Butanol Dehydration over V2O5-TiO2/MCM-41 Catalysts Prepared via Liquid Phase Atomic Layer Deposition
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
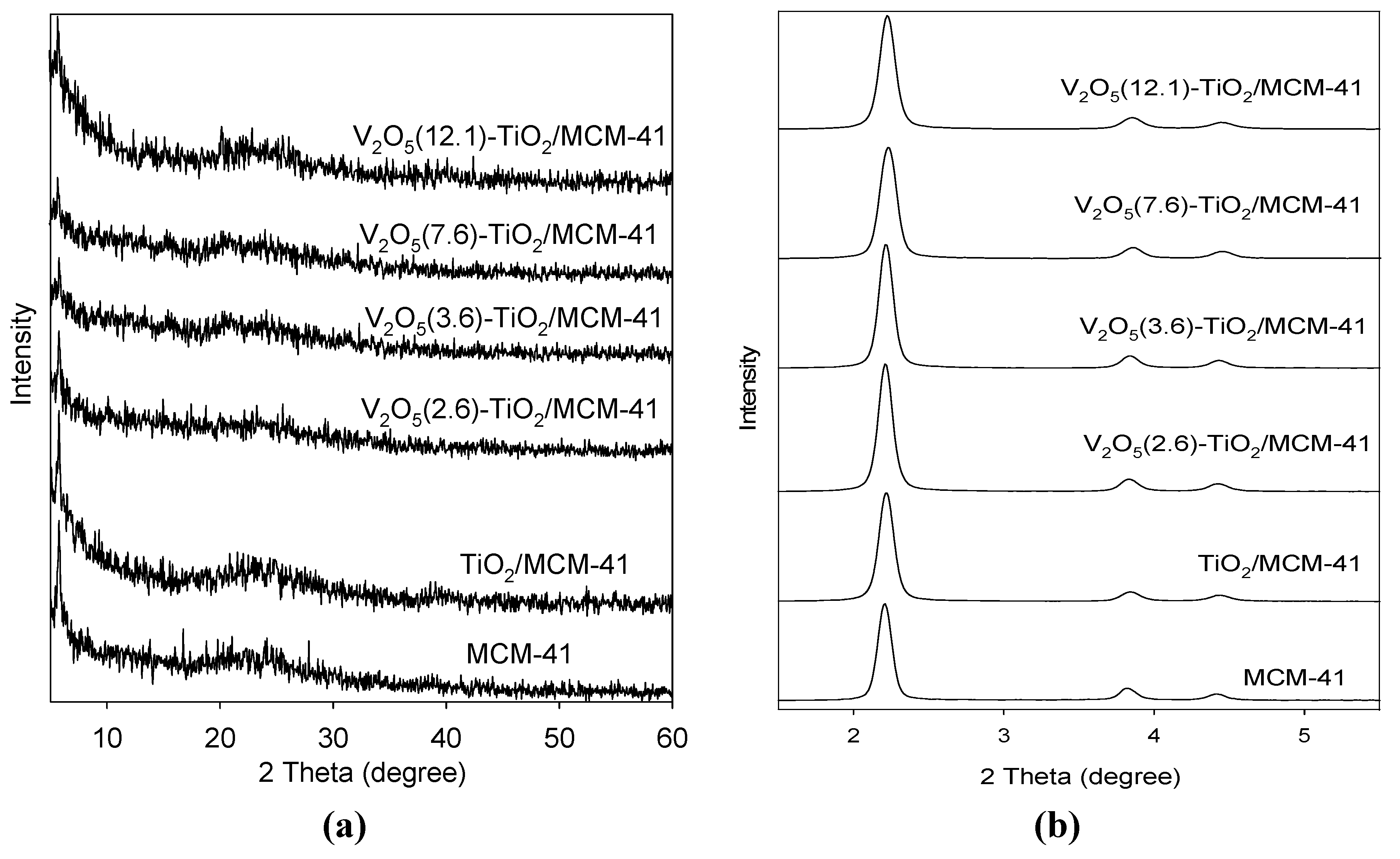
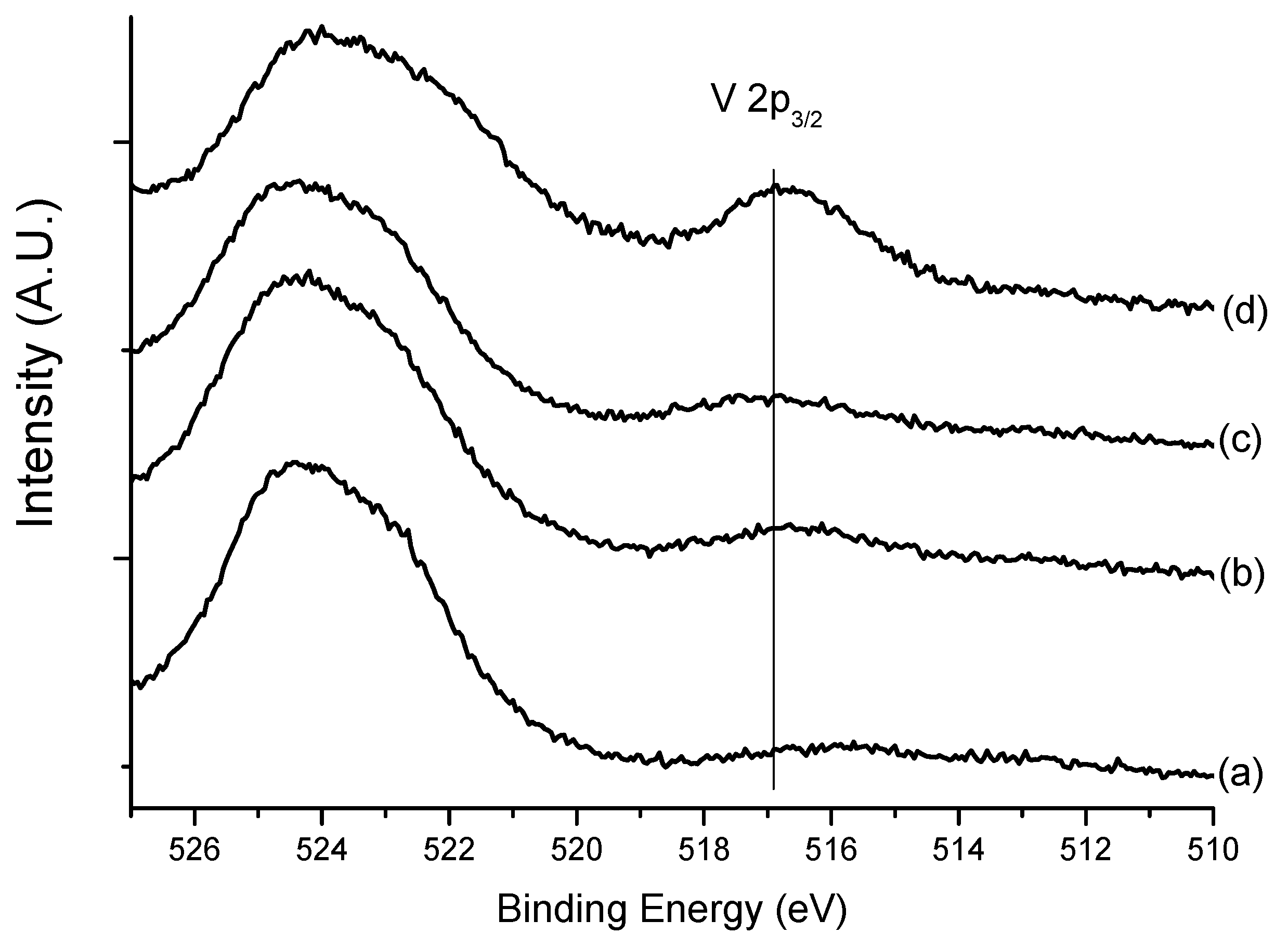
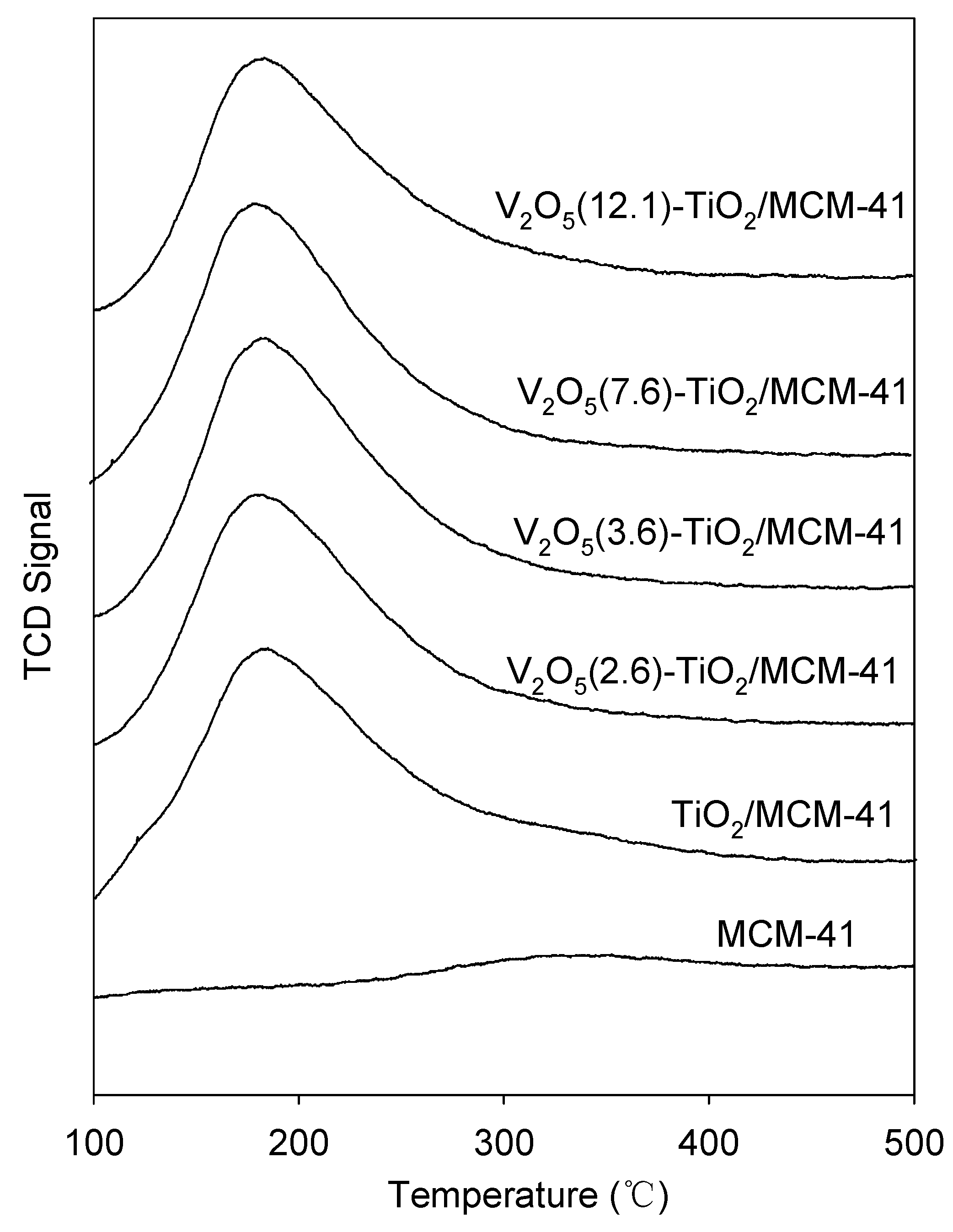
3.1. Catalyst Characterization
| Catalyst | TiO2 (wt %) | V2O5 (wt %) |
|---|---|---|
| TiO2/MCM-41 | 8.4 | – |
| V2O5(2.6)-TiO2/MCM-41 | 7.6 | 2.6 |
| V2O5(3.6)-TiO2/MCM-41 | 7.5 | 3.6 |
| V2O5(7.6)-TiO2/MCM-41 | 7.0 | 7.6 |
| V2O5(12.1)-TiO2/MCM-41 | 7.1 | 12.1 |

| Catalyst | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|
| MCM-41 | 1069 | 0.99 |
| TiO2/MCM-41 | 955 | 0.84 |
| V2O5(2.6)-TiO2/MCM-41 | 954 | 0.87 |
| V2O5(3.6)-TiO2/MCM-41 | 898 | 0.82 |
| V2O5(7.6)-TiO2/MCM-41 | 907 | 0.84 |
