Dehydration Process of Hofmann-Type Layered Solids
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis Procedure
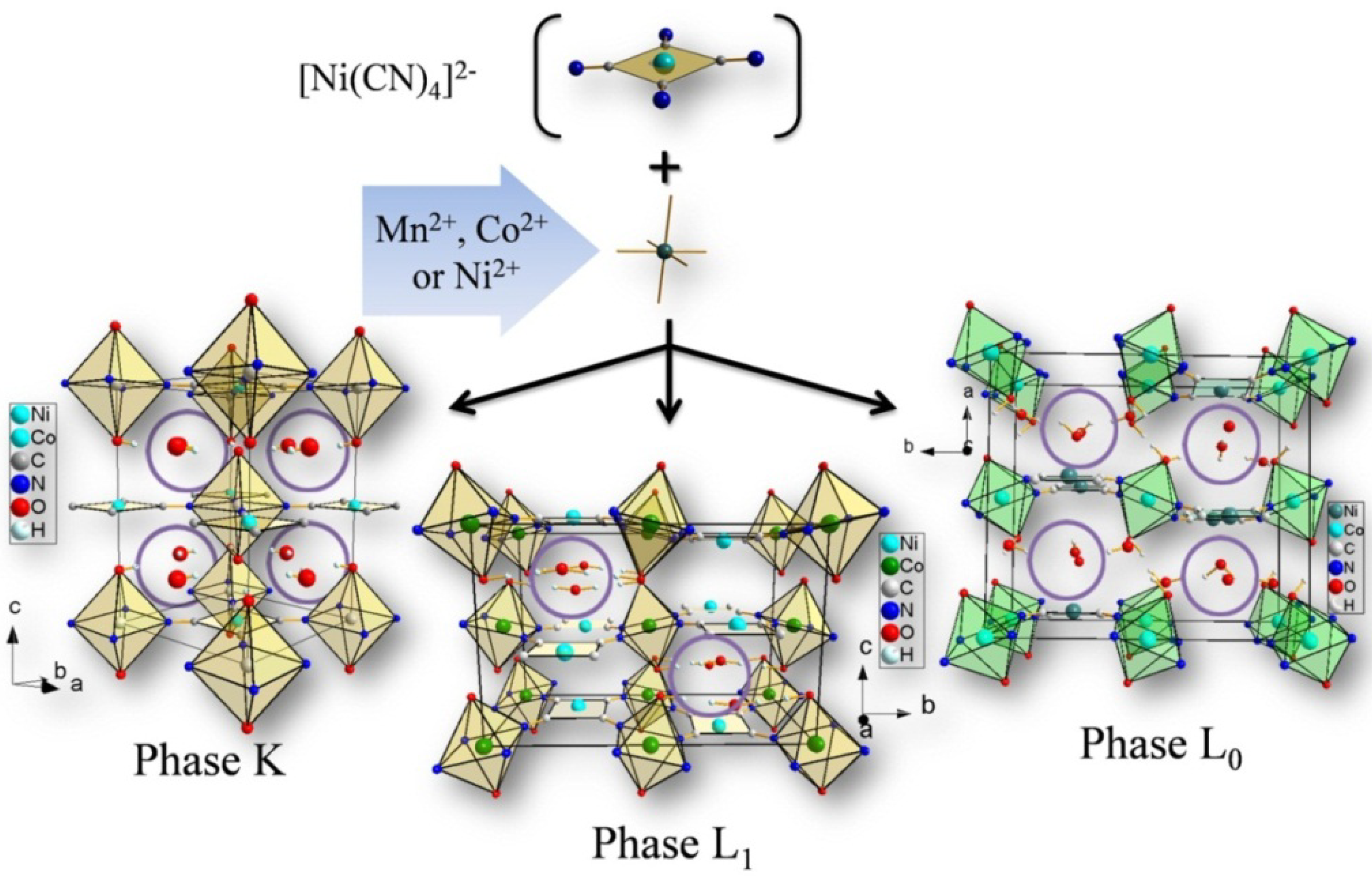
2.2.X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM)
2.3.Hi-Resolution and Modulated Thermogravimetric Analysis
3. Results and Discussion
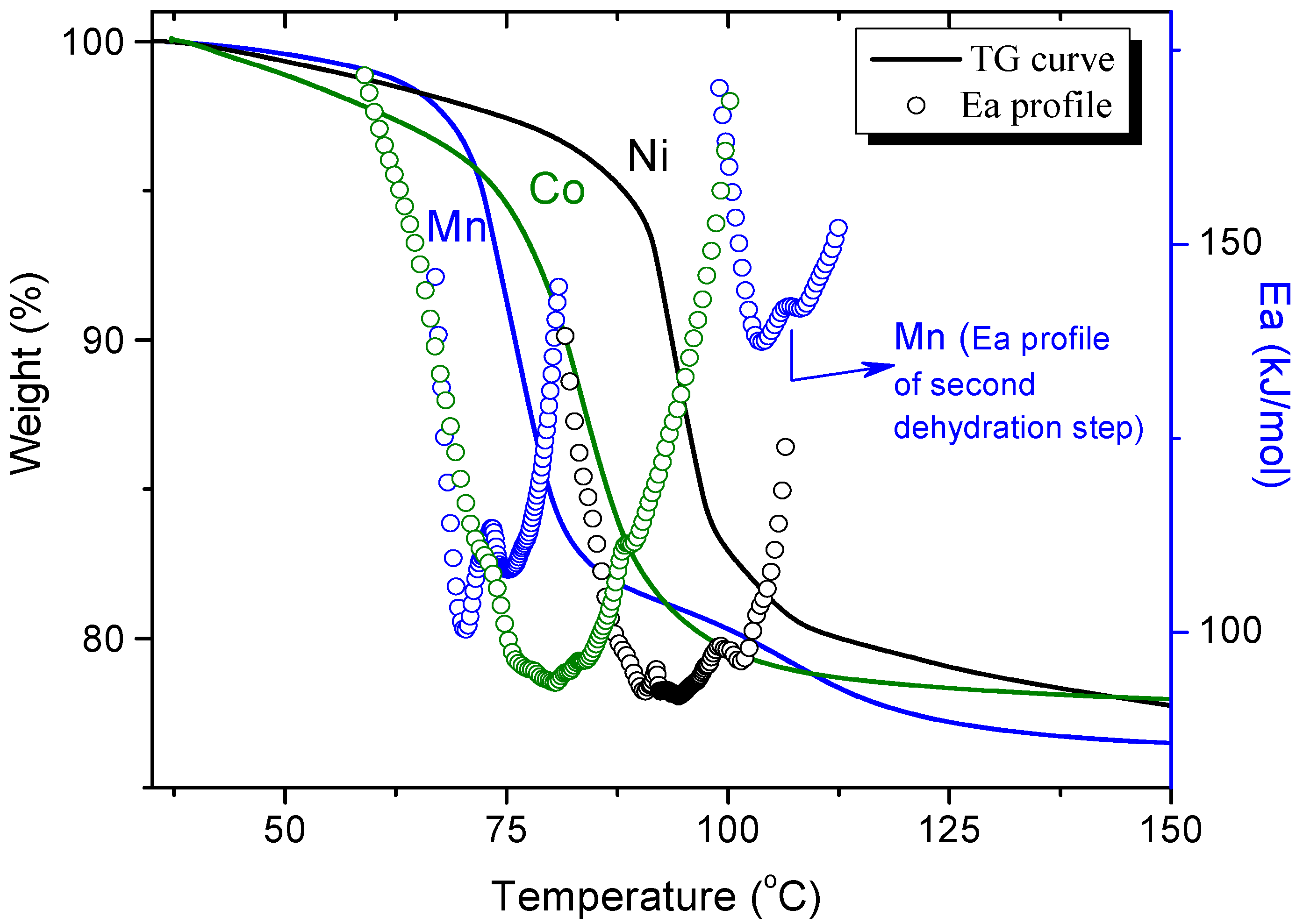
3.1. Dehydration with Different Transition Metals in the Same Structure Phase
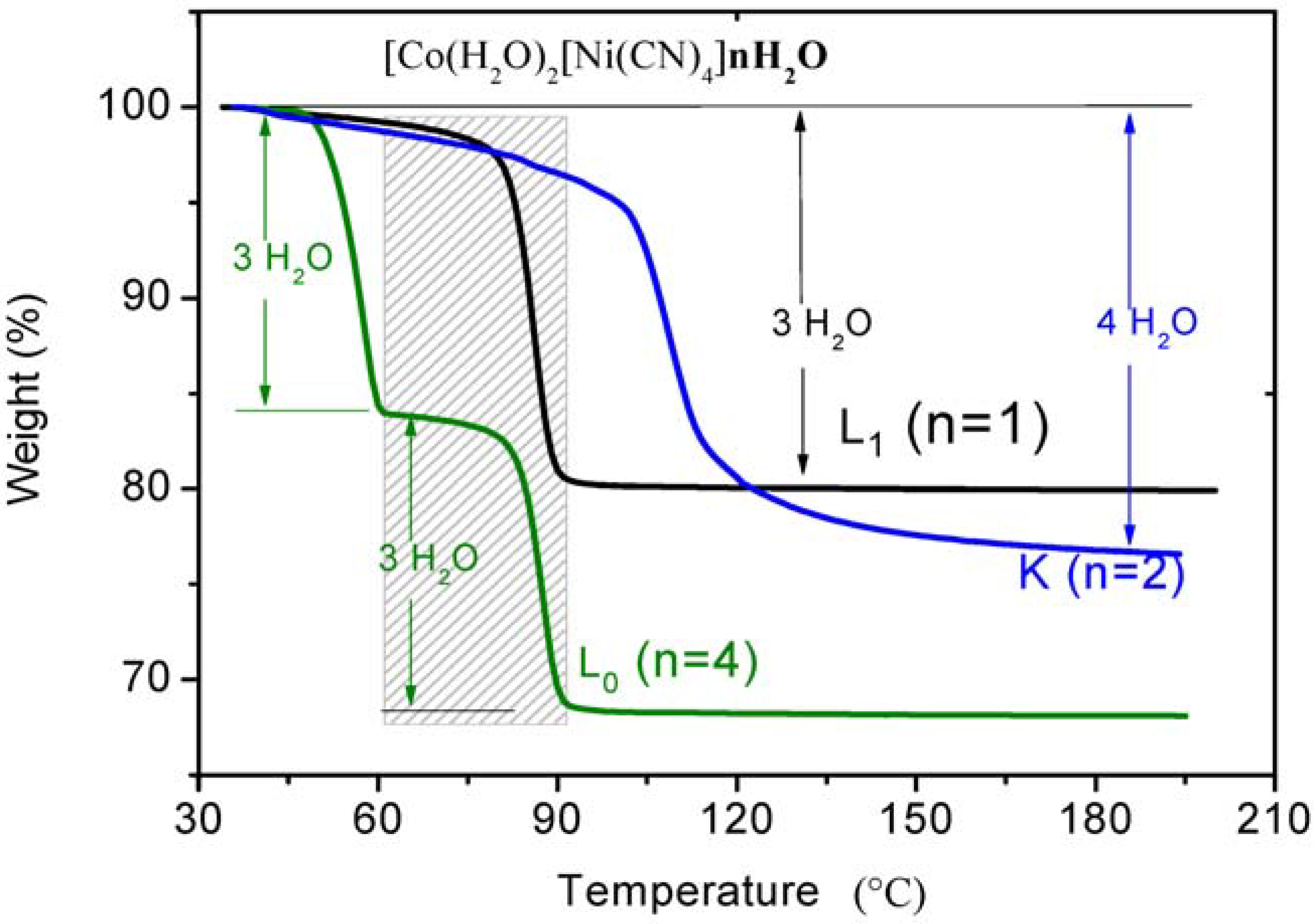
3.2. Dehydration with the Same Transition Metals in Different Phases
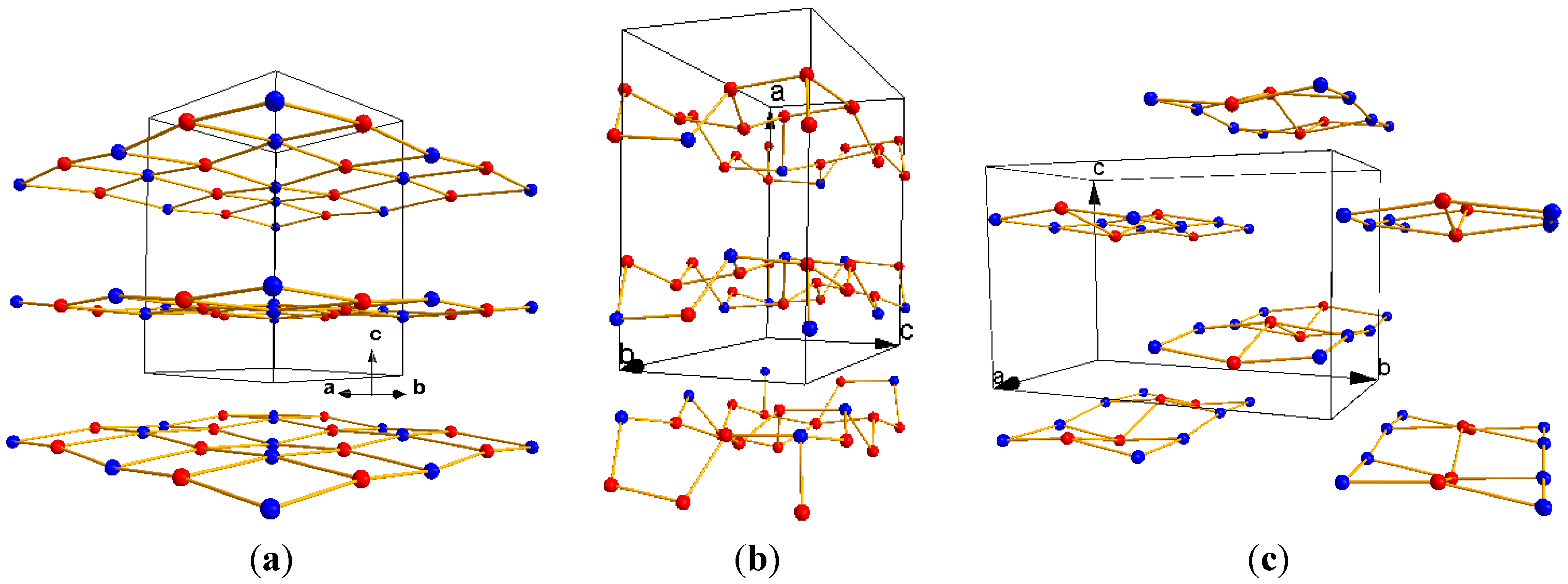
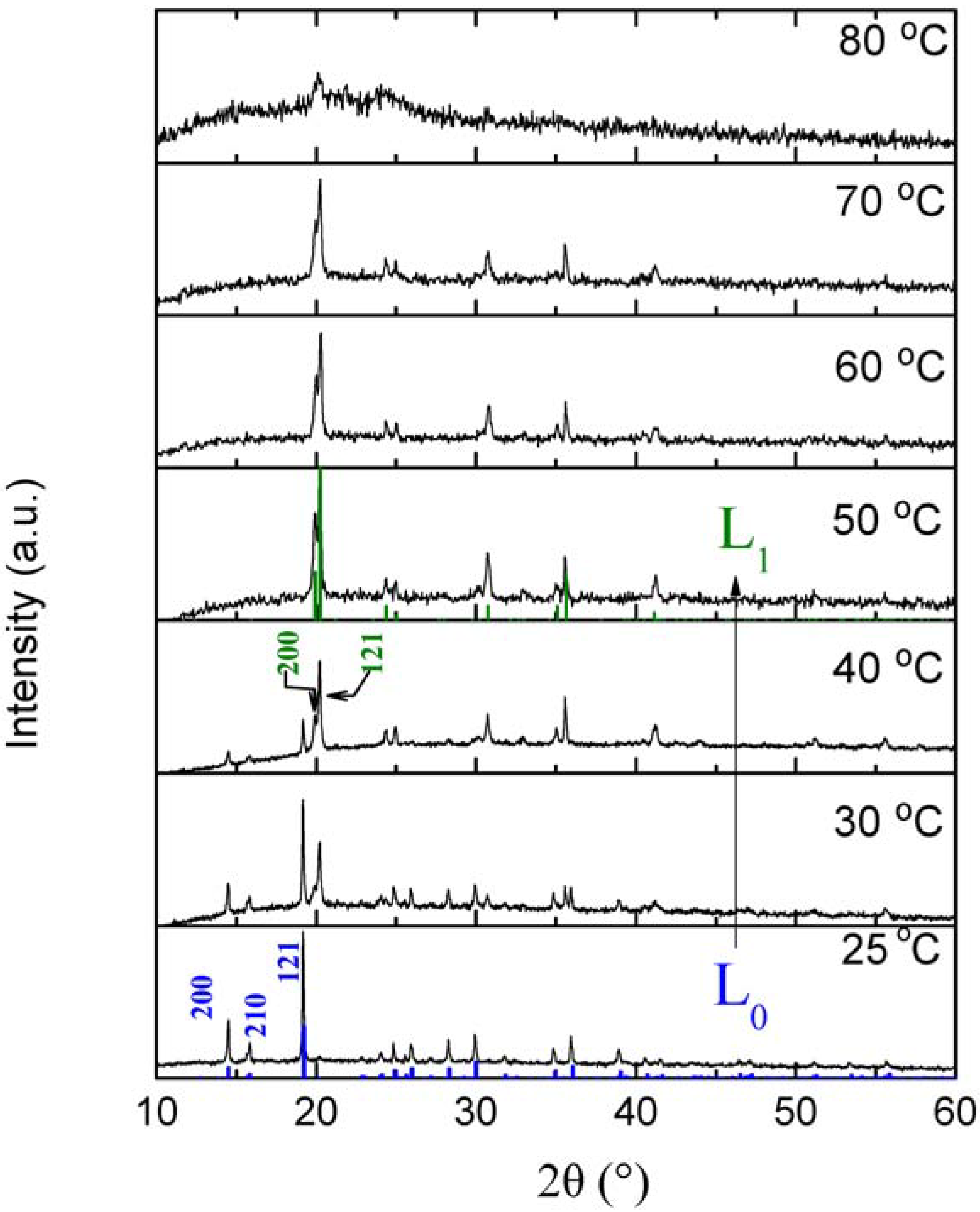
| L0 phase | L1 phase | |
|---|---|---|
| Cell Parameters (Å) | a = 12.195(2) | a = 7.115(2) |
| b = 13.885(3) | b = 14.264(3) | |
| c = 7.143(3) | c = 8.898(1) | |
| Volume (Å3) | 1209.5(3) | 903.0(2) |

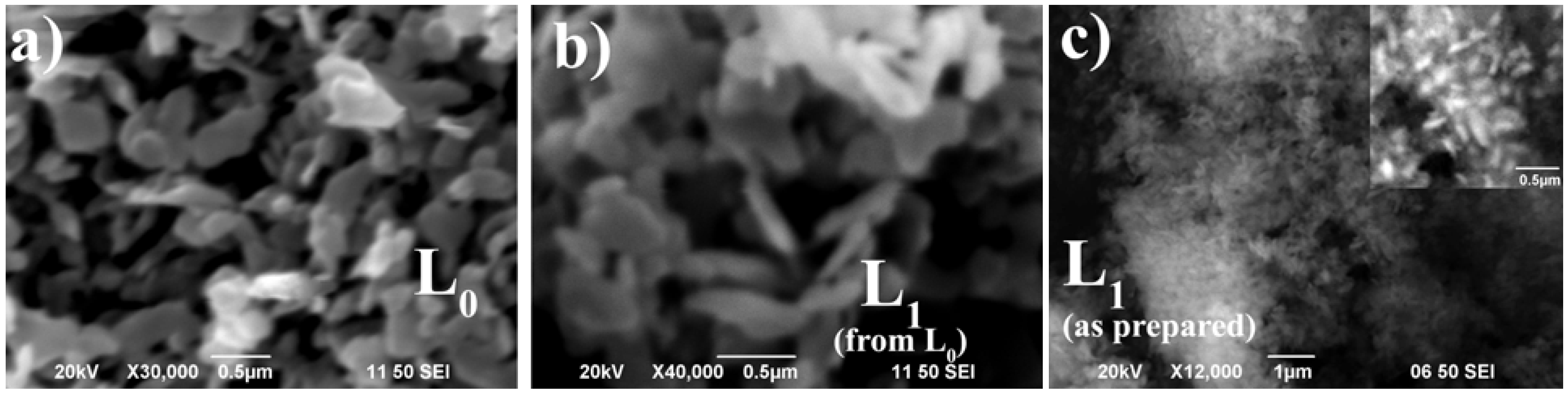

3.3. The Influence of Water Partial Pressure
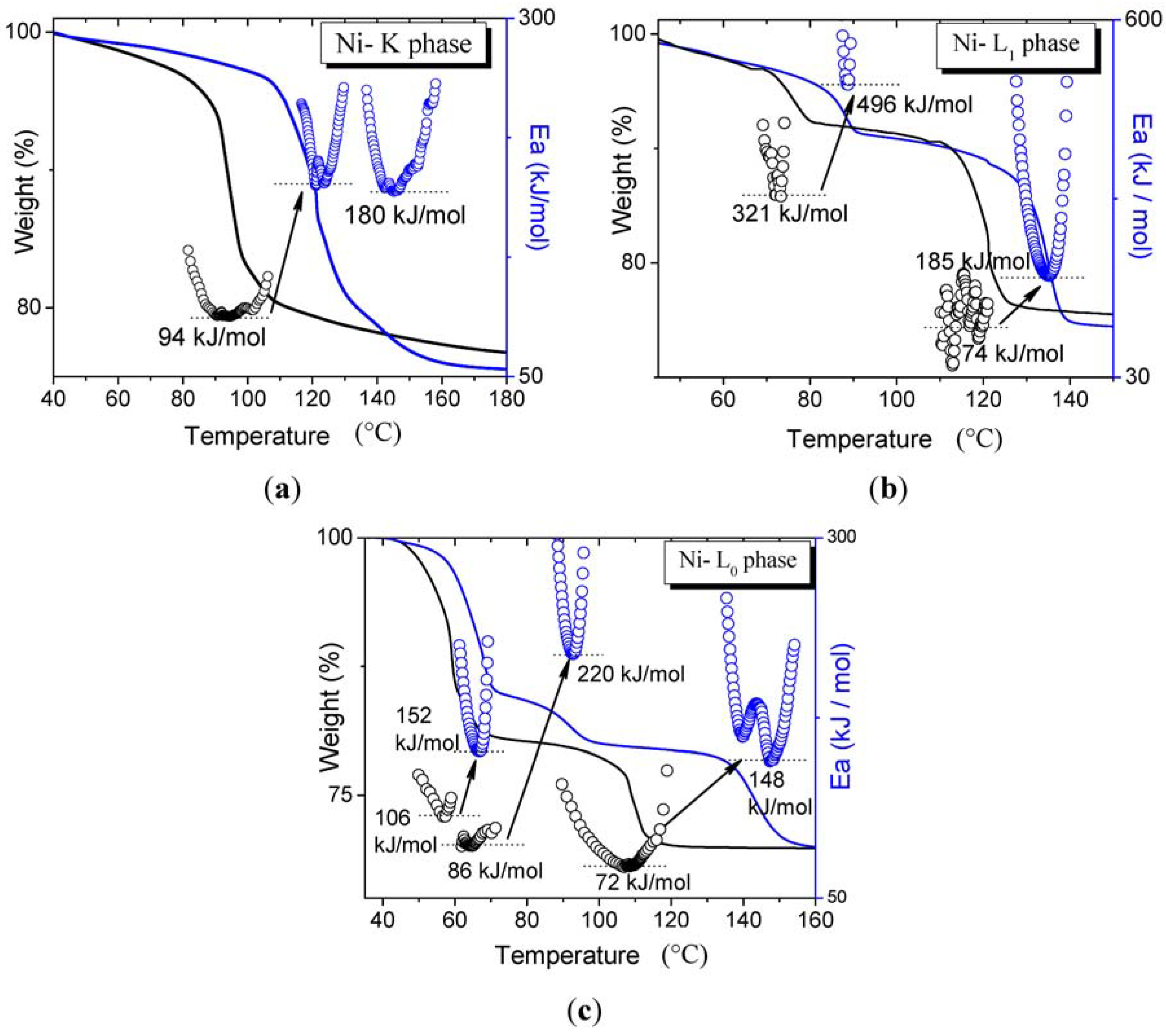
4. Conclusions
Acknowledgments
References
- Férey, G. Microporous solids: From organically templated inorganic skeletons to hybrid frameworks...ecumenism in chemistry. Chem. Mater. 2001, 13, 3084–3098. [Google Scholar] [CrossRef]
