Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials and Method
2.2. Experimental Setup and Procedure
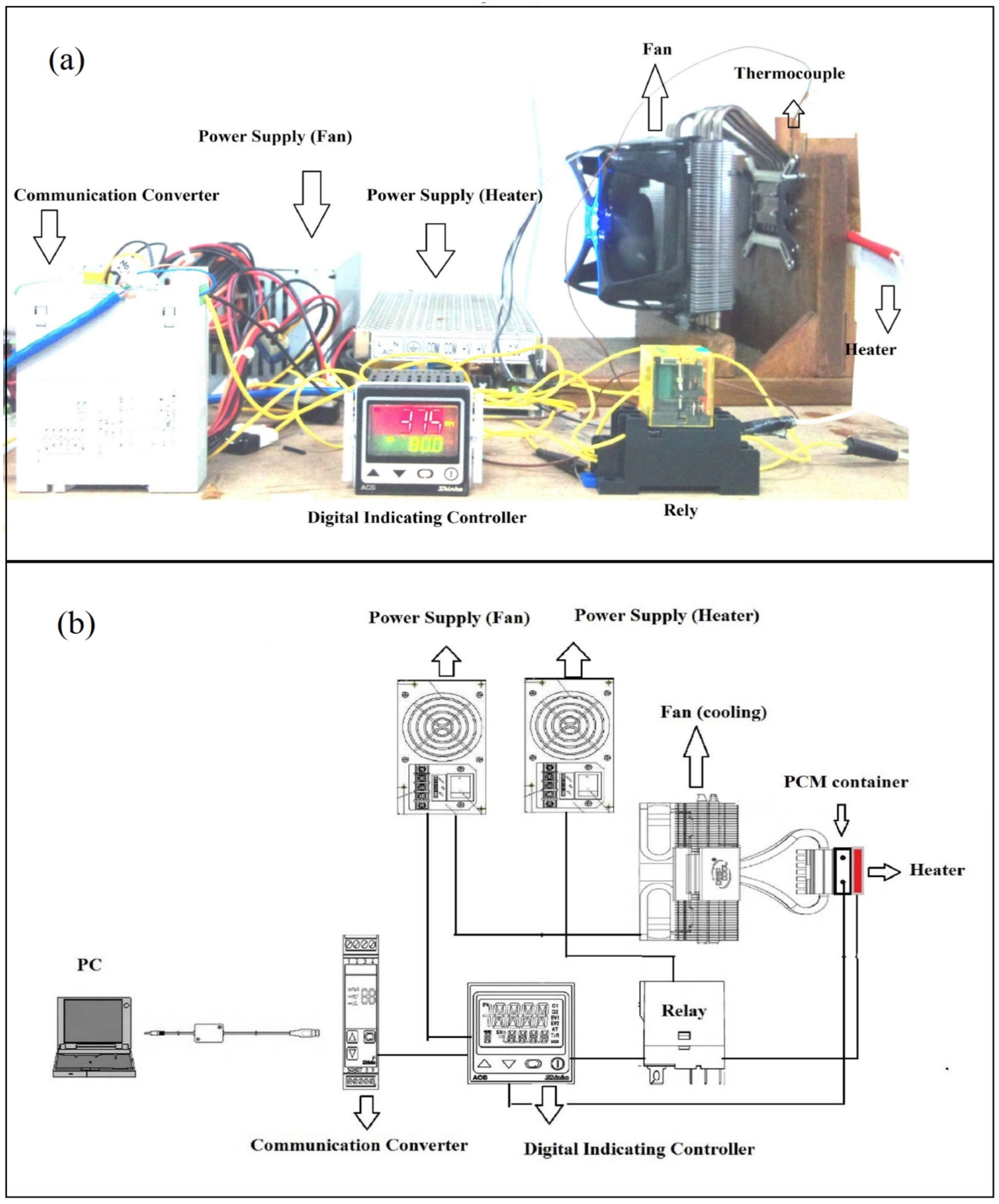
2.3. Characterization of Phase Change Material
3. Results and Discussions
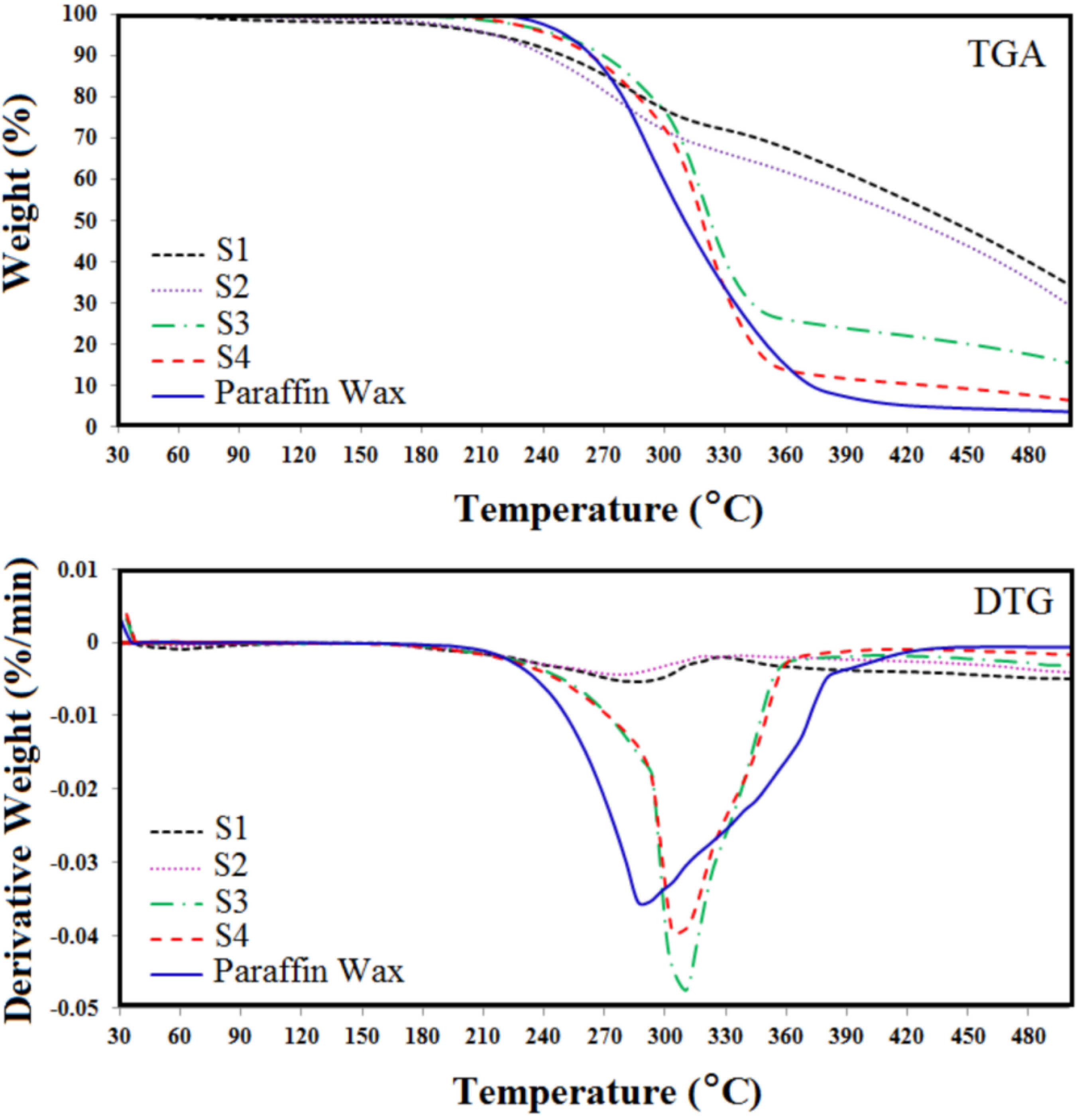
3.1. Thermogravimetry Test
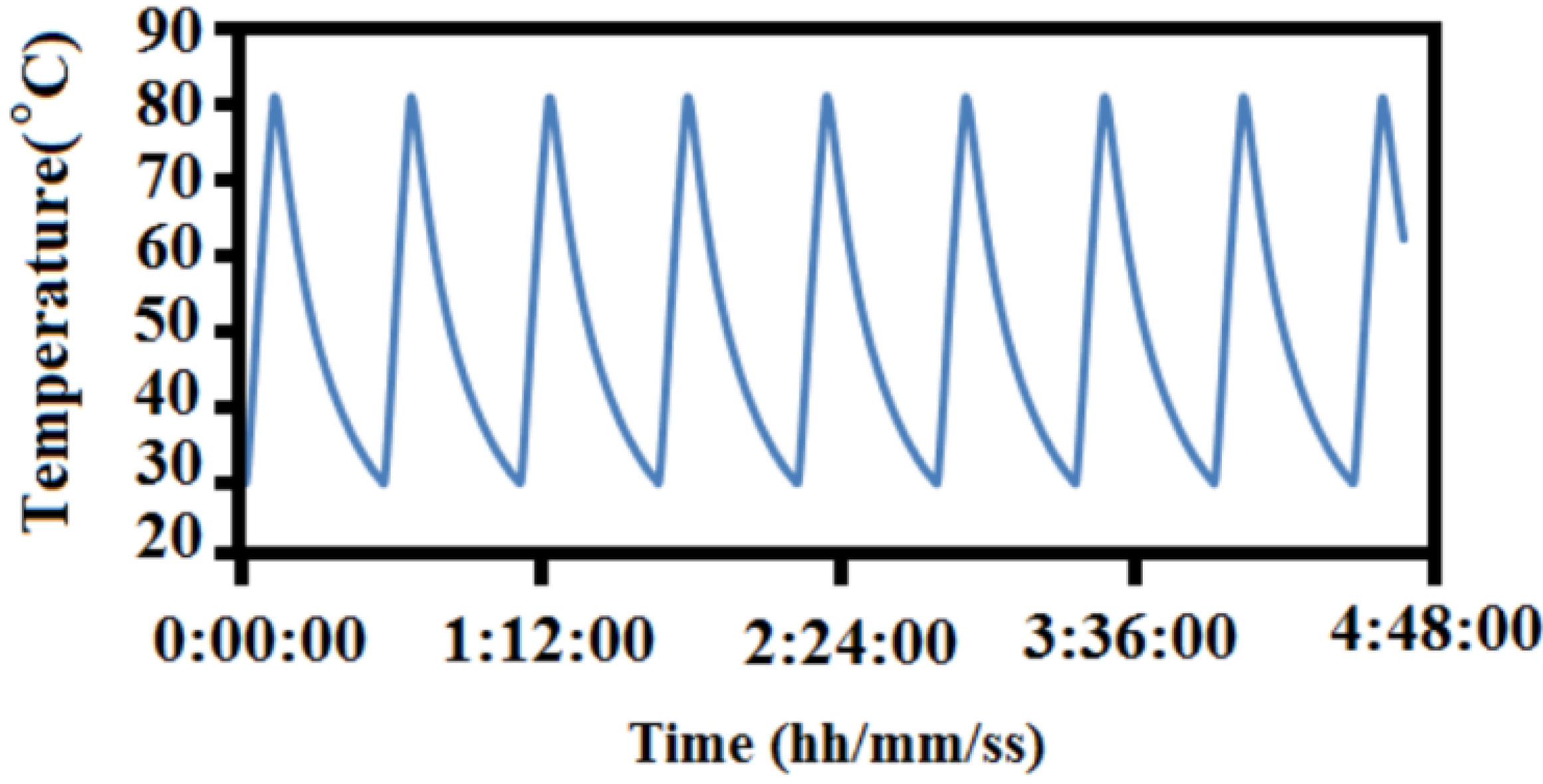
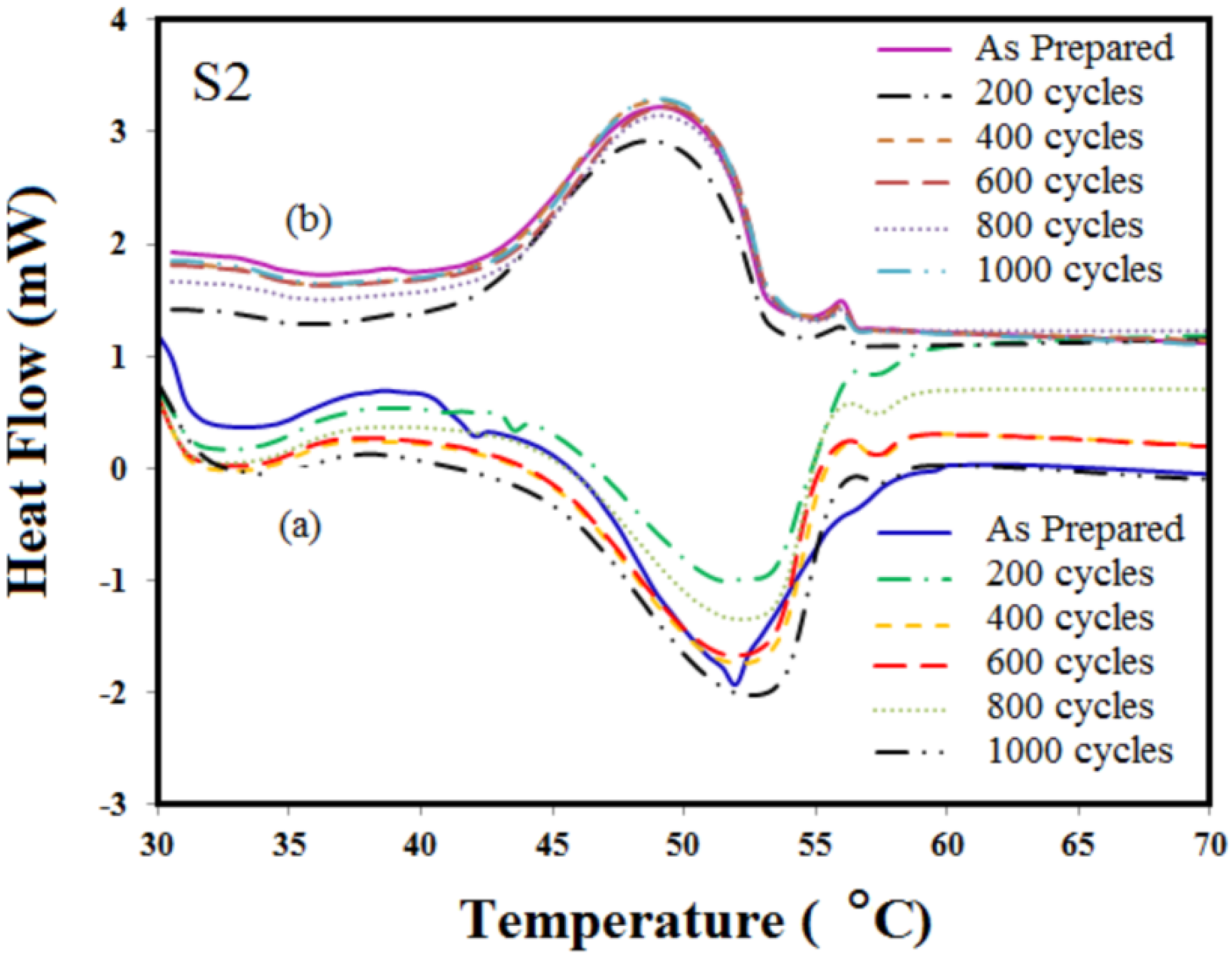
3.2. Repeated Thermal Cycling Test
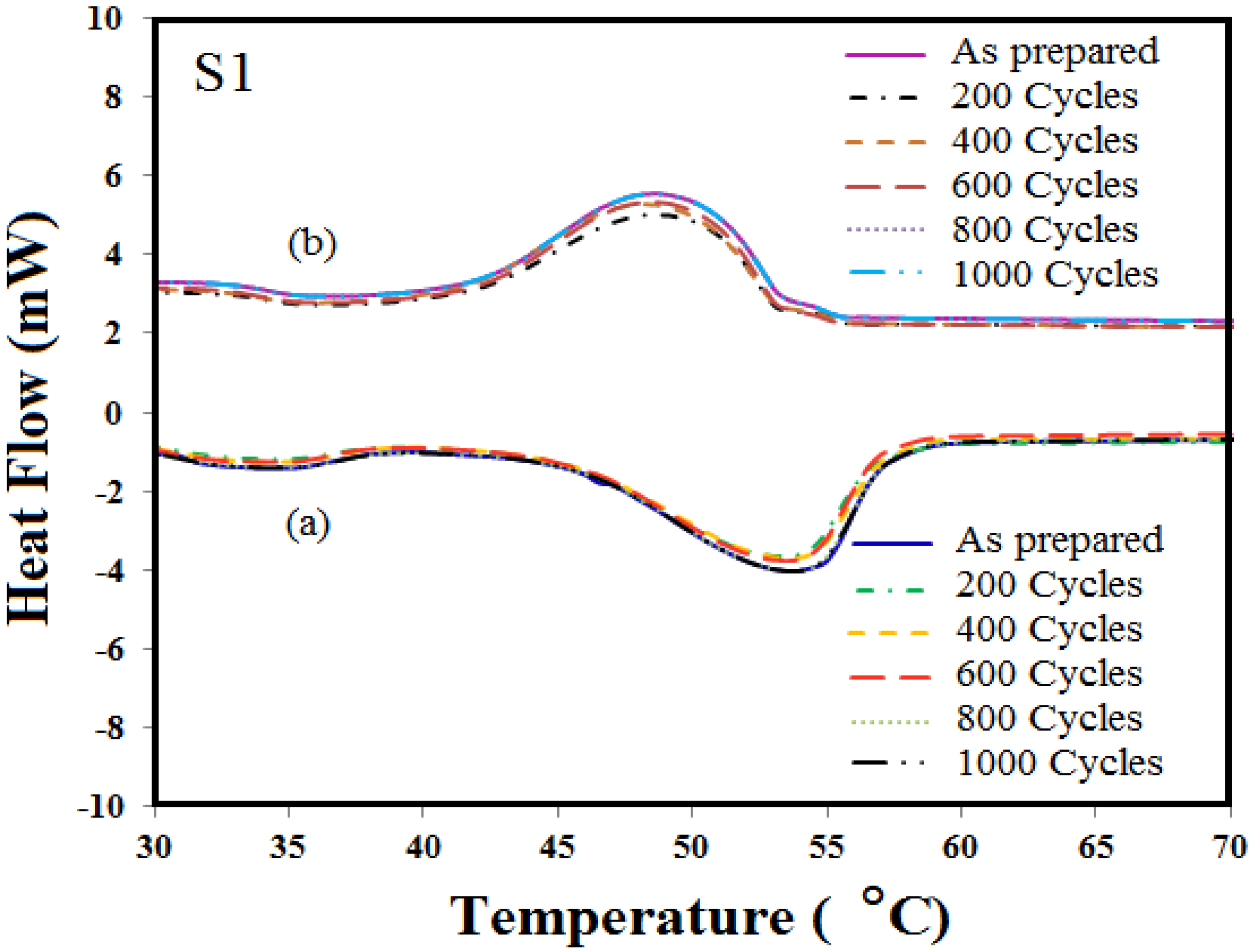
3.3. Thermal Properties of Microencapsulated Phase Change Material (MEPCM)
| Cycling number | Melting temperature (°C) | Melting latent heat (J/g) | Freezing temperature (ºC) | Freezing latent heat (J/g) |
|---|---|---|---|---|
| 0 | 53.2 | 31.0 | 46.4 | 32.6 |
| 200 | 53.2 | 30.6 | 44.9 | 31.5 |
| 400 | 53.3 | 30.0 | 45.2 | 31.5 |
| 600 | 53.3 | 30.3 | 45.1 | 30.6 |
| 800 | 53.5 | 30.6 | 45.5 | 30.7 |
