Fabrication of Cellulose Film with Enhanced Mechanical Properties in Ionic Liquid 1-Allyl-3-methylimidaxolium Chloride (AmimCl)
Abstract
:1. Introduction
2. Results and Discussion
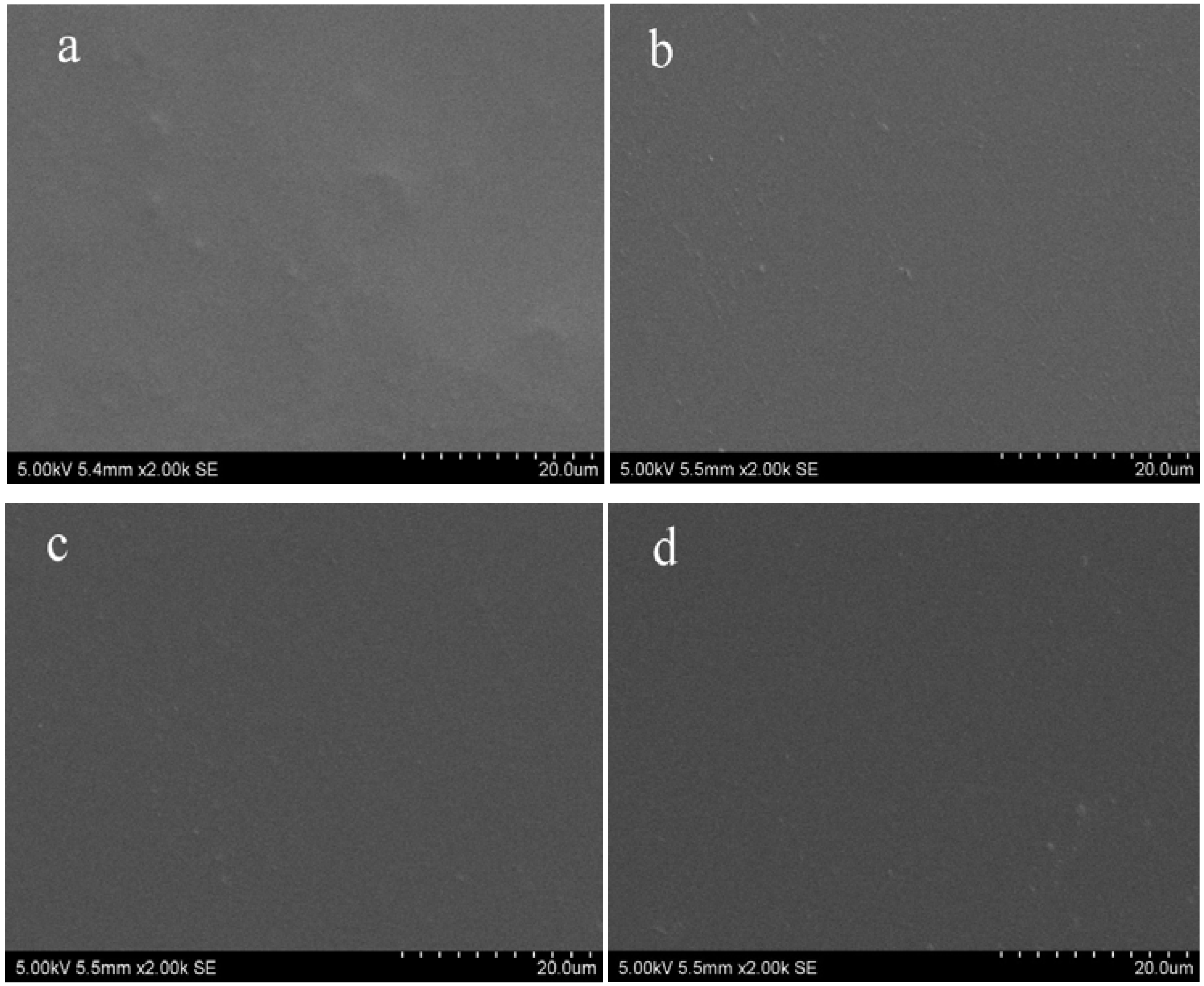
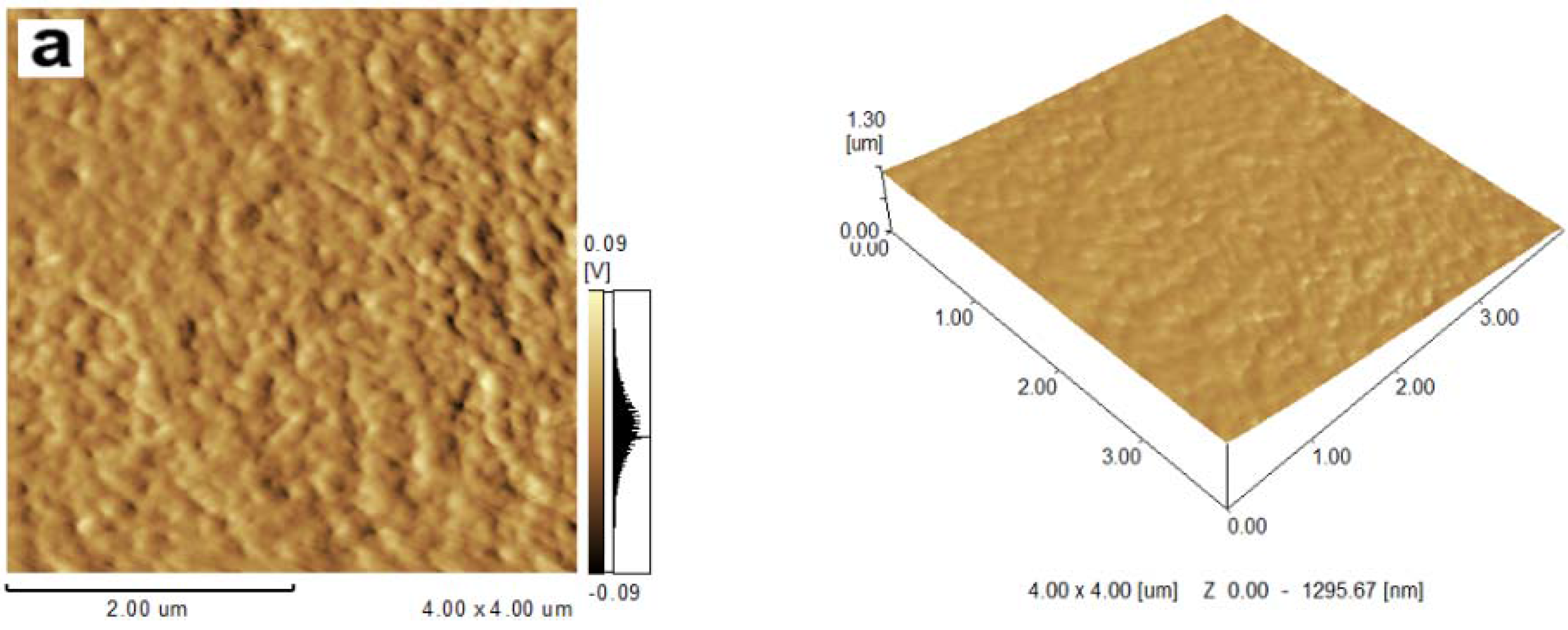
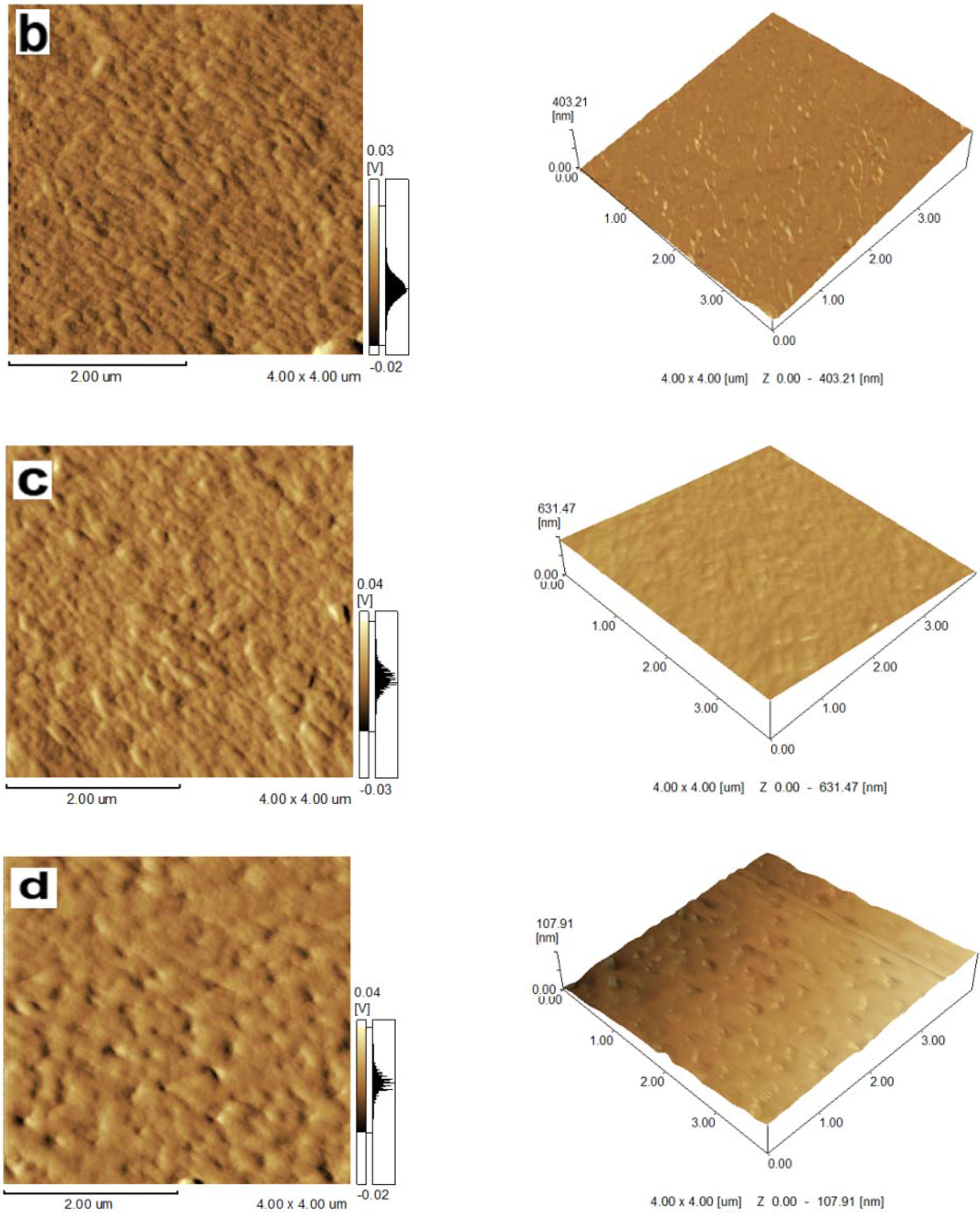
2.1. Topography of Films
2.2. Water Contact Angle
| Samples | Contact angle (degree/◦) |
|---|---|
| Control sample | 36.4 |
| Cellulose film plasticized with sorbitol | 59.4 |
| Cellulose film plasticized with glycerol | 74.4 |
| Cellulose film plasticized with CMC | 74.7 |
2.3. FTIR Spectra

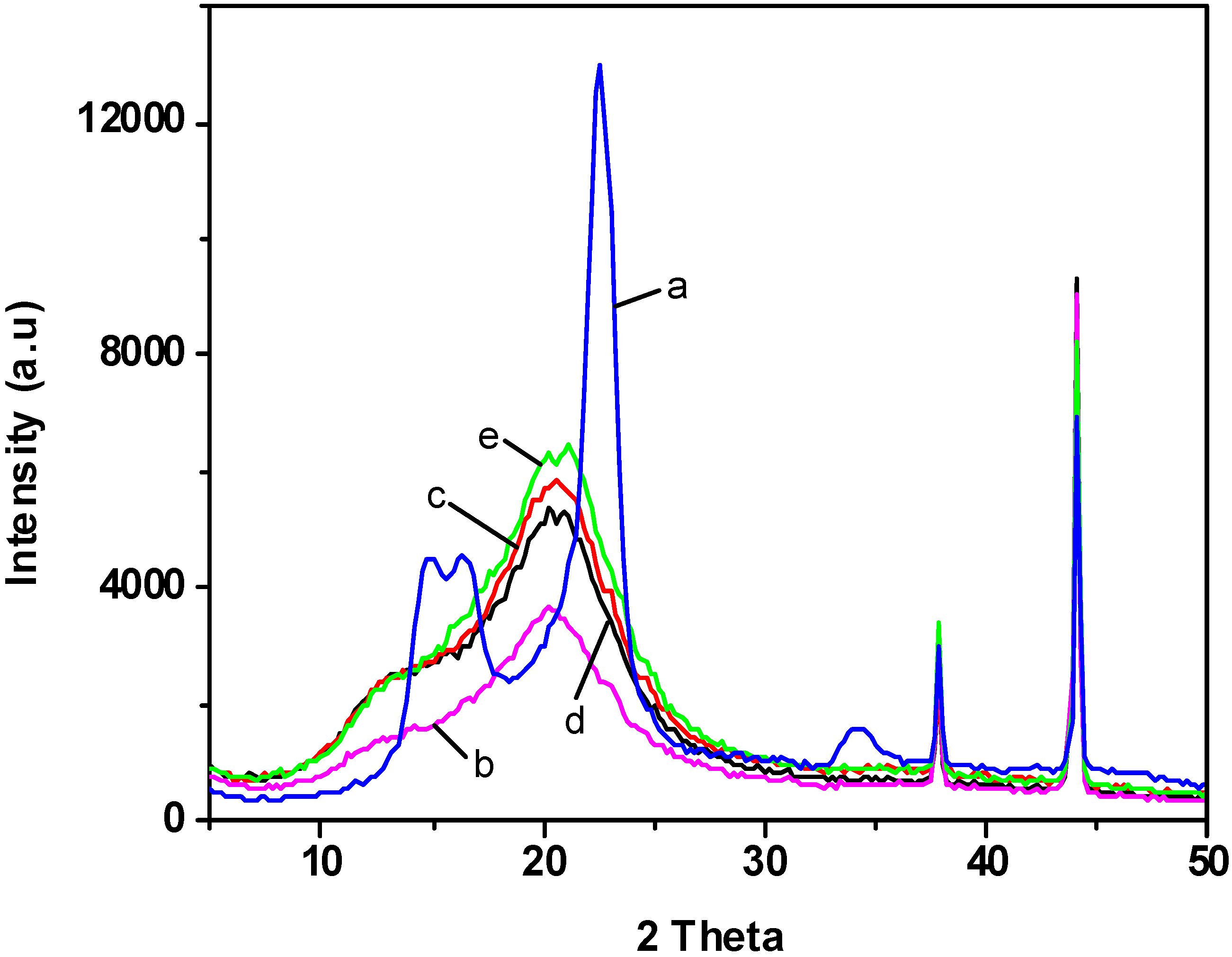
2.4. X-ray Diffraction
2.5. Thermogravimetric Analysis
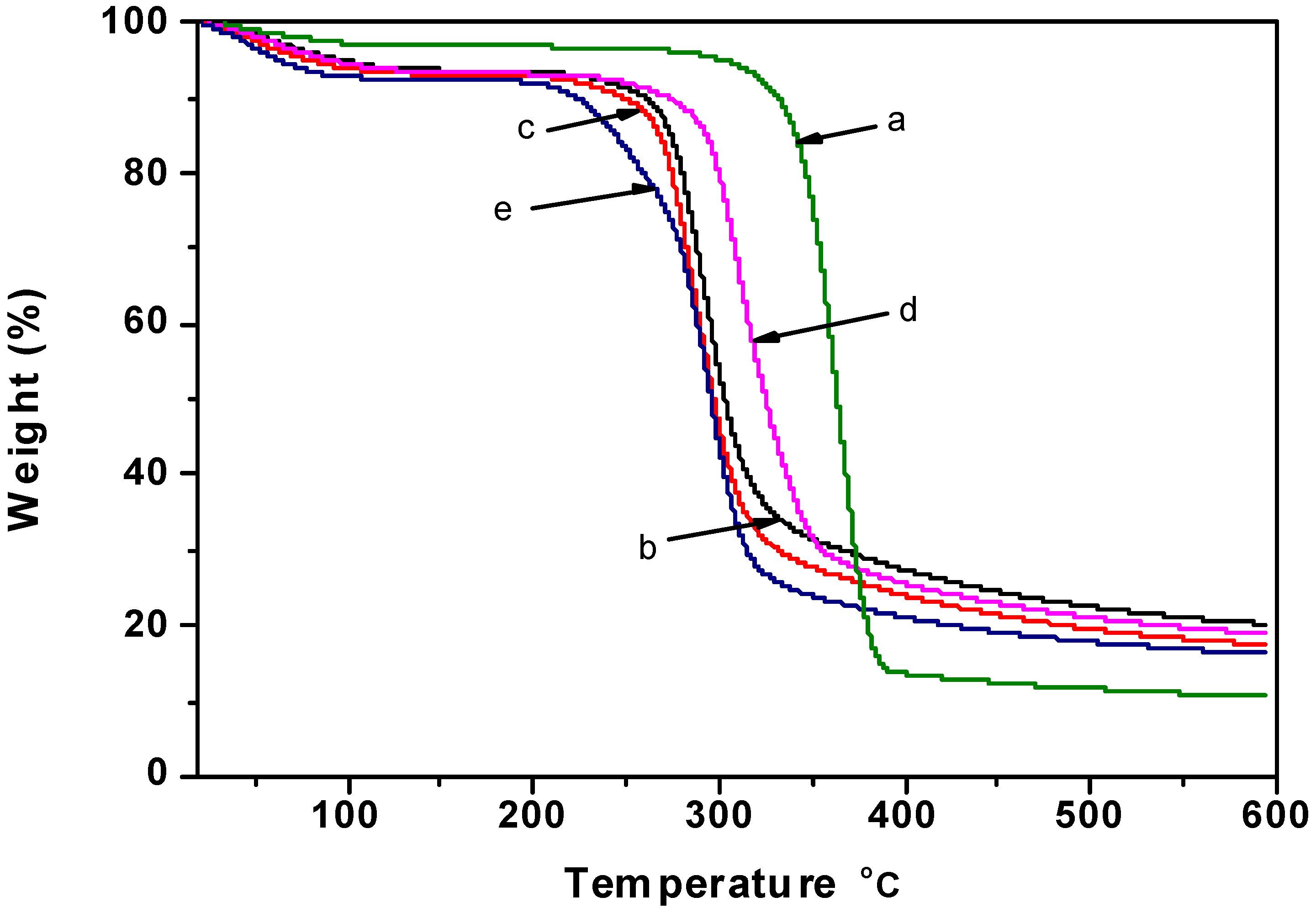
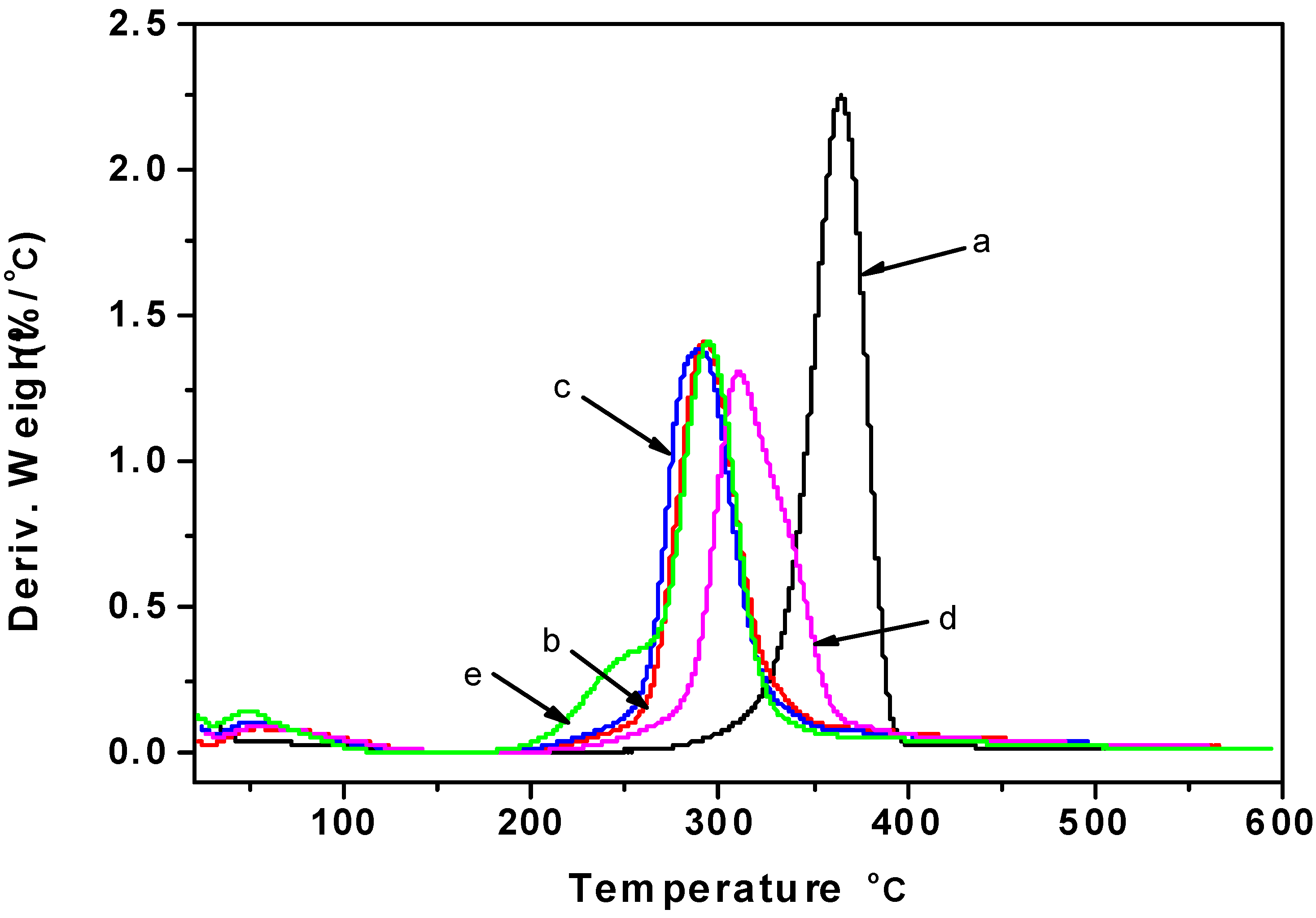
2.6. 13C CP/MAS NMR Spectra
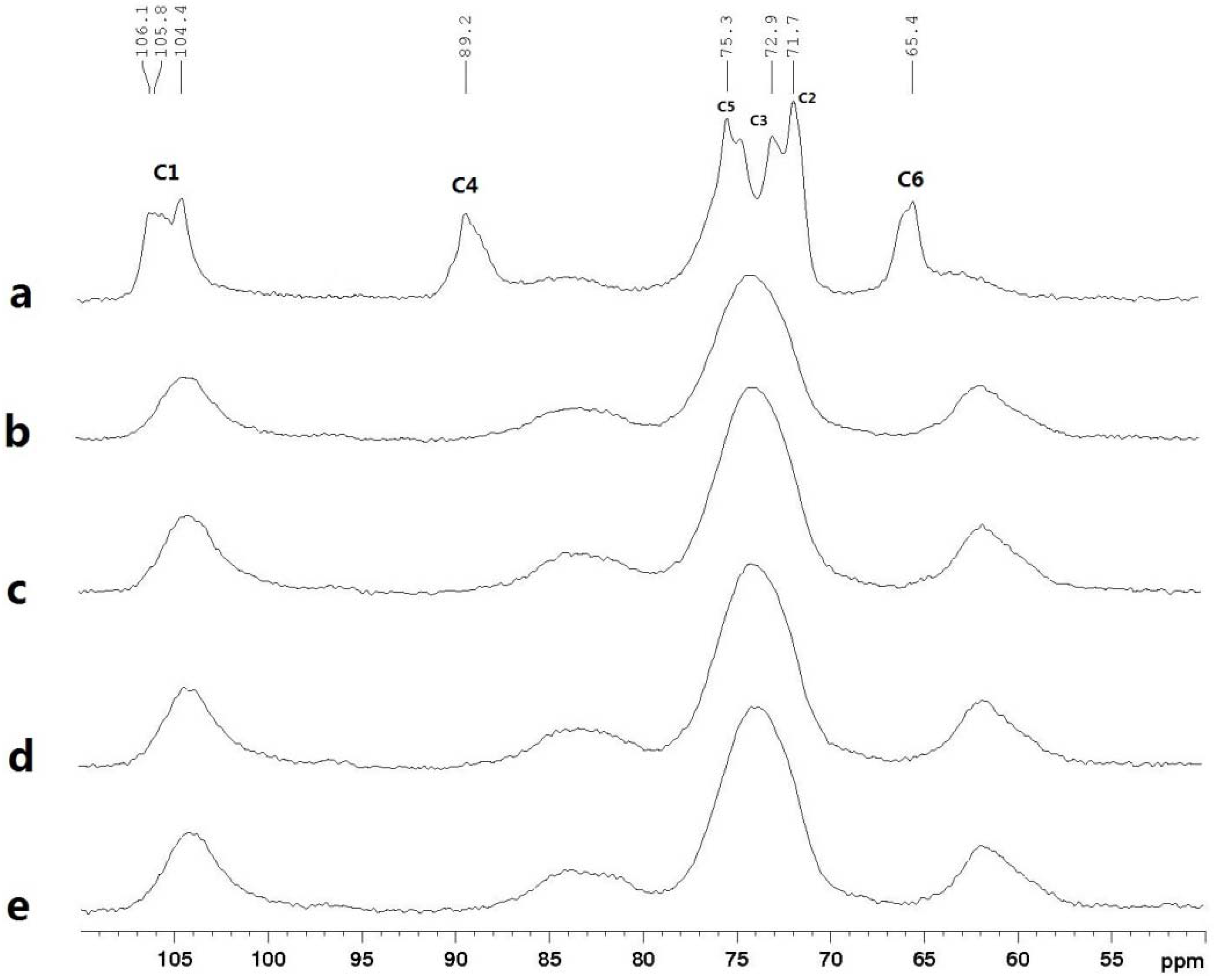
2.7. Mechanical Properties

3. Experimental Section
3.1. Materials
3.2. Preparation of Regenerated Cellulose Film
3.3. Characterization
3.3.1. Thickness
3.3.2. FTIR Spectra
3.3.3. Scanning Electron Microscopy
3.3.4. Atomic Force Microscopy
3.3.5. X-ray Diffraction
3.3.6. Thermogravimetric Analysis