- Paul, A.; Wright, M.D. Microporous Framework Solids; RSC Materials Monographs; Royal Society of Chemistry: London, UK, 2008; Volume 4. [Google Scholar]
- Chang, C.D.; Silvestri, A.J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 1977, 47, 249–259. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, catenation, and variation of the metal oxide and organic. Linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Côté, P.; Benin, A.I.; Ockwig, N.W.; Keeffe, M.O.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- El-Kaderi, H.M.; Yaghi, O.M.; Hunt, J.R.; Mendoza-Cortes, J.L.; Côté, A.P.; Taylor, R.E.; Keeffe, M.O. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Wang, Y.; Leng, Y.; Xie, L.; Li, X. Hydrogen storage properties of [M(Py){Ni(CN)4}] (M = Fe, Co, Ni). Int. J. Hydrog. Energy 2007, 32, 3411–3415. [Google Scholar] [CrossRef]
- Culp, J.T.; Natesakhawat, S.; Smith, M.R.; Bittner, E.; Matranga, C.; Bockrath, B.Y. Hydrogen storage properties of rigid three-dimensional hofmann clathrate derivatives: The effects of pore size. J. Phys. Chem. C 2008, 112, 7079–7083. [Google Scholar] [CrossRef]
- Culp, J.T.; Smith, M.R.; Bittner, E.; Bockrath, B. Hysteresis in the physisorption of CO2 and N2 in a flexible pillared layer nickel cyanide. J. Am. Chem. Soc. 2008, 130, 12427–12434. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, S.; Yoshikawa, H.; Sano, Y.; Iwamoto, T. Inorganic–organic hybrid molecular architectures of cyanometalate host and organic guest systems: Specific behavior of the guests. Acc. Chem. Res. 2005, 38, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Büttner, H.G.; Kearley, G.J.; Howard, C.J.; Fillaux, F. Structure of the Hofmann clathrates Ni(NH3)2Ni(CN)4·2C6D6 and Zn(NH3)2Ni(CN)4·2C6H6. Acta Cryst. 1994, B50, 431–435. [Google Scholar] [CrossRef]
- Dunbar, K.R.; Heintz, R.A. Chemistry of transition metal cyanide compounds: Modern perspectives. Inorg. Chem. 1997, 45, 283–391. [Google Scholar]
- González, M.; Lemus-Santana, A.A.; Rodríguez-Hernández, J.; Knobel, M.; Reguera, E. π–π interactions and magnetic properties in a series of hybrid inorganic–organic crystals. J. Solid State Chem. 2013, 197, 317–322. [Google Scholar] [CrossRef]