| 1000 | 53.4 | 30.5 | 45.4 | 30.7 |
| Cycling number | Melting temperature (°C) | Melting latent heat (J/g) | Freezing temperature (ºC) | Freezing latent heat (J/g) |
|---|---|---|---|---|
| 0 | 53.8 | 65.1 | 44.9 | 66.4 |
| 200 | 53.2 | 62.1 | 44 | 63.5 |
| 400 | 53.3 | 61 | 44.1 | 62 |
| 600 | 53.3 | 61 | 44 | 62 |
| 800 | 53.3 | 61 | 44.2 | 61.2 |
| 1000 | 53.4 | 60.5 | 46.1 | 61 |
| Sample Name | Paraffin wax loading (%) | Encapsulation ratio (%) | Encapsulation efficiency (%) | ||
|---|---|---|---|---|---|
| Paraffin wax | 131.92 | 132.31 | – | – | – |
| S1 | 31 | 32.6 | 10 | 23.4 | 24.0 |
| S2 | 65.1 | 66.4 | 20 | 49.3 | 49.7 |
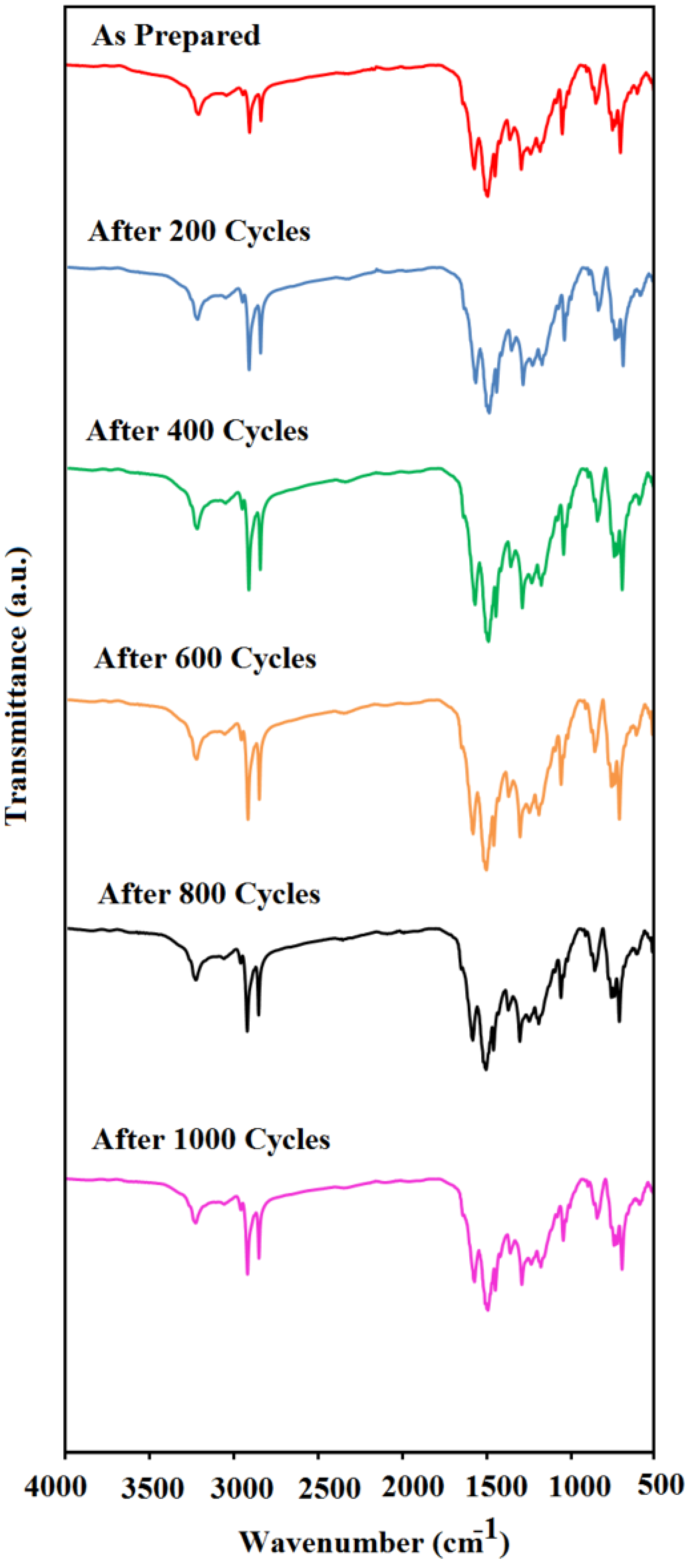
3.4. Structure Stability of MEPCM
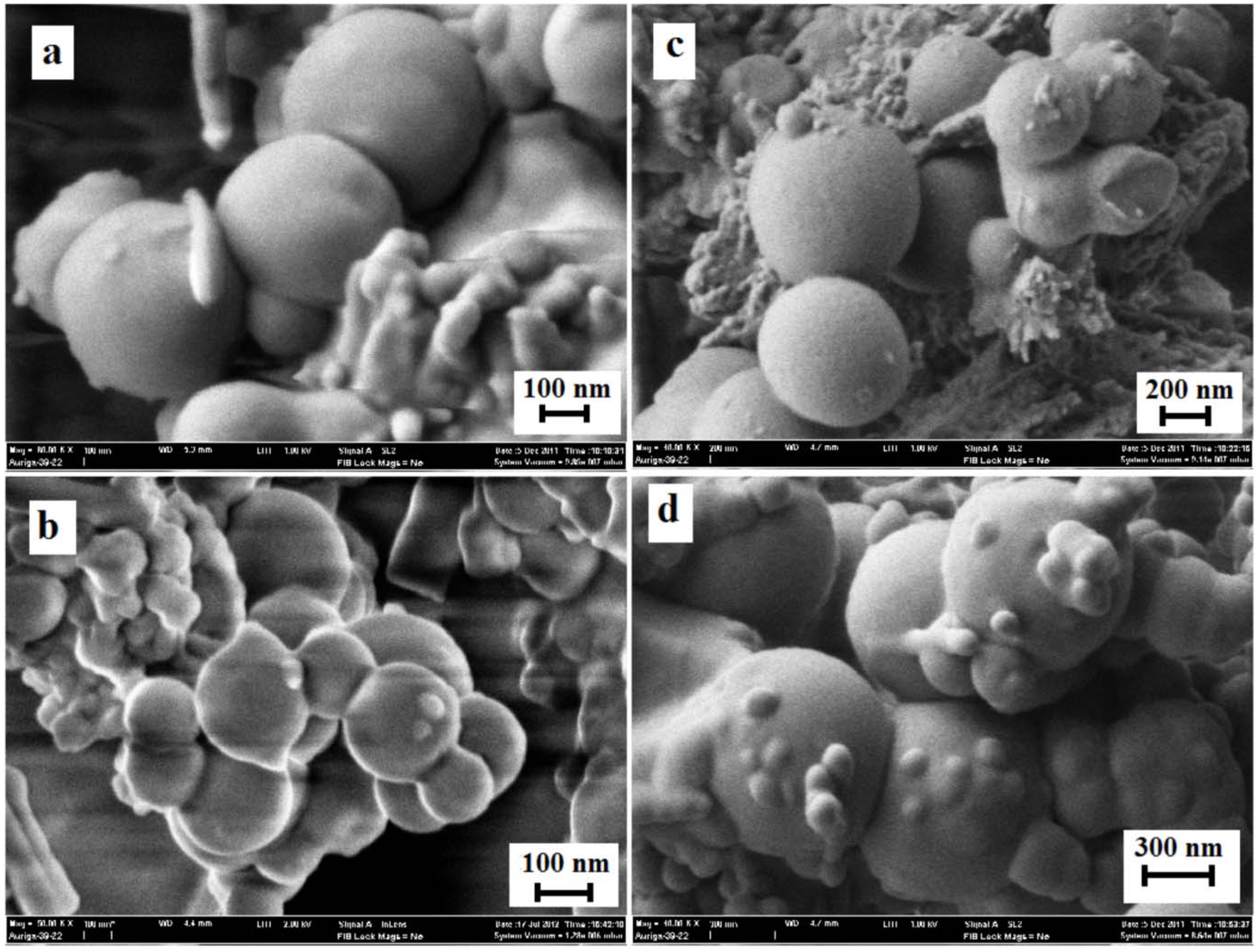
3.5. Surface Morphology of MEPCM
4. Conclusions
Acknowledgement
