Production and Characterization of a New Bacterial Cellulose/Poly(Vinyl Alcohol) Nanocomposite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Characterization of BC and BC/PVA Membranes
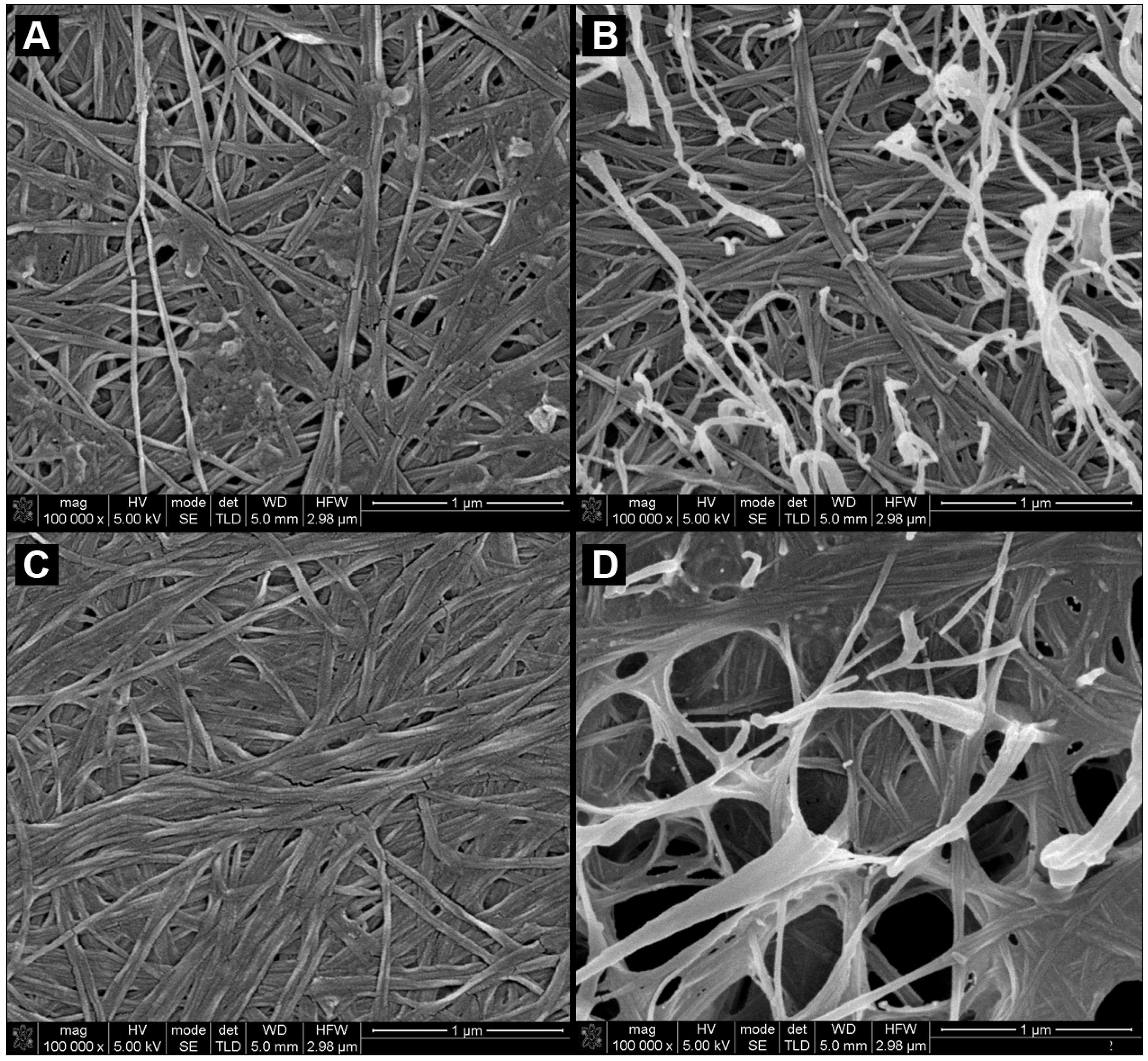
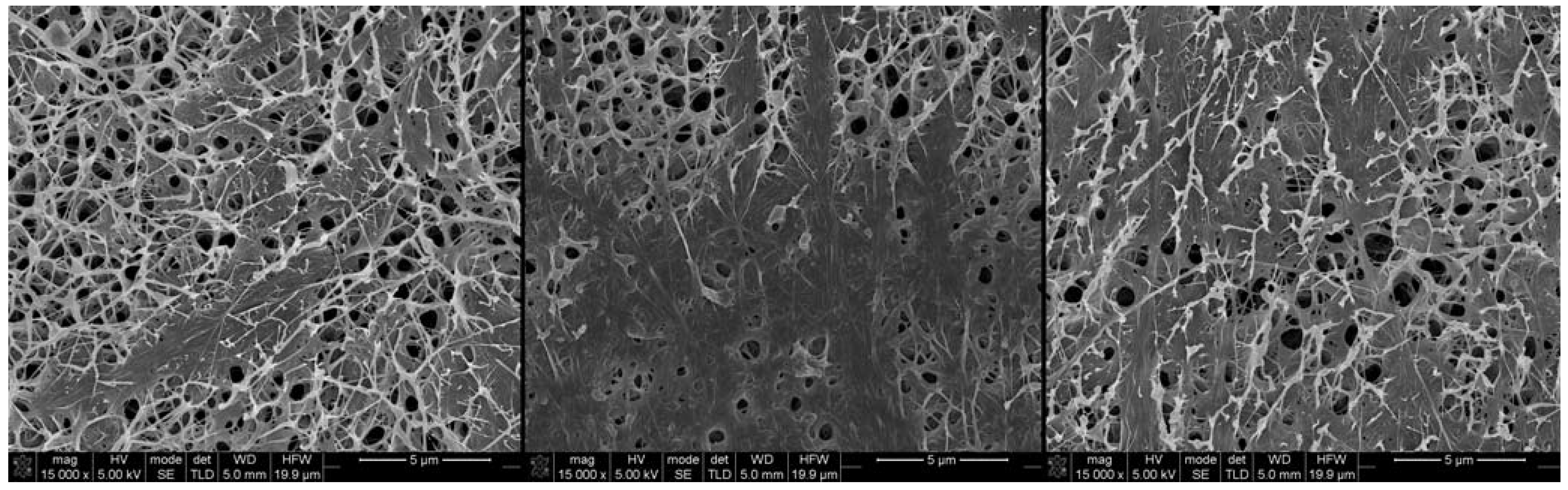
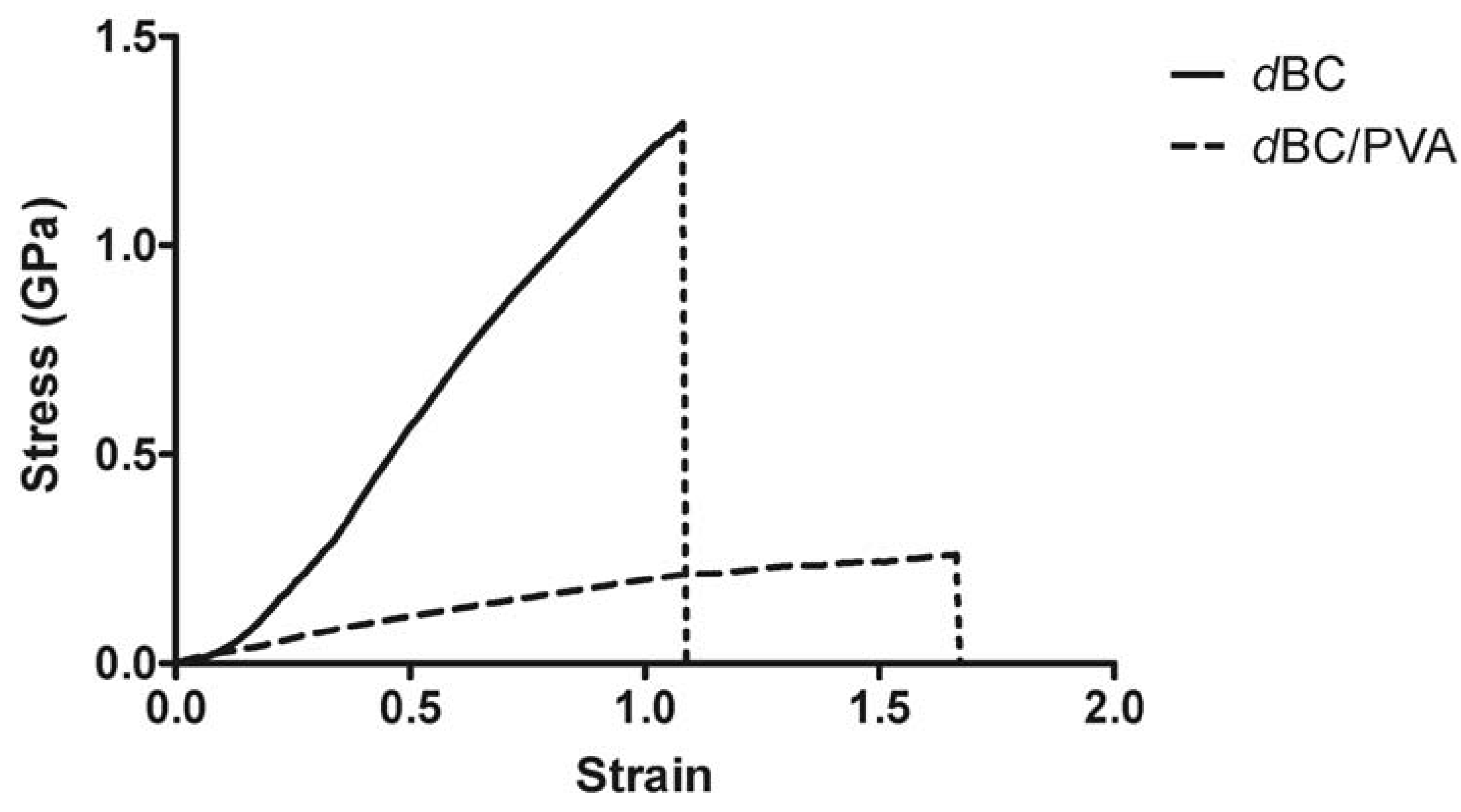
2.2. Mechanical Characterization of Dry BC and Dry BC/PVA Membranes
2.3. Diffusion Assays
| PEG Mw (Da) | Diffusion coefficient (cm2/sec) | |||
|---|---|---|---|---|
| ndBC | dBC | ndBC/PVA | dBC/PVA | |
| 900 | 7.40 × 10−5 | 1.12 × 10−7 | 4.48 × 10−5 | 1.16 × 10−5 |
| 8000 | 2.71 × 10−5 | 1.79 × 10−8 | 1.28 × 10−5 | 1.88 × 10−6 |
| 35,000 | 2.27 × 10−5 | 1.53 × 10−8 | 1.10 × 10−5 | 1.68 × 10−6 |
| 100,000 | 1.95 × 10−5 | 1.41 × 10−8 | 9.82 × 10−6 | 1.50 × 10−6 |
3. Experimental Section
3.1. Bacterial Cellulose and Bacterial Cellulose/Polyvinyl Alcohol
3.2. SEM Imaging
3.3. Stress-Strain Analysis
3.4. Diffusion Assays
3.5. Reagents
4. Conclusions
Acknowledgments
References
- Klemm, D.; Schumann, D.; Kramer, F.; Hessler, N.; Koth, D.; Sultanova, B. Nanocellulose materials—Different cellulose, different functionality. Macromol. Symp. 2009, 280, 60–71. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2006, 8, 1–12. [Google Scholar] [CrossRef]
- Sokolnicki, A.M.; Fisher, R.J.; Harrah, T.P.; Kaplan, D.L. Permeability of bacterial cellulose membranes. J. Membr. Sci. 2006, 272, 15–27. [Google Scholar] [CrossRef]
