelen, J.C. 2.5% efficient organic plastic solar cells. Appl. Phys. Lett. 2001, 78, 841–843. [Google Scholar] [CrossRef]
- Egbe, D.A.M.; Bader, C.; Klemm, E.; Ding, L.; Karasz, F.E.; Grummt, U.-W.; Birckner, E. Influence of the conjugation pattern on the photophysical properties of Alkoxy-substituted PE/PV hybrid polymers. Macromolecules 2003, 36, 9303–9312. [Google Scholar] [CrossRef]
- van Duren, J.K.J.; Yang, X.; Loos, J.; Bulle-Lieuwma, C.W.T.; Sieval, A.B.; Hummelen, J.C.; Janssen, R.A.J. Relating the morphology of poly(p-phenylene vinylene)/methanofullerene blends to solar-cell performance. Adv. Funct. Mater. 2004, 14, 425–434. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Braun, D.; Zhang, C.; Sardanov, V.; Heeger, A.J.; Stucky, G.; Wudl, F. Semiconducting polymer-buckminsterfullerene heterojunctions: Diodes, photodiodes, and photovoltaic cells. Appl. Phys. Lett. 1993, 62, 585–587. [Google Scholar] [CrossRef]
- Halls, J.J.M.; Friend, R.H. The photovoltaic effect in a poly(p-phenylenevinylene)/perylene heterojunction. Synth. Met. 1997, 85, 1307–1308. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Henckens, A.; Knipper, M.; Polec, I.; Manca, J.; Lutsen, L.; Vanderzande, D. Poly(thienylene vinylene) derivatives as low band gap polymers for photovoltaic applications. Thin Solid Films 2004, 451–452, 572–579. [Google Scholar] [CrossRef]
- Smith, A.P.; Smith, R.R.; Taylor, B.E.; Durstock, M.F. An investigation of Poly(thienylene vinylene) in organic photovoltaic devices. Chem. Mater. 2004, 16, 4687–4692. [Google Scholar] [CrossRef]
- Huo, L.; Chen, T.L.; Zhou, Y.; Hou, J.; Chen, H.-Y.; Yang, Y.; Li, Y. Improvement of photoluminescent and photovoltaic properties of poly(thienylene vinylene) by carboxylate substitution. Macromolecules 2009, 42, 4377–4380. [Google Scholar] [CrossRef]
- Fuchigami, H.; Tsumura, A.; Koezuka, H. Polythienylenevinylene thin-film transistor with high carrier mobility. Appl. Phys. Lett. 1993, 63, 1372–1374. [Google Scholar] [CrossRef]
- Wan, M.; Wu, W.; Sang, G.; Zou, Y.; Liu, Y.; Li, Y. Poly(thienylene-vinylene-thienylene) with cyano substituent: Synthesis and application in field-effect transistor and polymer solar cell. J. Polym. Sci. A Polym. Chem. 2009, 47, 4028–4036. [Google Scholar] [CrossRef]
- Thompson, B.C.; Kim, Y.-G.; McCarley, T.D.; Reynolds, J.R. Soluble narrow band gap and blue propylenedioxythiophene-cyanovinylene polymers as multifunctional materials for photovoltaic and electrochromic applications. J. Am. Chem. Soc. 2006, 128, 12714–12725. [Google Scholar] [CrossRef] [PubMed]
- Colladet, K.; Fourier, S.; Cleij, T.J.; Lusten, L.; Gelan, J.; Vanderzande, D.; Nguyen, L.H.; Neugebauer, H.; Sariciftci, S.; Aguirre, A.; et al. Low band gap donor-acceptor conjugated polymers toward organic solar cells applications. Macromolecules 2007, 40, 65–72. [Google Scholar] [CrossRef]
- Galand, E.M.; Kim, Y.-G.; Mwaura, J.K.; Jones, A.G.; McCarley, T.D.; Shrotriya, V.; Yang, Y.; Reynolds, J.R. Optimization of narrow band-gap propylenedioxythiophene: cyanovinylene copolymers for optoelectronic applications. Macromolecules 2006, 39, 9132–9142. [Google Scholar] [CrossRef]
- Mei, J.; Heston, N.C.; Vasilyeva, S.V.; Reynolds, J.R. A Facile approach to defect-free vinylene-linked benzothiadiazole-thiophene low-bandgap conjugated polymers for organic electronics. Macromolecules 2009, 42, 1482–1487. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Vasilyeva, S.V.; Ellinger, S.; McCarley, T.D.; Reynolds, J.R. Unsaturated linkages in dioxythiophene−benzothiadiazole donor-acceptor electrochromic polymers: The key role of conformational freedom. Macromolecules 2009, 42, 3694–3706. [Google Scholar] [CrossRef]
