Redox Response of Reduced Graphene Oxide-Modified Glassy Carbon Electrodes to Hydrogen Peroxide and Hydrazine
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Apparatus
2.3. Deposition of rGO on the Surface of a GC Electrode
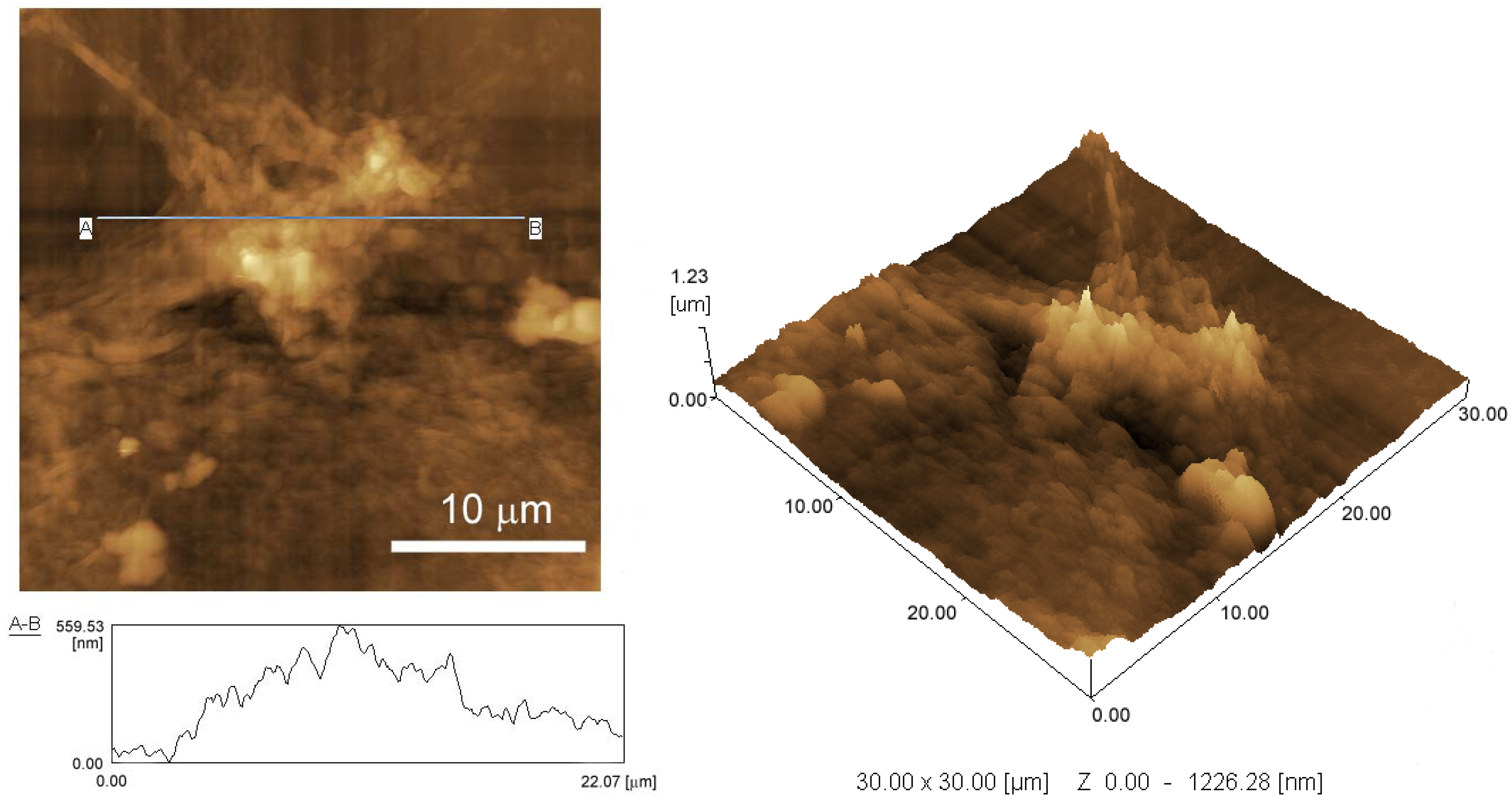
2.4. AFM and SEM imaging
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Preparation of rGO-Modified GC Electrode
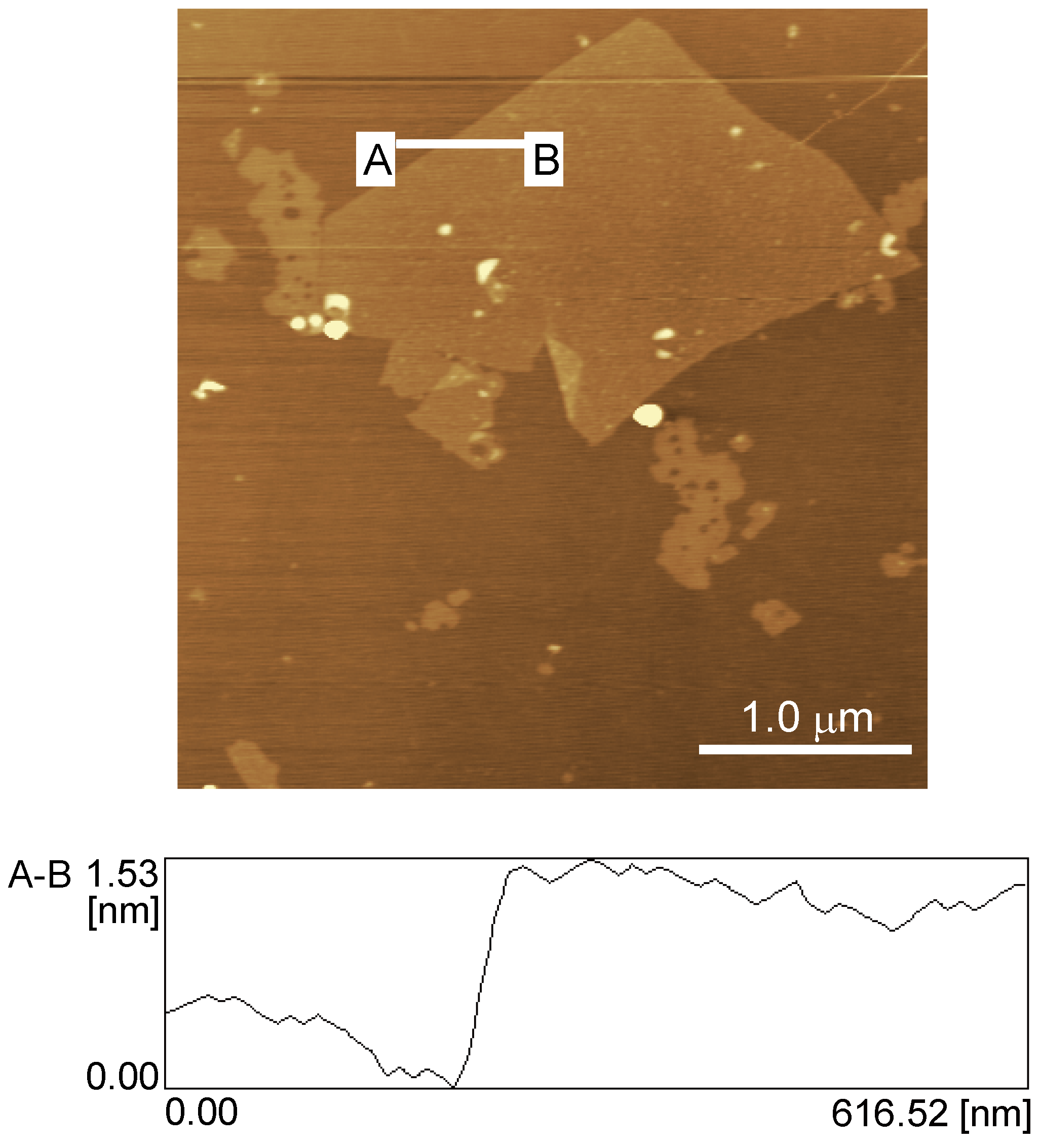
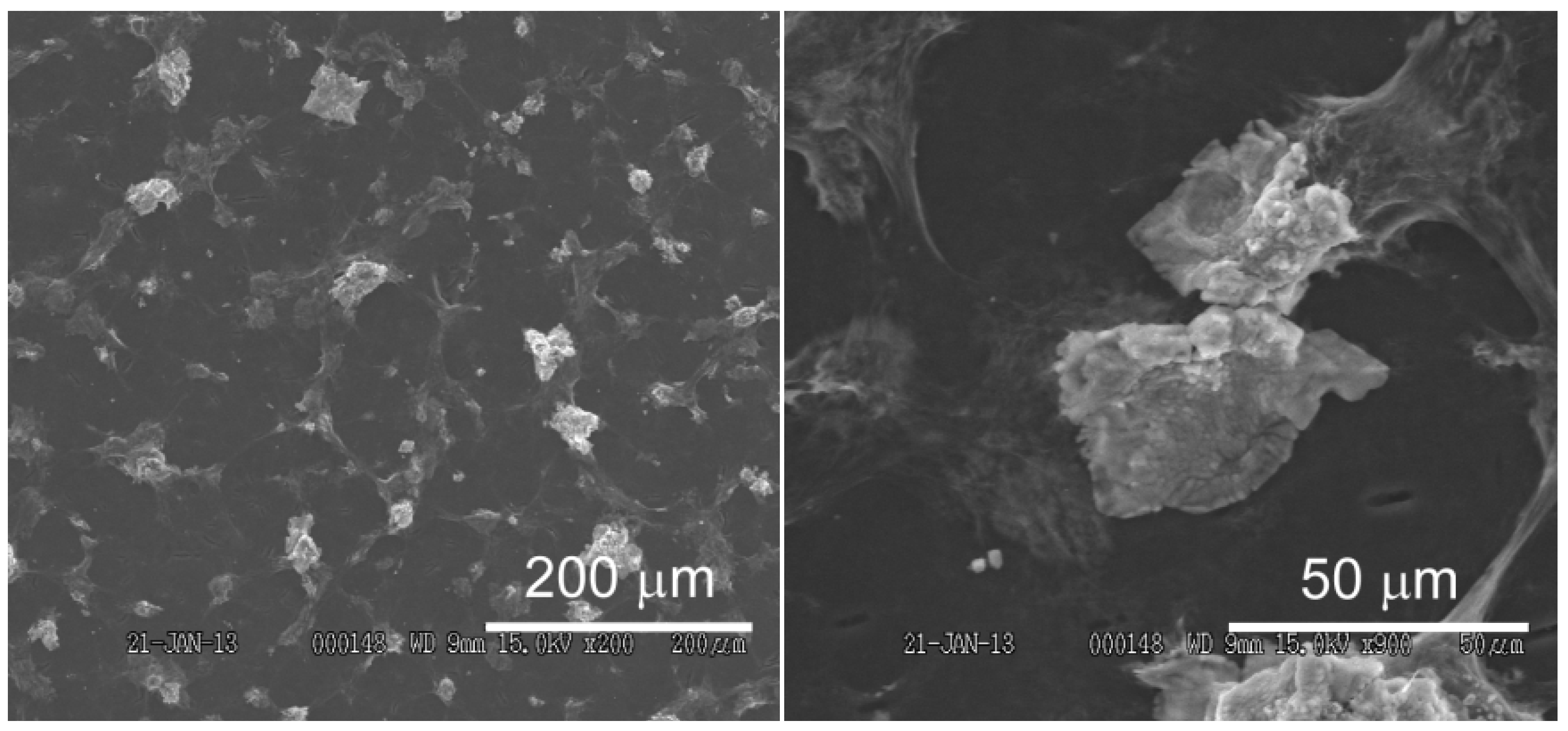
3.2. Redox Reactions of H2O2 and Hydrazine on an rGO-Modified Electrode
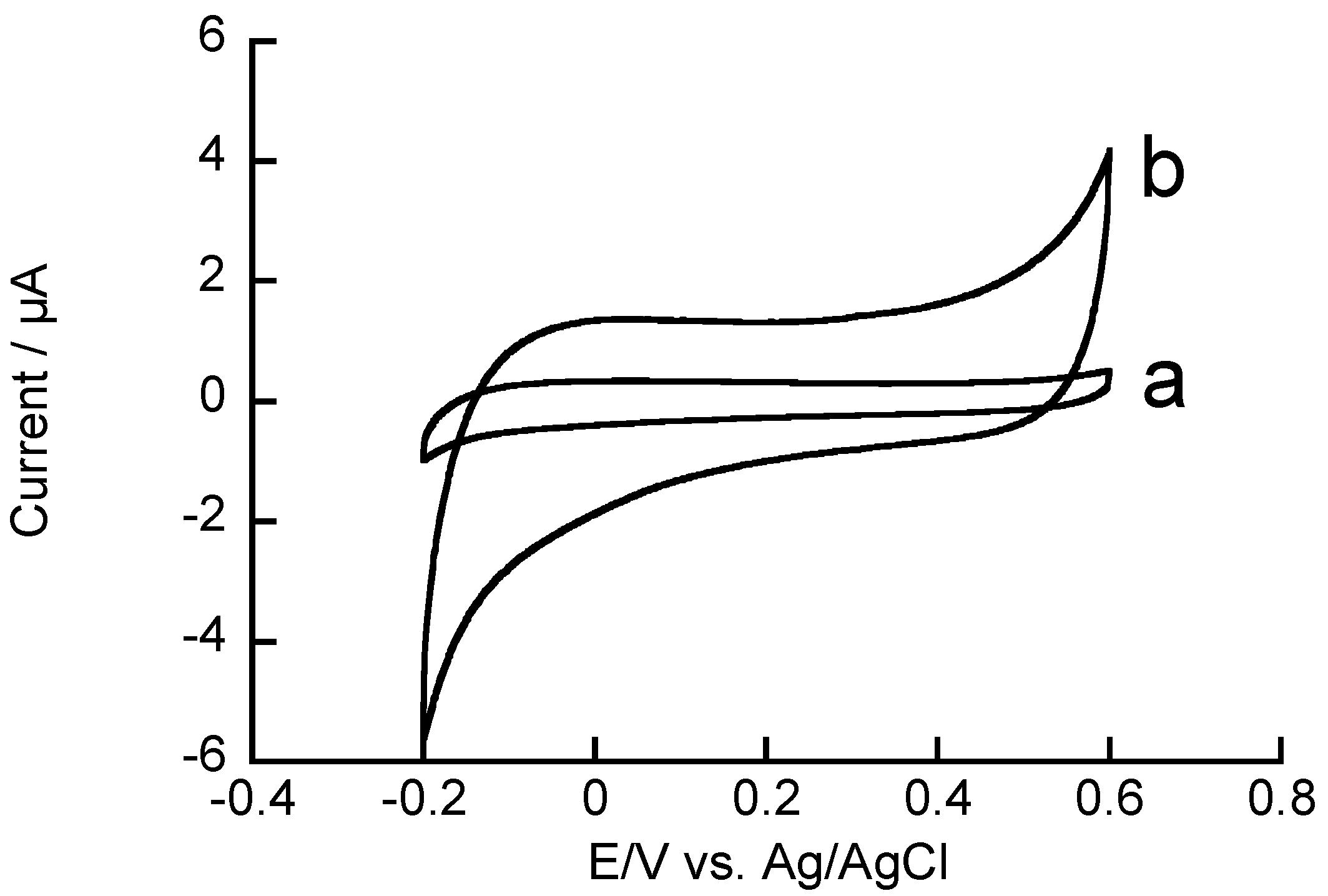
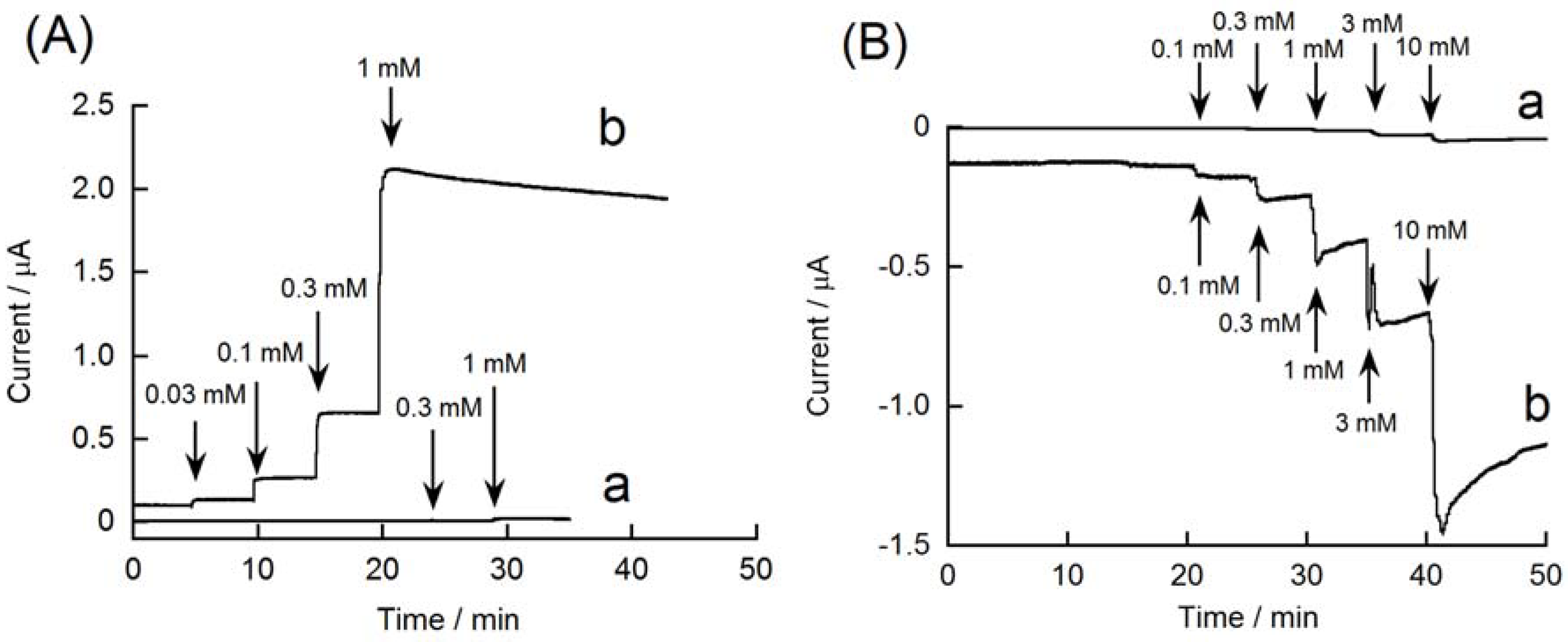
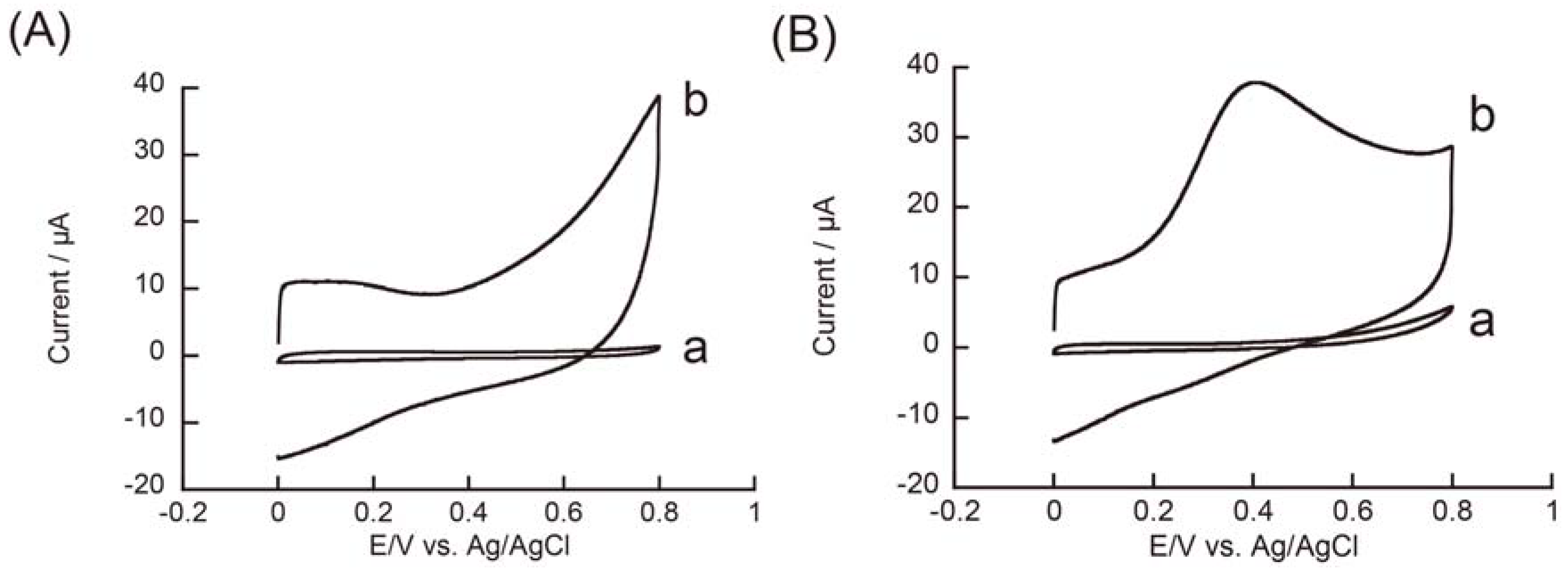
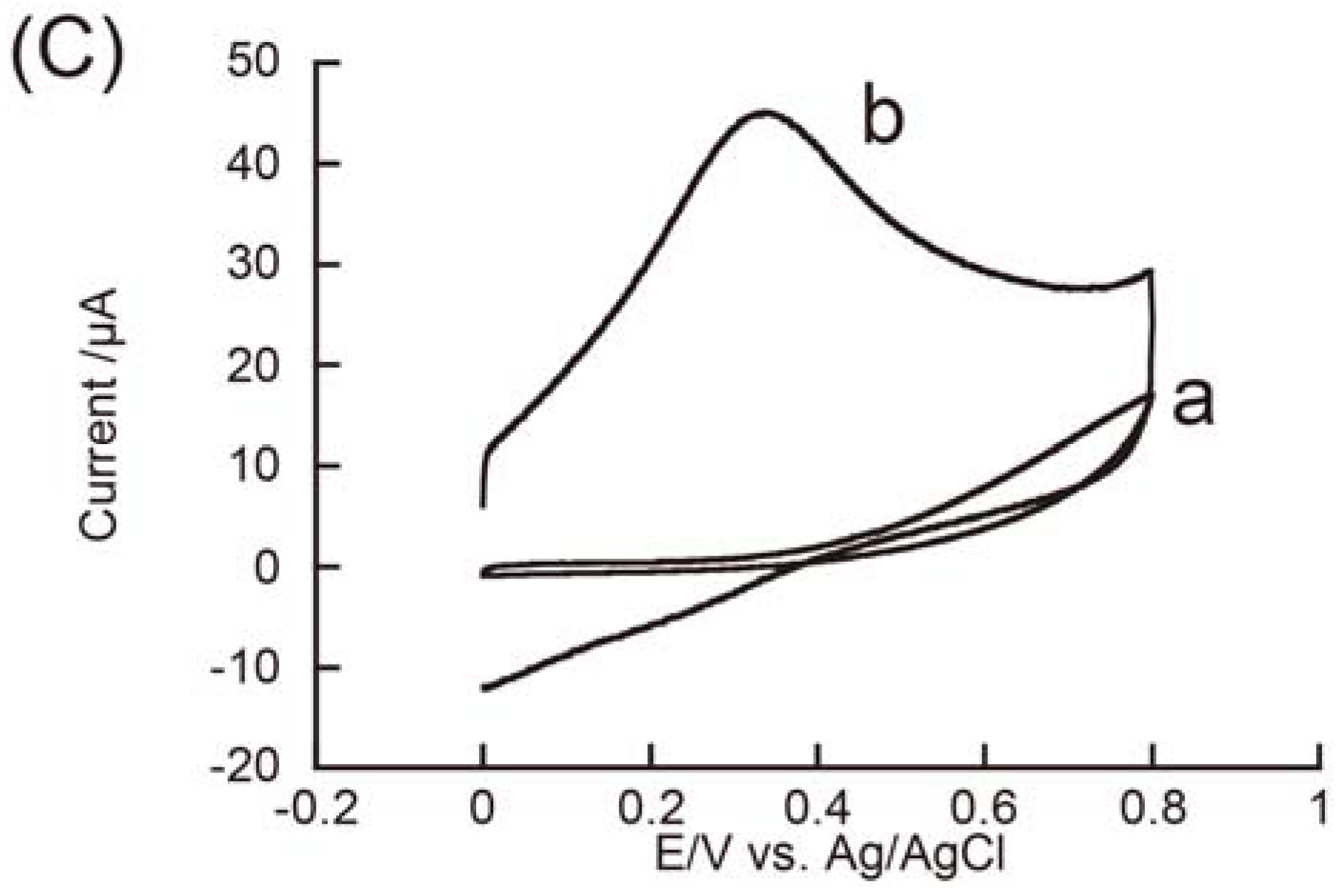


4. Conclusions
Acknowledgments
References
- Zayats, M.; Katz, E.; Willner, I. Electrical contacting of flavoenzymes and NAD(P)+-dependent enzymes by reconstitution and affinity interactions on phenylboronic acid monolayers associated with Au-electrodes. J. Am. Chem. Soc. 2002, 124, 14724–14735. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, Y.; Huang, J.; Zhao, Z.; Xu, X.; Anzai, J.; Osa, T.; Chen, Q. Amperometric choline biosensors prepared by layer-by-layer deposition of choline oxidase on the Prussian blue-modified platinum electrode. Talanta 2006, 70, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Anzai, J. Redox reactions of ferricyanide ions in layer-by-layer deposited polysaccharide films: A significant effect of the type of polycation in the films. Langmuir 2007, 23, 7378–7384. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer films: Effects of electrochemically inactive surface layers on the redox properties. Langmuir 2003, 19, 4043–4046. [Google Scholar] [CrossRef]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar] [CrossRef]
- Ge, C.; Doherty, W.J., III; Mendes, S.B.; Armstrong, N.R.; Saavedra, S.S. Voltammetric and waveguide spectroelectrochemical characterization of ultrathin poly(aniline)/poly(acrylic acid) films self-assembled on indium-tin oxide. Talanta 2005, 65, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kodama, D.; Naka, Y.; Anzai, J. Electrochemically induced disintegration of layer-by-layer-assembled thin films composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2006, 7, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Recksiedler, C.L.; Deore, B.A.; Freund, M.S. A novel layer-by-layer approach for the fabrication of conducting polymer/RNA multilayer films for controlled release. Langmuir 2006, 22, 2811–2815. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer construction of protein architectures through avidin-biotin and lectin-sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Leech, D.; Kavanagh, P.; Shuhmann, W. Enzymatic fuel cells: Recent progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Budnikov, H.C.; Evtugyn, G.A.; Gennady, A.; Porfireva, A.V. Electrochemical DNA sensors based on electropolymerized materials. Talanta 2012, 102, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Anzai, J. Use of polymeric indicator for electrochemical DNA sensors: Poly(4-vinylpyridine) derivative bearing [Os(5,6-dimethyl-1,10-phenanthroline)2Cl]2+. Anal. Chem. 2004, 76, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Musameh, M. Electrochemical detection of trace insulin at carbon-nanotube-modified electrodes. Anal. Chim. Acta 2004, 511, 33–36. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. Trend Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Rivas, G.A.; Rubianes, M.D.; Rodriguez, M.C.; Ferreyra, N.F.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon nanotubes for electrochemical biosensing. Talanta 2007, 74, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Mita, D.G.; Attanasio, A.; Arduini, F.; Diano, N.; Grano, V.; Bencivenga, U.; Rossi, S.; Amine, A.; Moscone, D. Enzymatic determination of BPA by means of tyrosinase immobilized on different carbon carriers. Biosens. Bioelectron. 