- Mathey, Y.; Mazieres, C. Les phases cyanures de nickel (II) hydrates. Can. J. Chem. 1974, 52, 3637–3644. [Google Scholar] [CrossRef]
- Lemus, A.A.; Rodriguez Hernandez, J.; del Castillo, L.F.; Basterrechea, M.; Reguera, E. Unique coordination of pyrazine in T[Ni(CN)4]·2pyz with T = Mn, Zn, Cd. J. Solid State Chem. 2009, 182, 757–766. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Lemus-Santana, A.A.; Vargas, C.N.; Reguera, E. Three structural modifications in the series of layered solids T(H2O)2[Ni(CN)4]·xH2O with T = Mn, Co, Ni: Their nature and crystal structures. Comptes Rendus Chim. 2012, 15, 350–355. [Google Scholar] [CrossRef]
- Menzinger, M.; Wolfgang, R. The meaning and use of the arrhenius activation energy. Angew. Chem. Int. Ed. 1969, 8, 438–444. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Kinetics in solids. Annu. Rev. Phys. Chem. 1997, 48, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Linnert, W. Isokinetic relationships at the thermal decomposition of tetranuclear copper(II)-complexes. Int. J. Chem. Kinet. 1995, 27, 597–604. [Google Scholar] [CrossRef]