- Liu, B.; Najari, A.; Pan, C.; Leclerc, M.; Xiao, D.; Zou, Y. New low bandgap dithienylbenzothiadiazole vinylene based copolymers: synthesis and photovoltaic properties. Macromol. Rapid Commun. 2010, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, L.; Li, H.; Li, Z.; Xu, B.; Wen, S.; Tian, W. Energy Level and Molecular Structure Engineering of conjugated donor−acceptor copolymers for photovoltaic applications. Macromolecules 2009, 42, 4491–4499. [Google Scholar] [CrossRef]
- Wen, S.; Pei, J.; Zhou, Y.; Li, P.; Xue, L.; Li, Y.; Xu, B.; Tian, W. Synthesis of 4,7-Diphenyl-2,1,3-Benzothiadiazole-based copolymers and their photovoltaic applications. Macromolecules 2009, 42, 4977–4984. [Google Scholar] [CrossRef]
- Abbotto, A.; Calderon, E.H.; Dangate, M.S.; de Angelis, F.; Manfredi, N.; Mari, C.M.; Marinzi, C.; Mosconi, E.; Muccini, M.; Ruffo, R.; et al. Pyridine-EDOT heteroarylene-vinylene donor-acceptor polymers. Macromolecules 2010, 43, 9698–9713. [Google Scholar] [CrossRef]
- Abbotto, A.; Seri, M.; Dangate, M.S.; de Angelis, F.; Manfredi, N.; Mosconi, E.; Bolognesi, M.; Ruffo, R.; Salamone, M.M.; Muccini, M. A vinylene-linked benzo[1,2-b:4,5-b']dithiophene-2,1,3-benzothiadiazole low-bandgap polymer. J. Polym. Sci. A Polym. Chem. 2012, 50, 2829–2840. [Google Scholar] [CrossRef]
- Cardone, A.; Martinelli, C.; Losurdo, M.; Dilonardo, E.; Bruno, G.; Scavia, G.; Destri, S.; Cosma, P.; Salamandra, L.; Reale, A.; et al. Fluoro-Functionalization of vinylene units in a polyarylenevinylene for polymer solar cells. J. Mater. Chem. A 2013, 1, 715–727. [Google Scholar] [CrossRef]
- Piacenza, M.; Comoretto, D.; Burger, M.; Morandi, V.; Marabelli, F.; Martinelli, C.; Farinola, G.M.; Cardone, A.; Gigli, G.; Della Sala, F. Raman spectra of poly(p-phenylenevinylene)s with fluorinated vinylene units: Evidence of inter-ring distortion. ChemPhysChem 2009, 10, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G.; Babudri, F.; Cardone, A.; Martinelli, C.; Farinola, G.M.; Naso, F.; Büchel, M. Impact of fluorinated vinylene units on supramolecular organization and optical properties of poly(p-phenylenedifluorovinylene) thin films as a class of blue band gap conjugated polymers. Polymer 2008, 49, 4133–4140. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Cardone, A.; Martinelli, C.; Farinola, G.M.; Babudri, F.; Naso, F.; Büchel, M.; Bruno, G. Blue-gap poly(p-phenylenevinylene)s with fluorinated double bonds: interplay between supramolecular organization and optical properties in thin films. Adv. Mater. 2009, 21, 1115–1120. [Google Scholar] [CrossRef]
- Scherf, U.; List, E.J.W. Semiconducting polyfluorenes-Towards reliable structure-property relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Grice, A.W.; Bradley, D.D.C.; Bernius, M.T.; Inbasekaran, M.; Wu, W.W.; Woo, E.P. High brightness and efficiency blue light-emitting polymer diodes. Appl. Phys. Lett. 1998, 73, 629–631. [Google Scholar] [CrossRef]
- Parker, L.D.; Pei, Q.; Marrocco, M. Efficient blue electroluminescence from a fluorinated polyquinoline. Appl. Phys. Lett. 1994, 65, 1272–1274. [Google Scholar] [CrossRef]
- Leuze, M.; Hohloch, M.; Hanack, M. Electrochemical investigation on PPV model compounds. Chem. Mater. 2002, 14, 3339–3342. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martinelli, C.; Farinola, G.M.; Pinto, V.; Cardone, A. Synthetic Aspects and Electro-Optical Properties of Fluorinated Arylenevinylenes for Luminescence and Photovoltaics. Materials 2013, 6, 1205-1236. https://doi.org/10.3390/ma6041205
Martinelli C, Farinola GM, Pinto V, Cardone A. Synthetic Aspects and Electro-Optical Properties of Fluorinated Arylenevinylenes for Luminescence and Photovoltaics. Materials. 2013; 6(4):1205-1236. https://doi.org/10.3390/ma6041205
Chicago/Turabian StyleMartinelli, Carmela, Gianluca M. Farinola, Vita Pinto, and Antonio Cardone. 2013. "Synthetic Aspects and Electro-Optical Properties of Fluorinated Arylenevinylenes for Luminescence and Photovoltaics" Materials 6, no. 4: 1205-1236. https://doi.org/10.3390/ma6041205