- Andersson, J.; Stenhamre, H.; Bäckdahl, H.; Gatenholm, P. Behavior of human chondrocytes in engineered porous bacterial cellulose scaffolds. J. Biomed. Mater. Res. Part A 2010, 94, 1124–1132. [Google Scholar]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, O.M.; Patel, M.; Booker, J.; Markowitz, L. Effectiveness of a biocellulose wound dressing for the treatment of chronic venous leg ulcers: Results of a single center randomized study involving 24 patients. Wounds 2004, 16, 224–233. [Google Scholar]
- Luan, J.; Wu, J.; Zheng, Y.; Song, W.; Wang, G.; Guo, J.; Ding, X. Impregnation of silver sulfadiazine into bacterial cellulose for antimicrobial and biocompatible wound dressing. Biomed. Mater. 2012, 7. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; Novaes, A.B. Bone formation over a TiAl6V4(IMZ) implant placed into an extraction socket in association with membrane therapy (Gengiflex). Clin. Oral Implant. Res. 1993, 4, 106–110. [Google Scholar] [CrossRef]
- Dos Anjos, B.; Novaes, A.B., Jr.; Meffert, R.; Barboza, E.P. Clinical comparison of cellulose and expanded polytetrafluoroethylene membranes in the treatment of class II furcations in mandibular molars with 6-month re-entry. J. Periodontol. 1998, 69, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Andrade, F.K.; Silva, J.P.; Carvalho, M.; Coutinho, E.M.S.; Soares, R.; Gama, F.M. Studies on the hemocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2011, 98, 554–566. [Google Scholar] [CrossRef]
- Backdahl, H.; Risberg, B.; Gatenholm, P. Observations on bacterial cellulose tube formation for application as vascular graft. Mater. Sci. Eng. C 2011, 31, 14–21. [Google Scholar] [CrossRef]
- Grasso, D.; Strevett, K.; Fisher, R. Uncoupling mass transfer limitations of gaseous substrates in microbial systems. Chem. Eng. J. Biochem. Eng. J. 1995, 59, 195–204. [Google Scholar] [CrossRef]