- Bertrand, G.; Lallemant, M.; Watelle, G. Propos sur l’interpretation de l’energie d’activation experimentale Cas des decompositions endothermiques solide-gaz. J. Therm. Anal. 1978, 13, 525–542. [Google Scholar] [CrossRef]
- He, G.; Riedl, B.; Aït-Kadi, A. Model-free kinetics: Curing behavior of phenol formaldehyde resins by differential scanning calorimetry. J. Appl. Polym. Sci. 2003, 87, 433–440. [Google Scholar] [CrossRef]
- Vyazovkin, S.V.; Lesnikovich, A.I. An approach to the solution of the inverse kinetic problem in the case of complex processes: Part 1. Methods employing a series of thermoanalytical curves. Thermochim. Acta 1990, 165, 273–280. [Google Scholar] [CrossRef]
- Rodriguez-Gattorno, G.; Martinez-Hernández, A.; Aleman-Vazquez, L.O.; Torres-Garcia, E. Structural and thermal study of carbon-modified molybdenum sub-oxide catalysts. Appl. Catal. A 2007, 321, 117–124. [Google Scholar] [CrossRef]
- Darriet, B.; Rat, M.; Galy, J.; Hagenmuller, P. Sur quelques nouveaux pyrochlores des systemes MTO3 WO3 et MTO3 TeO3 (M = K, Rb, Cs, Tl; T = Nb, Ta). Mater. Res. Bull. 1971, 6, 1305–1316. [Google Scholar] [CrossRef]