References
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Solar Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Kaygusuz, K.; Sari, A. Thermal energy storage system using a technical grade paraffin wax as latent heat energy storage material. Energy Sources 2005, 27, 1535–1546. [Google Scholar] [CrossRef]
- Farid, M.M.; Kim, Y.; Kansawa, A. Thermal Performance of a Heat Storage Module using PCM’s with Different Melting Temperature: Experimental. J. Solar Energy Eng. 1990, 112, 125–131. [Google Scholar] [CrossRef]
- Farid, M.M.; Kanzawa, A. Thermal Performance of a Heat Storage Module using PCM’s with Different Melting Temperatures: Mathematical Modeling. J. Solar Energy Eng. 1989, 111, 152–157. [Google Scholar] [CrossRef]
- Farid, M.M.; Mohamed, A.K. Effect of natural convection on the process of melting and solidification of paraffin wax. Chem. Eng. Commun. 1987, 57, 297–316. [Google Scholar] [CrossRef]
- Himran, S.; Suwono, A.; Mansoori, G.A. Characterization of Alkanes and Paraffin Waxes for Application as Phase Change Energy Storage Medium. Energy Sources 1994, 16, 117–128. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. Thermal cycle testing of calcium chloride hexahydrate as a possible PCM for latent heat storage. Solar Energy Mater. Solar Cells 2008, 92, 891–899. [Google Scholar] [CrossRef]
- Sharma, S.D.; Buddhi, D.; Sawhney, R.L. Accelerated thermal cycle test of latent heat-storage materials. Solar Energy 1999, 66, 483–490. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.D.; Buddhi, D. Accelerated thermal cycle test of acetamide, stearic acid and paraffin wax for solar thermal latent heat storage applications. Energy Convers. Manag. 