| V2O5(12.1)-TiO2/MCM-41 | 859 | 0.78 |
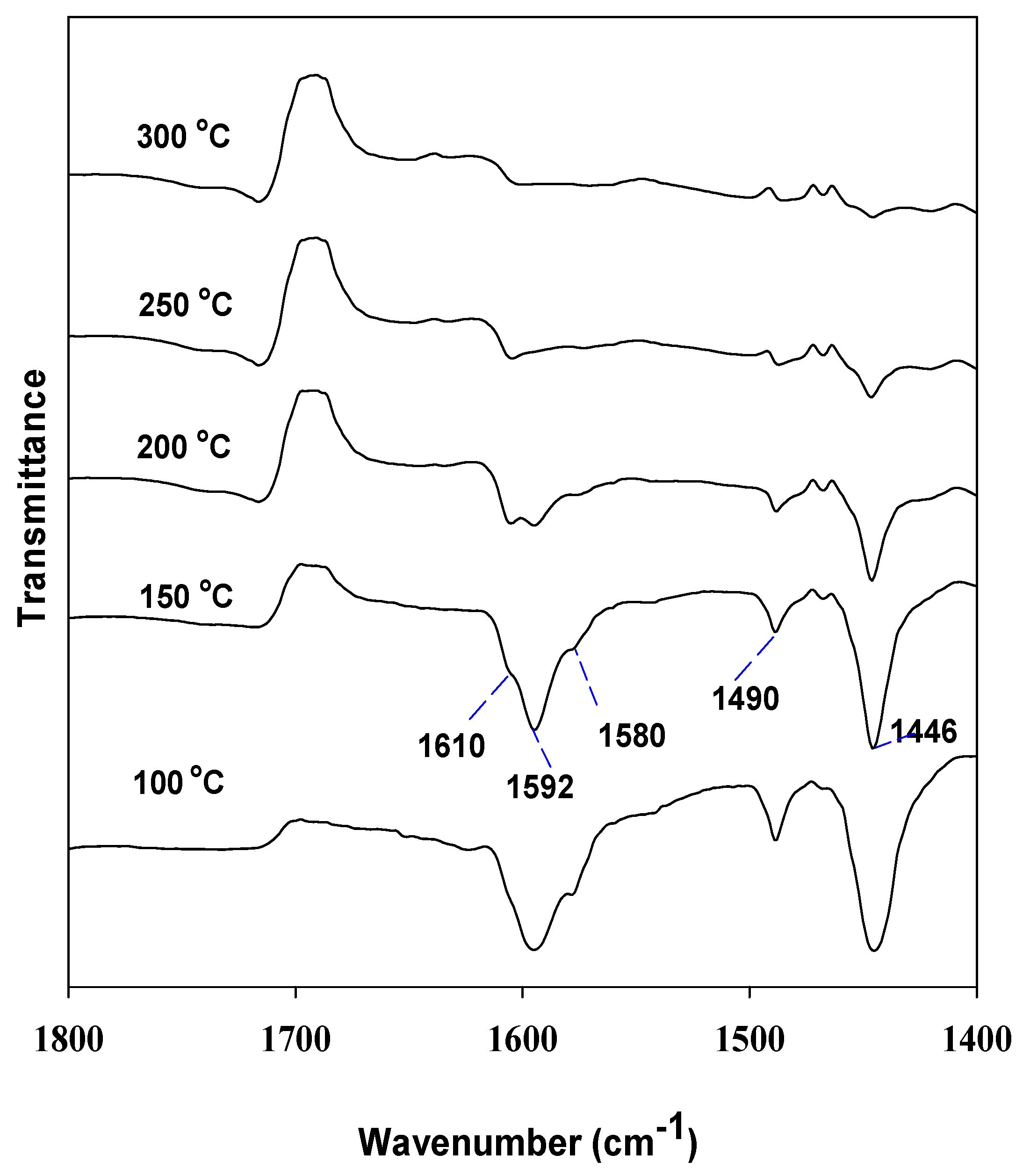

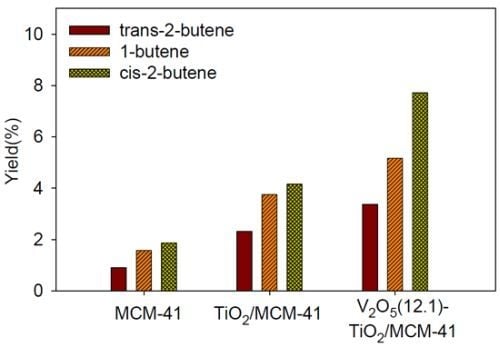
3.2. 2-Butanol Dehydration
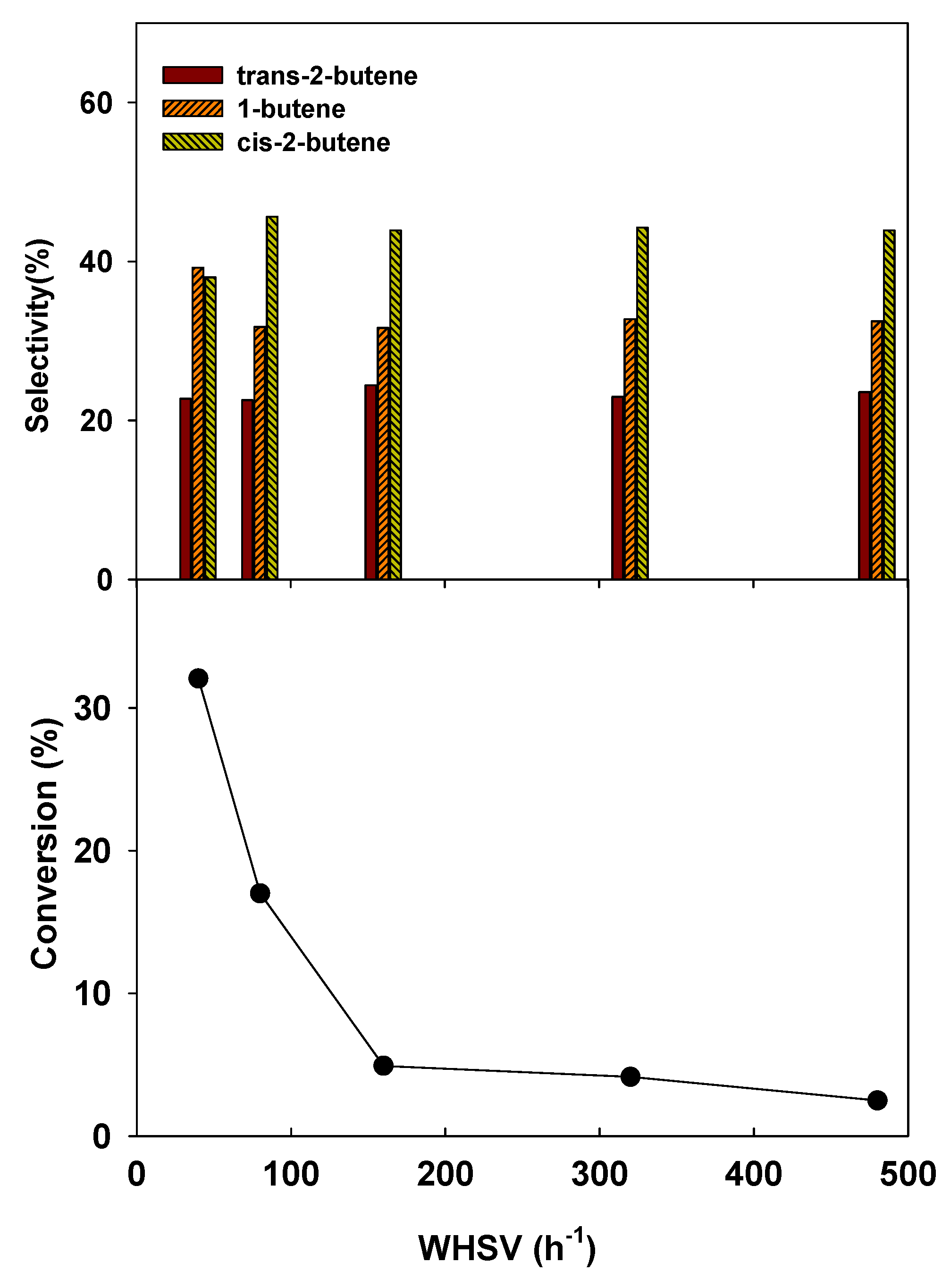
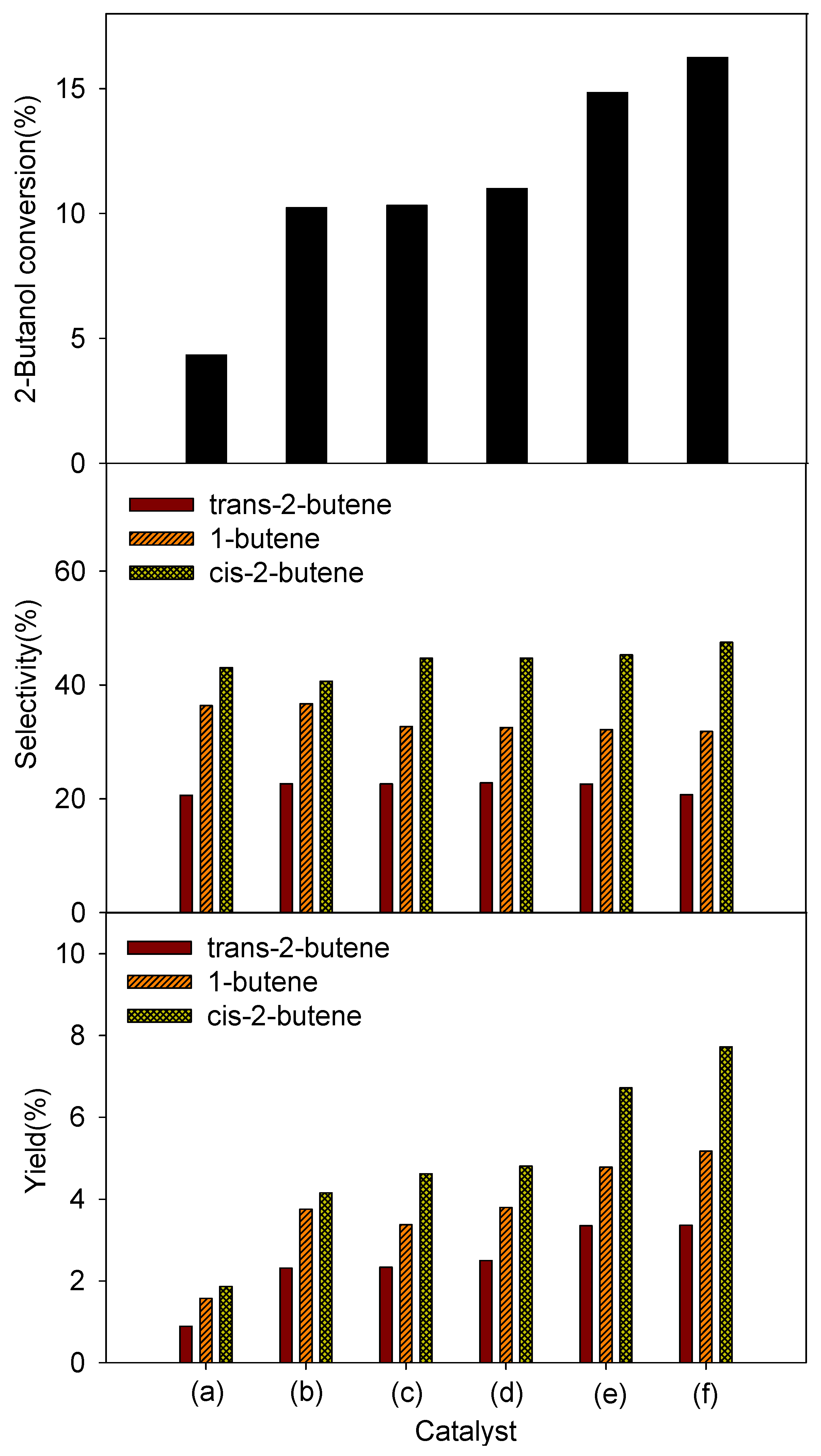
4. Conclusions
Acknowledgments
References
- Bañares, M.A.; Martı́nez-Huerta, M.V.; Gao, X.; Fierro, J.L.G.; Wachs, I.E. Dynamic behavior of supported vanadia catalysts in the selective oxidation of ethane: In situ Raman, UV-Vis DRS and reactivity studies. Catal. Today 2000, 61, 295–301. [Google Scholar] [CrossRef]
- Briand, L.E.; Tkachenko, O.P.; Guraya, M.; Gao, X.; Wachs, I.E.; Grunert, W. Surface-analytical studies of supported vanadium oxide monolayer catalysts. J. Phys. Chem. B 2004, 108, 4823–4830. [Google Scholar] [CrossRef]