- Zhang, Y. Electronegativities of elements in valence states and their applications. Inorg. Chem. 1982, 21, 3886–3889. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Holt, J.B.; Cutlert, I.B.; Wadsworth, M.E. Kinetics of the Thermal Dehydration of Hydrous Silicates. In Proceedings of the Twelfth National Conference on Clays and Clay Minerals; International Series of Monographs on Earth Sciences; Symposium Publications Division, Pergamon Press: Atlanta, GA, USA, 1964; Volume 19, pp. 55–67. [Google Scholar]
- Koga, N.; Tanaka, H. Accommodation of the actual solid-state process in the kinetic model function. I. Significance of the non-integral kinetic exponents. J. Therm. Anal. 1994, 41, 455–469. [Google Scholar] [CrossRef]
- Valente, J.S.; Rodriguez-Gattorno, G.; Valle-Orta, M.; Torres-García, E. Thermal decomposition kinetics of MgAl layered double hydroxides. Mater. Chem. Phys. 2012, 133, 621–629. [Google Scholar] [CrossRef]
- Xantheas, S.S. Cooperativity and hydrogen bonding network in water clusters. Chem. Phys. 2000, 258, 225–231. [Google Scholar] [CrossRef]
- Rivelino, R.; Canuto, S. Theoretical study of mixed hydrogen-bonded complexes: H2O–HCN–H2O and H2O–HCN–HCN–H2O. J. Phys. Chem. A 2001, 105, 11260–11265. [Google Scholar] [CrossRef]