2007, 23, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Allafchian, A.R.; Razaei, B. A sensitive and selective voltammetric sensor based on multiwall carbon nanotubes decorated with MgCr2O4 for the determination of azithromycin. Colloid Surf. B 2013, 103, 468–474. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M. Simultaneous voltammetric determination of tyrosine and paracetamol using a carbon nanotube-graphene nanosheet nanocomposite modified electrode in human blood serum and pharmaceuticals. Colloid Surf. B 2013, 103, 84–93. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Shi, H.; Song, Z.; Zhao, Z.; Anzai, J.; Osa, T.; Chen, Q. Multi-walled carbon nanotube-based glucose biosensors prepared by a layer-by-layer technique. Mater. Sci. Eng. C 2006, 26, 113–117. [Google Scholar] [CrossRef]
- Song, Z.; Huang, J.; Wu, B.; Shi, H.; Anzai, J.; Chen, Q. Amperometric aqueous sol-gel biosensor for low-potential stable choline detection at multi-walled carbon nanotube modified platinum electrode. Sens. Actuators B 2006, 115, 626–633. [Google Scholar] [CrossRef]
- Huang, J.; Song, Z.; Li, J.; Yang, Y.; Shi, H.; Wu, B.; Anzai, J.; Osa, T.; Chen, Q. A highly-sensitive L-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Mater. Sci. Eng. C 2007, 27, 29–34. [Google Scholar] [CrossRef]