- Olthof, B.; Khodakov, A.; Bell, A.T.; Iglesia, E. Effects of support composition and pretreatment conditions on the structure of vanadia dispersed on SiO2, Al2O3, TiO2, ZrO2, and HfO2. J. Phys. Chem. B 2000, 104, 1516–1528. [Google Scholar] [CrossRef]
- Khodakov, A.; Olthof, B.; Bell, A.T.; Iglesia, E. Structure and catalytic properties of supported vanadium oxides: Support effects on oxidative dehydrogenation reactions. J. Catal. 1999, 181, 205–216. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, K.Y.; Choi, S.; Liu, J.; Wang, L.Q.; Peden, C.H.F. Grafting sulfated zirconia on mesoporous silica. Green Chem. 2007, 9, 540–544. [Google Scholar] [CrossRef]
- Ichinose, I.; Senzu, H.; Kunitake, T. A surface sol-gel process of TiO2 and other metal oxide films with molecular precision. Chem. Mater. 1997, 9, 1296–1298. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, S.; Kim, D.H.; Park, Y.K.; Jeon, J.K. Preparation of highly dispersed tungsten oxide on MCM-41 by atomic layer deposition and its application to butanol dehydration. J. Nanosci. Nanotechnol. 2012, 12, 6074–6079. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.H.; Jeong, K.E.; Park, Y.K.; Jeon, J.K. Catalytic characteristics of titanium oxide/MCM-41 synthesized by liquid phase atomic layer deposition. J. Nanosci. Nanotechnol. 2013, 13, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.E.; Kwak, J.H.; Hu, J.Z.; Wang, Y.; Peden, C.H.F. Effects of novel supports on the physical and catalytic properties of tungstophosphoric acid for alcohol dehydration reactions. Top. Catal. 2008, 49, 259–267. [Google Scholar] [CrossRef]