2002, 43, 1923–1930. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R. Thermal cycling test of few selected inorganic and organic phase change materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L. Phase change material and therir basic properties. In Thermal Energy Storage for Sustainable Energy Consumption; Paksoy, H., Ed.; Springer: Dordrecht, the Netherlands, 2007; Volume 234, pp. 257–277. [Google Scholar]
- Sari, A.; Kaygusuz, K. Thermal performance of palmitic acid as a phase change energy storage material. Energy Convers. Manag. 2002, 43, 863–876. [Google Scholar] [CrossRef]
- Uddin, M.S.; Zhu, H.J.; Hawlader, M.N.A. Effects of cyclic operation on the characteristics of a microencapsulated PCM storage material. Int. J. Solar Energy 2002, 22, 105–114. [Google Scholar] [CrossRef]
- Alkan, C.; Kaya, K.; SarI, A. Preparation, thermal properties and thermal reliability of form-stable paraffin/polypropylene composite for thermal energy storage. J. Polym. Environ. 2009, 17, 254–258. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Karaipekli, A.; Uzun, O. Microencapsulated n-octacosane as phase change material for thermal energy storage. Solar Energy 2009, 83, 1757–1763. [Google Scholar] [CrossRef]
- Ma, S.D.; Song, G.L.; Miao, Z.C.; Wang, D.W. Preparation and characterization of paraffin/PMMA core/shell structured microcapsules. Mater. Adv. Res. 2011, 239–242, 524–527. [Google Scholar]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C.; Silakhori, M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/graphene oxide composite. Energy Convers. Manag. 2013, 67, 275–282. [Google Scholar] [CrossRef]