3.3.7. 13C CP/MAS NMR Spectra
3.3.8. Tensile Strength Test
4. Conclusions
Acknowledgments
References
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Rodriguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Rogers, R.D. Ionic liquid-based preparation of cellulose-dendrimer films as solid supports for enzyme immobilization. Biomacromolecules 2008, 9, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.B.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules 2004, 5, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.S.; Chang, C.Y.; Zhang, L. Properties and applications of biodegradable transparent and photoluminescent cellulose films prepared via a green process. Green Chem. 2009, 11, 177–184. [Google Scholar] [CrossRef]
- Zhang, X.M.; Feng, J.X.; Liu, X.Q.; Zhu, J. Preparation and characterization of regenerated cellulose/poly (vinylidene fluoride) (PVDF) blend films. Carbohydr. Polym. 2012, 89, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Schlufter, K.; Liebert, T.; Heinze, T.; Budtova, T. Rheological properties of cellulose/ionic liquid solutions: From dilute to concentrated states. Biomacromolecules 2009, 10, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, S.; Wahit, M.U.; Ismail, A.F.; Yussuf, A.A. Preparation of regenerated cellulose/montmorillonite nanocomposite films via ionic liquids. Carbohydr. Polym. 2012, 88, 1251–1257. [Google Scholar] [CrossRef]
- Uragami, T.; Ohsumi, Y.; Sugihara, M. Studies on syntheses and permeabilities of special polymer membranes: 35. Preparation and permeation characteristics of chitin membranes. Polymer 1981, 22, 1155–1156. [Google Scholar] [CrossRef]
- Fink, H.P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from nmmo-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Le Moigne, N.; Navard, P. Dissolution mechanisms of wood cellulose fibres in naoh-water. Cellulose 2010, 17, 31–45. [Google Scholar]
- Cao, Y.; Wu, J.; Zhang, J.; Li, H.Q.; Zhang, Y.; He, J.S. Room temperature ionic liquids (RTILS): A new and versatile platform for cellulose processing and derivatization. Chem. Eng. J. 2009, 147, 13–21. [Google Scholar] [CrossRef]
- Matsumoto, H.; Yanagida, M.; Tanimoto, K.; Nomura, M.; Kitagawa, Y.; Miyazaki, Y. Highly conductive room temperature molten salts based on small trimethylalkylammonium