- West, M.; Braden, D.J.; Dumesic, J.A. Dehydration of butanol to butene over solid acid catalysts in high water environments. J. Catal. 2009, 262, 134–143. [Google Scholar] [CrossRef]
- Macht, J.; Carr, R.T.; Iglesia, E. Functional assessment of the strength of solid acid catalysts. J. Catal. 2009, 264, 54–66. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hajri, R.; Barri, S.A.; Chadwick, D. One-step dehydration and isomerisation of n-butanol to iso-butene over zeolite catalysts. Chem. Commun. 2010, 46, 4088–4090. [Google Scholar] [CrossRef]
- Jeon, J.K.; Lee, H.; Yim, J.H.; Kim, Y.S.; Lee, S.J.; Park, Y.K.; Shon, J.K.; Kim, J.M. Selective synthesis of 1-butene through positional isomerisation of 2-butene over mesoporous silica MCM-41. Catal. Lett. 2007, 119, 179–184. [Google Scholar] [CrossRef]
- Ryoo, R.; Kim, J.M. Structural order in MCM-41 controlled by shifting silicate polymerization equilibrium. J. Chem. Soc. Chem. Commun. 1995, 711–712. [Google Scholar]
- Park, Y.K.; Kim, S.J.; You, N.; Cho, J.; Lee, S.J.; Lee, J.H.; Jeon, J.K. MoO3/SiO2 catalysts for double bond migration of 2-butene. J. Ind. Eng. Chem. 2011, 17, 186–190. [Google Scholar] [CrossRef]
- Jeon, J.K.; Park, Y.K. Pyrolysis of an LDPE-LLDPE-EVA copolymer mixture over various mesoporous catalysts. Korean J. Chem. Eng. 2012, 29, 196–200. [Google Scholar] [CrossRef]
- Herrera, J.E.; Kwak, J.H.; Hu, J.Z.; Wang, Y.; Peden, C.H.F. Synthesis of nanodispersed oxides of vanadium, titanium, molybdenum, and tungsten on mesoporous silica using atomic layer deposition. Top. Catal. 2006, 39, 245–255. [Google Scholar] [CrossRef]