- Serafica, G.; Mormino, R.; Bungay, H. Inclusion of solid particles in bacterial cellulose. Appl. Microbiol. Biotechnol. 2002, 58, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.M.; Rawn, C.J. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Bodugoz-Senturk, H.; Macias, C.E.; Kung, J.H.; Muratoglu, O.K. Poly(vinyl alcohol)-acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials 2009, 30, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.-R.; Kim, J.; Lee, J.; Kim, Y.; Kim, J.; Chang, S.; Jin, S.; Kim, J.; Lyoo, W.; Han, S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. Aaps Pharmscitech 2010, 11, 1092–1103. [Google Scholar]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]
- Millon, L.E.; Guhados, G.; Wan, W. Anisotropic polyvinyl alcohol—Bacterial cellulose nanocomposite for biomedical applications. J. Biomed. Mater. Res. Part B 2008, 86, 444–452. [Google Scholar] [CrossRef]
- Gea, S.; Bilotti, E.; Reynolds, C.T.; Soykeabkeaw, N.; Peijs, T. Bacterial cellulose—Poly(vinyl alcohol) nanocomposites prepared by an in-situ process. Mater. Lett. 2010, 64, 901–904. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Kudo, S.; Otsuka, E.; Suzuki, A. Swelling behavior of chemically crosslinked PVA gels in mixed solvents. J. Polym. Sci. Part B 2010, 48, 1978–1986. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Sabapathy, S.N.; Bawa, A.S. Physico-mechanical properties of chemically treated bacterial (acetobacter xylinum) cellulose membrane. World J. Microbiol. Biotechnol. 2005, 21, 1323–1327. [Google Scholar] [CrossRef]