- Luck, W.A.P. The importance of cooperativity for the properties of liquid water. J. Mol. Struct. 1998, 448, 131–142. [Google Scholar] [CrossRef]
- Saenger, W. Structure and dynamics of water surrounding biomolecules. Ann. Rev. Biophys. Biophys. Chem. 1987, 16, 93–114. [Google Scholar] [CrossRef]
- Bürgi, H.B.; Dunitz, J.D. Can statistical analysis of structural parameters from different crystal environments lead to quantitative energy relationships? Acta Cryst. B 1988, 44, 445–448. [Google Scholar] [CrossRef]
- Lemus-Santana, A.A.; Rodríguez-Hernández, J.; González, M.; Demeshko, S.; Ávila, M.; Knobel, M.; Reguera, E. Synthesis and characterization of T[Ni(CN)4]·2pyz with T = Fe, Ni; pyz = pyrazine: Formation of T-pyz–Ni bridges. J. Solid State Chem. 2011, 184, 2124–2130. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Lemus-Santana, A.A.; Ortiz-Lopez, J.; Sandoval, S.J.; Reguera, E. Low temperature structural transformation in T[Ni(CN)4]·xpyz with x = 1, 2; T = Mn, Co, Ni, Zn, Cd; pyz = pyrazine. J. Solid State Chem. 2010, 183, 105–113. [Google Scholar] [CrossRef]
