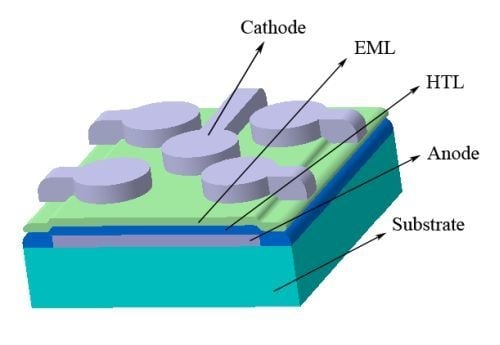
Effect of Solvents, Their Mixture and Thermal Annealing on the Performance of Solution Processed Polymer Light-Emitting Diodes
Abstract
:1. Introduction
2. Results and Discussion
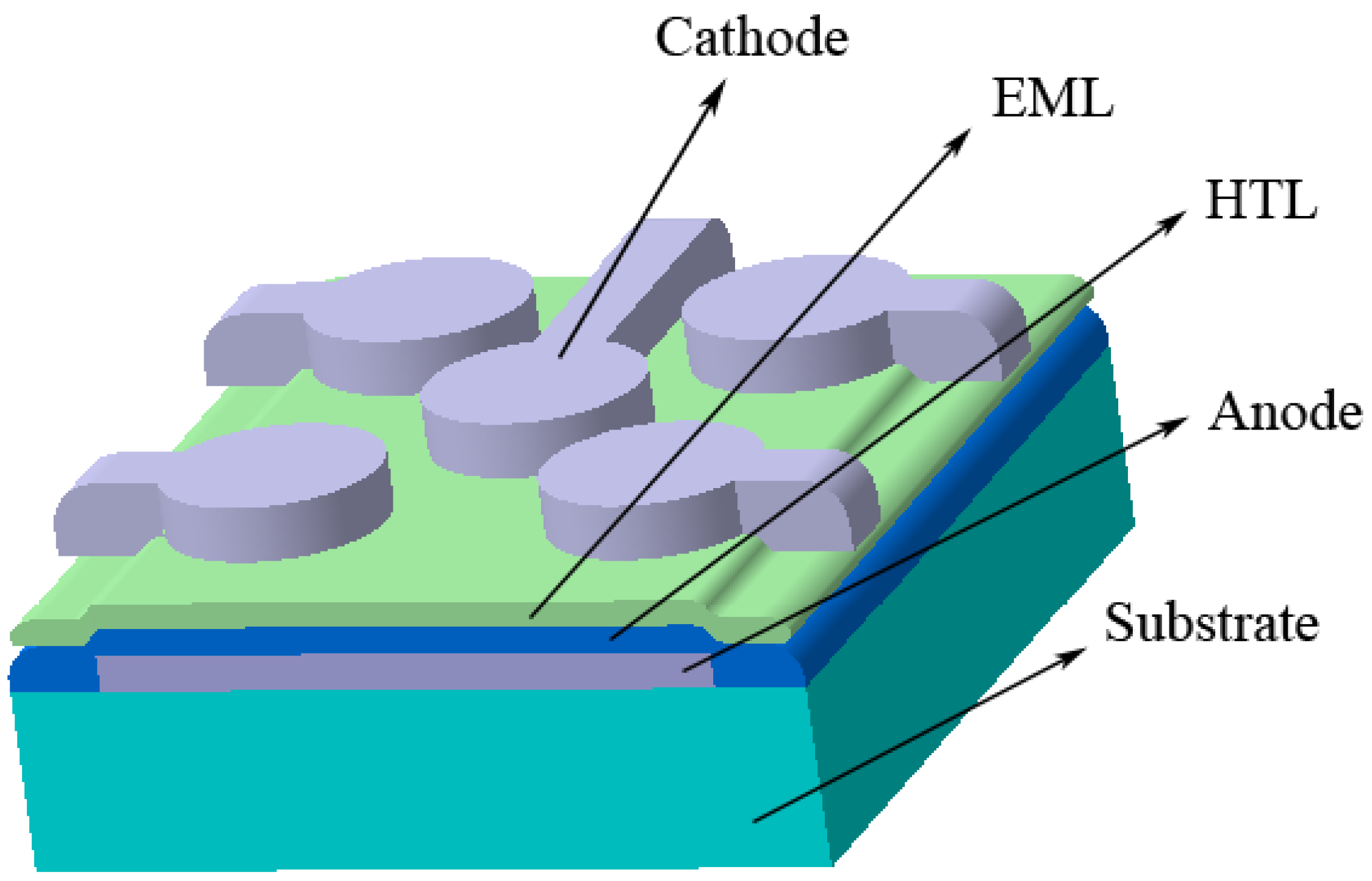
2.1. Solvent Mixture