- Shin, H.J.; Ryoo, R.; Liu, Z.; Terasaki, O. Template synthesis of asymmetrically mesostructured platinum networks. J. Am. Chem. Soc. 2001, 123, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Eden Prairie, MA, USA, 1992. [Google Scholar]
- Palomino, G.T.; Pascual, J.J.C.; Delgado, M.R.; Parra, J.B.; Arean, C.O. FT-IR studies on the acidity of gallium-substituted mesoporous MCM-41 silica. Mater. Chem. Phys. 2001, 85, 145–150. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Al-Sagheer, F.A.; Pasupulety, L. In situ FTIR spectra of pyridine adsorbed on SiO2-Al2O3, TiO2, ZrO2 and CeO2: General considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf. A Physicochem. Eng. Aspects 2001, 190, 261–274. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choi, H.; Bae, J.-H.; Kim, D.H.; Park, Y.-K.; Jeon, J.-K. Butanol Dehydration over V2O5-TiO2/MCM-41 Catalysts Prepared via Liquid Phase Atomic Layer Deposition. Materials 2013, 6, 1718-1729. https://doi.org/10.3390/ma6051718
Choi H, Bae J-H, Kim DH, Park Y-K, Jeon J-K. Butanol Dehydration over V2O5-TiO2/MCM-41 Catalysts Prepared via Liquid Phase Atomic Layer Deposition. Materials. 2013; 6(5):1718-1729. https://doi.org/10.3390/ma6051718
Chicago/Turabian StyleChoi, Hyeonhee, Jung-Hyun Bae, Do Heui Kim, Young-Kwon Park, and Jong-Ki Jeon. 2013. "Butanol Dehydration over V2O5-TiO2/MCM-41 Catalysts Prepared via Liquid Phase Atomic Layer Deposition" Materials 6, no. 5: 1718-1729. https://doi.org/10.3390/ma6051718