- Bodin, A.; Concaro, S.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 2007, 1, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Young, R.J.; Lindström, T.; Sampson, W.W.; Eichhorn, S.J. Effective Young’s modulus of bacterial and microfibrillated cellulose fibrils in fibrous networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Sian, C.; Gea, S.; Nishino, T.; Peijs, T. All-cellulose nanocomposites by surface selective dissolution of bacterial cellulose. Cellulose 2009, 16, 435–444. [Google Scholar] [CrossRef]
- Yasuda, H.; Lamaze, C.E.; Peterlin, A. Diffusive and hydraulic permeabilities of water in water-swollen polymer membranes. J. Polym. Sci. Part A 1971, 9, 1117–1131. [Google Scholar] [CrossRef]
- Fisher, R.J. Diffusion with immobilization in membranes: Transport and failure mechanisms. Prog. Clin. Biol. Res. 1989, 292, 129–151. [Google Scholar] [PubMed]
- Fang, Y.-E.; Cheng, Q.; Lu, X.-B. Kinetics of in vitro drug release from chitosan/gelatin hybrid membranes. J. Appl. Polym. Sci. 1998, 68, 1751–1758. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leitão, A.F.; Silva, J.P.; Dourado, F.; Gama, M. Production and Characterization of a New Bacterial Cellulose/Poly(Vinyl Alcohol) Nanocomposite. Materials 2013, 6, 1956-1966. https://doi.org/10.3390/ma6051956
Leitão AF, Silva JP, Dourado F, Gama M. Production and Characterization of a New Bacterial Cellulose/Poly(Vinyl Alcohol) Nanocomposite. Materials. 2013; 6(5):1956-1966. https://doi.org/10.3390/ma6051956
Chicago/Turabian StyleLeitão, Alexandre F., João Pedro Silva, Fernando Dourado, and Miguel Gama. 2013. "Production and Characterization of a New Bacterial Cellulose/Poly(Vinyl Alcohol) Nanocomposite" Materials 6, no. 5: 1956-1966. https://doi.org/10.3390/ma6051956