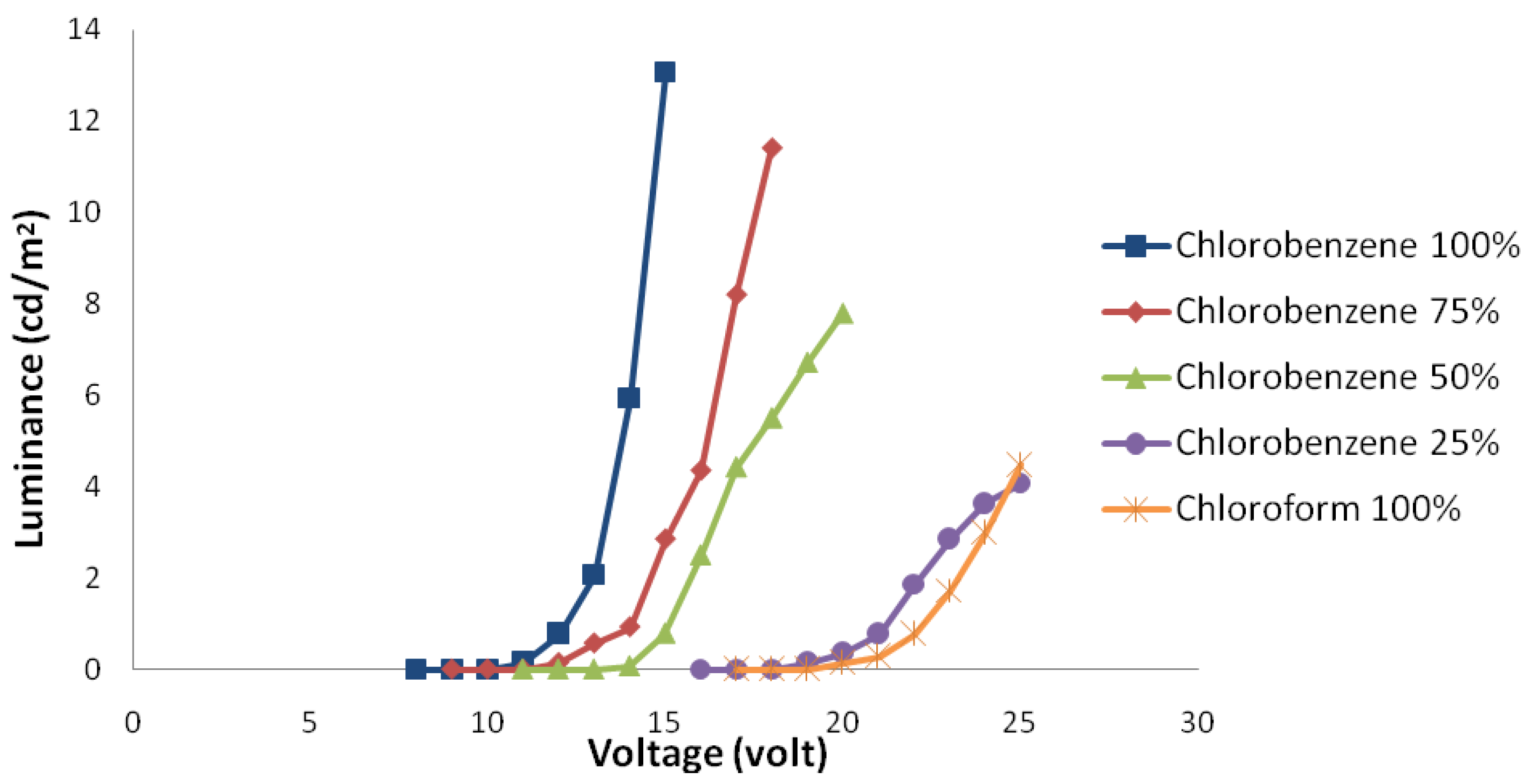
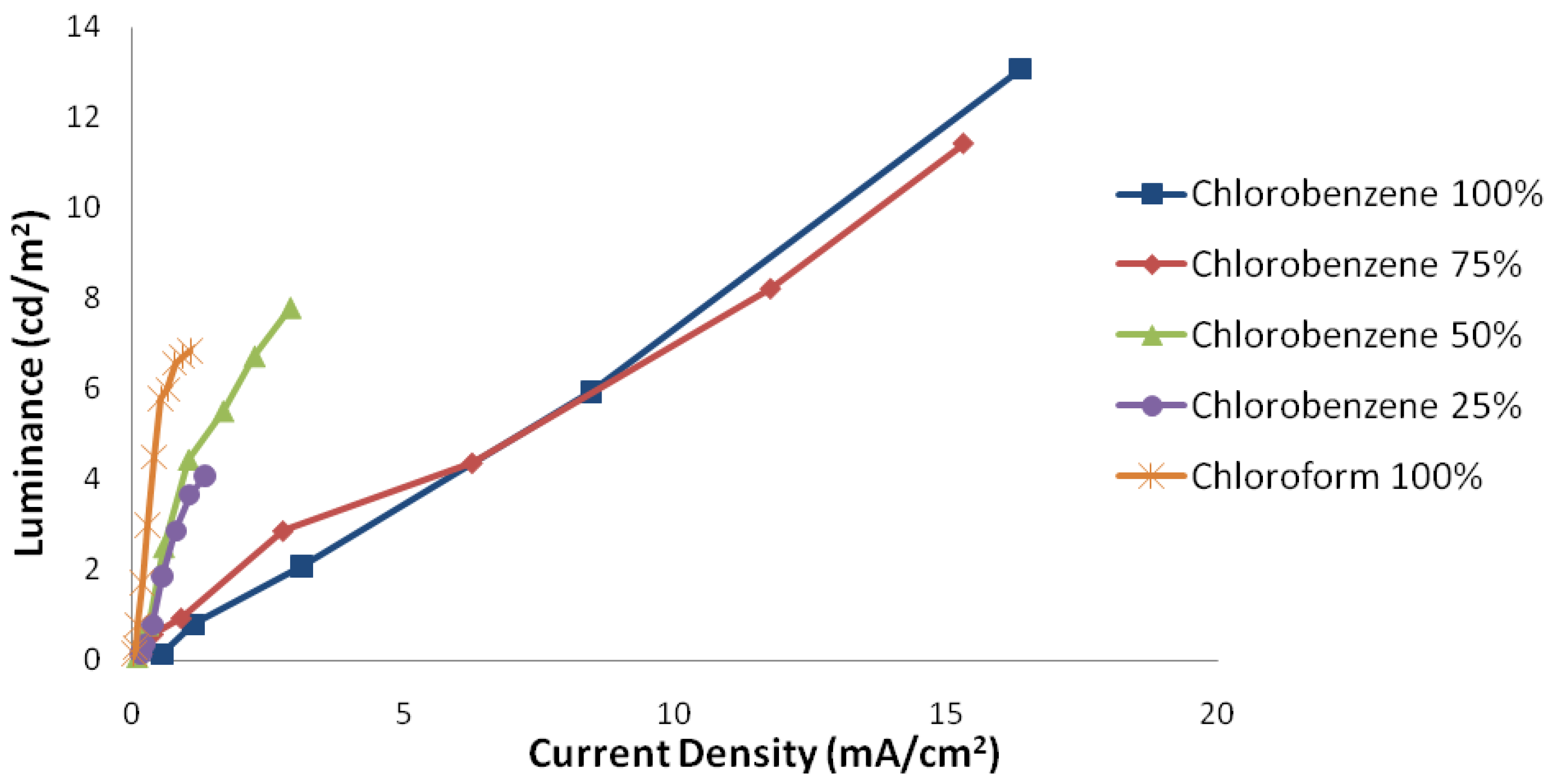
2.1.1. The Effect of Layer Thicknesses
2.1.2. The Effect of Layer Surface Uniformities