- Munge, B.; Liu, G.; Collins, G.; Wang, J. Multiple enzyme layers on carbon nanotubes for electrochemical detection down to 80 DNA copies. Anal. Chem. 2005, 77, 4662–4666. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.B.; Chen, X.J.; Tay, B.K.; Khor, K.A. Transparent and flexible glucose biosensor via layer-by-layer assembly of multi-wall carbon nanotubes and glucose oxidase. Electrochem. Commun. 2007, 9, 1269–1275. [Google Scholar] [CrossRef]
- Chavez-Valdez, A.; Shaffer, M.S.P.; Boccaccini, A.R. Applications of graphene electrophoretic deposition: A review. J. Phys. Chem. B 2013, 117, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Inagaki, M.; Kim, Y.A.; Endo, M. Graphene: Preparation and structural perfection. J. Mater. Chem. 2011, 21, 3280–3294. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Munro, L.J.; Kampouris, D.K.; Banks, C.E. Electrochemistry of grapheme: Not such a beneficial electrode materials? RSC Adv. 2011, 1, 978–988. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Ratinac, K.R.; Yang, W.; Gooding, J.J.; Thordarson, P.; Braet, F. Graphene and related materials in electrochemical sensing. Electroanalysis 2011, 23, 803–826. [Google Scholar] [CrossRef]