- Pan, L.; Tao, Q.; Zhang, S.; Wang, S.; Zhang, J.; Wang, Z.; Zhang, Z. Preparation, characterization and thermal properties of micro-encapsulated phase change materials. Solar Energy Mater. Solar Cells 2011, 98, 66–70. [Google Scholar] [CrossRef]
- Gemeay, A.H.; Mansour, I.A.; El-Sharkawy, R.G.; Zaki, A.B. Preparation and characterization of polyaniline/manganese dioxide composites via oxidative polymerization: Effect of acids. Eur. Polym. J. 2005, 41, 2575–2583. [Google Scholar] [CrossRef]
- Zhang, G.H.; Bon, S.A.F.; Zhao, C.Y. Synthesis, characterization and thermal properties of novel nanoencapsulated phase change materials for thermal energy storage. Solar Energy 2012, 86, 1149–1154. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, E.; Barmar, M.; Kish, M.H. Preparation of phase-change material microcapsules with paraffin or camel fat cores: Application to fabrics. Iran. Polym. J. 2010, 19, 277–286. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silakhori, M.; Naghavi, M.S.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Mehrali, M. Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage. Materials 2013, 6, 1608-1620. https://doi.org/10.3390/ma6051608
Silakhori M, Naghavi MS, Metselaar HSC, Mahlia TMI, Fauzi H, Mehrali M. Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage. Materials. 2013; 6(5):1608-1620. https://doi.org/10.3390/ma6051608
Chicago/Turabian StyleSilakhori, Mahyar, Mohammad Sajad Naghavi, Hendrik Simon Cornelis Metselaar, Teuku Meurah Indra Mahlia, Hadi Fauzi, and Mohammad Mehrali. 2013. "Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage" Materials 6, no. 5: 1608-1620. https://doi.org/10.3390/ma6051608