cations and bis(trifluoromethylsulfonyl)imide. Chem. Lett. 2000, 922–923. [Google Scholar]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Fukaya, Y.; Sugimoto, A.; Ohno, H. Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formates. Biomacromolecules 2006, 7, 3295–3297. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Z.I. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Addition of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide to improve the thermal stability of regenerated cellulose. J. Appl. Polym. Sci. 2011, 121, 750–755. [Google Scholar] [CrossRef]
- Xiong, R.Y.; Hameed, N.; Guo, Q.P. Cellulose/polycaprolactone blends regenerated from ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2012, 90, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Zhang, J.; He, J.S. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.S.; Ren, Q.; Guo, M. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.G.; Zhang, Z.N.; Wu, J.; Zhang, J.; He, H.S. Regenerated-cellulose/multiwalled-carbon-nanotube composite fibers with enhanced mechanical properties prepared with the ionic liquid 1-allyl-3-methylimidazolium chloride. Adv. Mater. 2007, 19, 698–704. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Yi, C.; Kim, J. Electrical and electromechanical properties of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide-blended cellulose. Ionics 2011, 17, 41–47. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Influence of residual ionic liquid on the thermal stability and electromechanical behavior of cellulose regenerated from 1-ethyl-3-methylimidazolium acetate. Fibers Polym. 2012, 13, 289–294. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Takegawa, A.; Murakami, M.; Kaneko, Y.; Kadokawa, J. Preparation of chitin/cellulose composite gels and films with ionic liquids. Carbohydr. Polym. 2010, 79, 85–90. [Google Scholar] [CrossRef]
- Lawton, J.W.; Fanta, G.F. Glycerol-plasticized films prepared from starch poly(vinyl alcohol) mixtures—Effect of poly(ethylene-co-acrylic acid). Carbohydr. Polym. 1994, 23, 275–280. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.S.; Laine, J.; Rojas, O.J. Modification of cellulose films by adsorption of CMC and chitosan for controlled attachment of biomolecules. Biomacromolecules 2011, 12, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.W.; Ren, J.L.; Zhong, L.X.; Sun, R.C. Nanocomposite films based on xylan-rich hemicelluloses and cellulose nanofibers with enhanced mechanical properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Hameed, N.; Guo, Q.P.; Tay, F.H.; Kazarian, S.G. Blends of cellulose and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) prepared from the ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2011, 86, 94–104. [Google Scholar] [CrossRef]
- Yin, J.B.; Luo, K.; Chen, X.S.; Khutoryanskiy, V.V. Miscibility studies of the blends of chitosan with some cellulose ethers. Carbohydr. Polym. 2006, 63, 238–244. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Carrillo, A.; Colom, X.; Sunol, J.J.; Saurina, J. Structural ftir analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Siroky, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance fourier-transform infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Mathew, A.P.; Oksman, K. All-cellulose composites by partial dissolution in the ionic liquid 1-butyl-3-methylimidazolium chloride. Compos. A Appl. S 2009, 40, 2031–2037. [Google Scholar] [CrossRef]
- Han, D.L.; Yan, L.F. Preparation of all-cellulose composite by selective dissolving of cellulose surface in PEG/NaOH aqueous solution. Carbohydr. Polym. 2010, 79, 614–619. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal structure and hydrogen bonding system in cellulose I (alpha), from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef] [PubMed]
- Langan, P.; Nishiyama, Y.; Chanzy, H. A revised structure and hydrogen-bonding system in cellulose ii from a neutron fiber diffraction analysis. J. Am. Chem. Soc. 1999, 121, 9940–9946. [Google Scholar] [CrossRef]
- Ishikawa, A.; Okano, T.; Sugiyama, J. Fine structure and tensile properties of ramie fibres in the crystalline form of cellulose I, II, III1 and IV1. Polymer 1997, 38, 463–468. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.P.; Yan, R.; Chen, H.P.; Lee, D.H.; Zheng, C.G. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Horii, F.; Hirai, A.; Kitamaru, R. CP/MAS C-13 nmr-spectra of the crystalline components of native celluloses. Macromolecules 1987, 20, 2117–2120. [Google Scholar] [CrossRef]