- Yeo, M.Y.; Park, S.Y.; In, I. Temperature-dependent optical transmittance of chemically reduced graphene oxide/hydroxypropyl cellulose assembly. Chem. Lett. 2012, 41, 197–199. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Q.; Zhu, X.; Li, C.; Xu, M.; Liang, Y. Reduction of graphene oxide via ascorbic acid and its application for simultaneous detection of dopamine and ascorbic acid. Int. J. Electrochem. Sci. 2012, 7, 5172–5184. [Google Scholar]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2012, 20, 743–748. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, Y.; Kong, J.; Zou, C.; Li, C.M.; Lu, X.; Ma, J.; Boey, F.Y.C.; Chen, X. Assembly of graphene sheets into hierarchical structures for high-performance energy storage. ACS Nano 2011, 5, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Muthuswamy, N.; de la Fuente, J.L.G.; Ochal, P.; Giri, R.; Raaen, S.; Sunde, S.; Ronning, M.; Chen, D. Towards a highly-efficient fuel-cell catalyst: Optimization of Pt particle size, supports and surface-oxygen group concentration. Phys. Chem. Chem. Phys. 2013, 15, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Parashar, V.; Parashar, R.; Prakash, R.; Ramteke, P.W.; Pandey, A.C. Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale 2011, 3, 4104–4108. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Raichur, A.M. Near-infrared light-responsive graphene oxide composite multilayer capsules: a novel route for remote controlled drug delivery. Chem. Commun. 2013, 49, 734–736. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, T.; Zhang, Z.; Jiang, Z.; Ma, Y. Self-assembly of multilayered functional films based on graphene oxide sheets for controlled release. J. Mater. Chem. 2011, 21, 3471–3476. [Google Scholar] [CrossRef]
- Hammers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Qian, Q.; Wang, F.; Xie, X. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, W.E. Morphology and physical properties of poly(ethylene oxide) loaded graphene nanocomposites prepared by two different techniques. Eur. Polym. J. 2011, 47, 1534–1540. [Google Scholar] [CrossRef]
- Banks, C.E.; Compton, R.G. Exploring the electrocatalytic sites of carbon nanotubes for NADH detection: an edge plane pyrolytic graphite electrode study. Analyst 2005, 130, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, Z.; Fu, W.; Yang, H. Construction of 3D electrochemically reduced grapheme oxide-silver nanocomposite film and application as nonenzymatic hydrogen peroxide sensor. Electrochem. Commun. 2013, 27, 1–4. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Guo, Z.; Xu, J.; Wang, H.; Zhai, K.; Zhuo, X. A novel hydrazine electrochemical sensor based on the high specific surface area graphene. Microchim. Acta 2010, 169, 1–6. [Google Scholar] [CrossRef]