- Martin, W.F.; Feller, D.; Dixon, D.A. Hydrogen bond energy of the water dimer. J. Phys. Chem. 1996, 100, 2993–2997. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, J.; Varlashkin, P.; Long, S.; Kidd, C. Dehydration, hydration behavior, and structural analysis of fenoprofen calcium. J. Pharm. Sci. 2001, 90, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.N.; Greer, R.T. Dehydration of serpentine: Heat of reaction and reaction kinetics at PH2O = 1 atm. Am. Mineral. 1965, 50, 450–464. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reyes-Martinez, O.; Torres-García, E.; Rodríguez-Gattorno, G.; Reguera, E. Dehydration Process of Hofmann-Type Layered Solids. Materials 2013, 6, 1452-1466. https://doi.org/10.3390/ma6041452
Reyes-Martinez O, Torres-García E, Rodríguez-Gattorno G, Reguera E. Dehydration Process of Hofmann-Type Layered Solids. Materials. 2013; 6(4):1452-1466. https://doi.org/10.3390/ma6041452
Chicago/Turabian StyleReyes-Martinez, Omar, Enelio Torres-García, Geonel Rodríguez-Gattorno, and Edilso Reguera. 2013. "Dehydration Process of Hofmann-Type Layered Solids" Materials 6, no. 4: 1452-1466. https://doi.org/10.3390/ma6041452