- Hesse, S.; Jager, C. Determination of the C-13 chemical shift anisotropies of cellulose I and cellulose II. Cellulose 2005, 12, 5–14. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, B.; Li, H.S.; Li, Y.Q.; Ou, S.Y. Green composite films composed of nanocrystalline cellulose and a cellulose matrix regenerated from functionalized ionic liquid solution. Carbohydr. Polym. 2011, 84, 383–389. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pang, J.; Liu, X.; Zhang, X.; Wu, Y.; Sun, R. Fabrication of Cellulose Film with Enhanced Mechanical Properties in Ionic Liquid 1-Allyl-3-methylimidaxolium Chloride (AmimCl). Materials 2013, 6, 1270-1284. https://doi.org/10.3390/ma6041270
Pang J, Liu X, Zhang X, Wu Y, Sun R. Fabrication of Cellulose Film with Enhanced Mechanical Properties in Ionic Liquid 1-Allyl-3-methylimidaxolium Chloride (AmimCl). Materials. 2013; 6(4):1270-1284. https://doi.org/10.3390/ma6041270
Chicago/Turabian StylePang, Jinhui, Xin Liu, Xueming Zhang, Yuying Wu, and Runcang Sun. 2013. "Fabrication of Cellulose Film with Enhanced Mechanical Properties in Ionic Liquid 1-Allyl-3-methylimidaxolium Chloride (AmimCl)" Materials 6, no. 4: 1270-1284. https://doi.org/10.3390/ma6041270