- Perez, E.F.; Neto, G.O.; Tanaka, A.A.; Kubota, L.T. Electrochemical sensor for hydrazine based on silica modified with nickel tetrasulfonated phthalocyanine. Electroanalysis 1998, 10, 111–115. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, Y.; Wang, Z.; Wei, W.; Li, B.; Zou, J.; Yang, N. Graphene nanoplatelets supported metal nanoparticles for electrochemical oxidation of hydrazine. Electrochem. Commun. 2013, 29, 29–32. [Google Scholar] [CrossRef]
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takahashi, S.; Abiko, N.; Anzai, J.-i. Redox Response of Reduced Graphene Oxide-Modified Glassy Carbon Electrodes to Hydrogen Peroxide and Hydrazine. Materials 2013, 6, 1840-1850. https://doi.org/10.3390/ma6051840
Takahashi S, Abiko N, Anzai J-i. Redox Response of Reduced Graphene Oxide-Modified Glassy Carbon Electrodes to Hydrogen Peroxide and Hydrazine. Materials. 2013; 6(5):1840-1850. https://doi.org/10.3390/ma6051840
Chicago/Turabian StyleTakahashi, Shigehiro, Naoyuki Abiko, and Jun-ichi Anzai. 2013. "Redox Response of Reduced Graphene Oxide-Modified Glassy Carbon Electrodes to Hydrogen Peroxide and Hydrazine" Materials 6, no. 5: 1840-1850. https://doi.org/10.3390/ma6051840