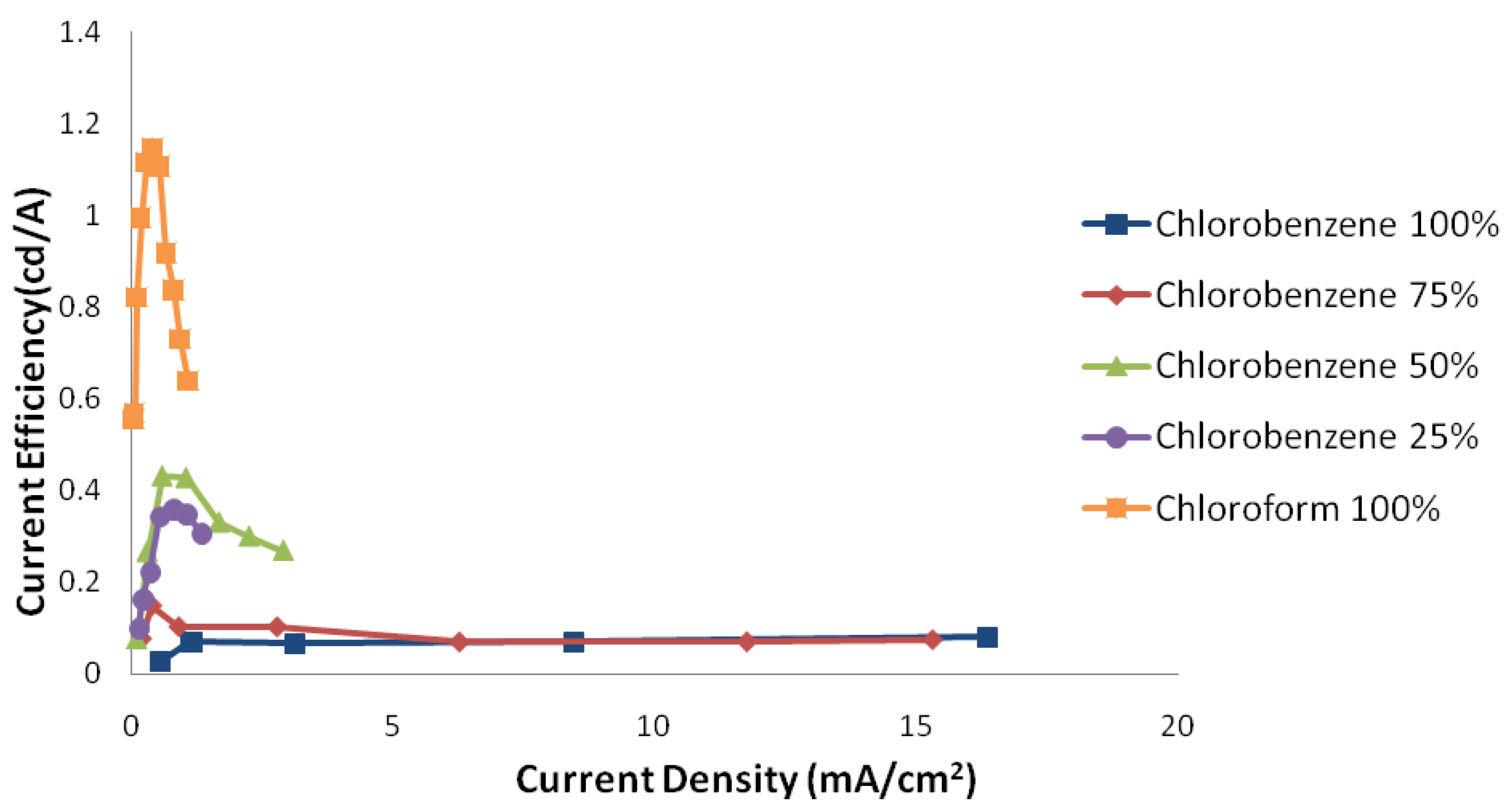
2.1.3. The Effect of Internal Nano-Structure Morphology

2.2. Thermal Annealing Process

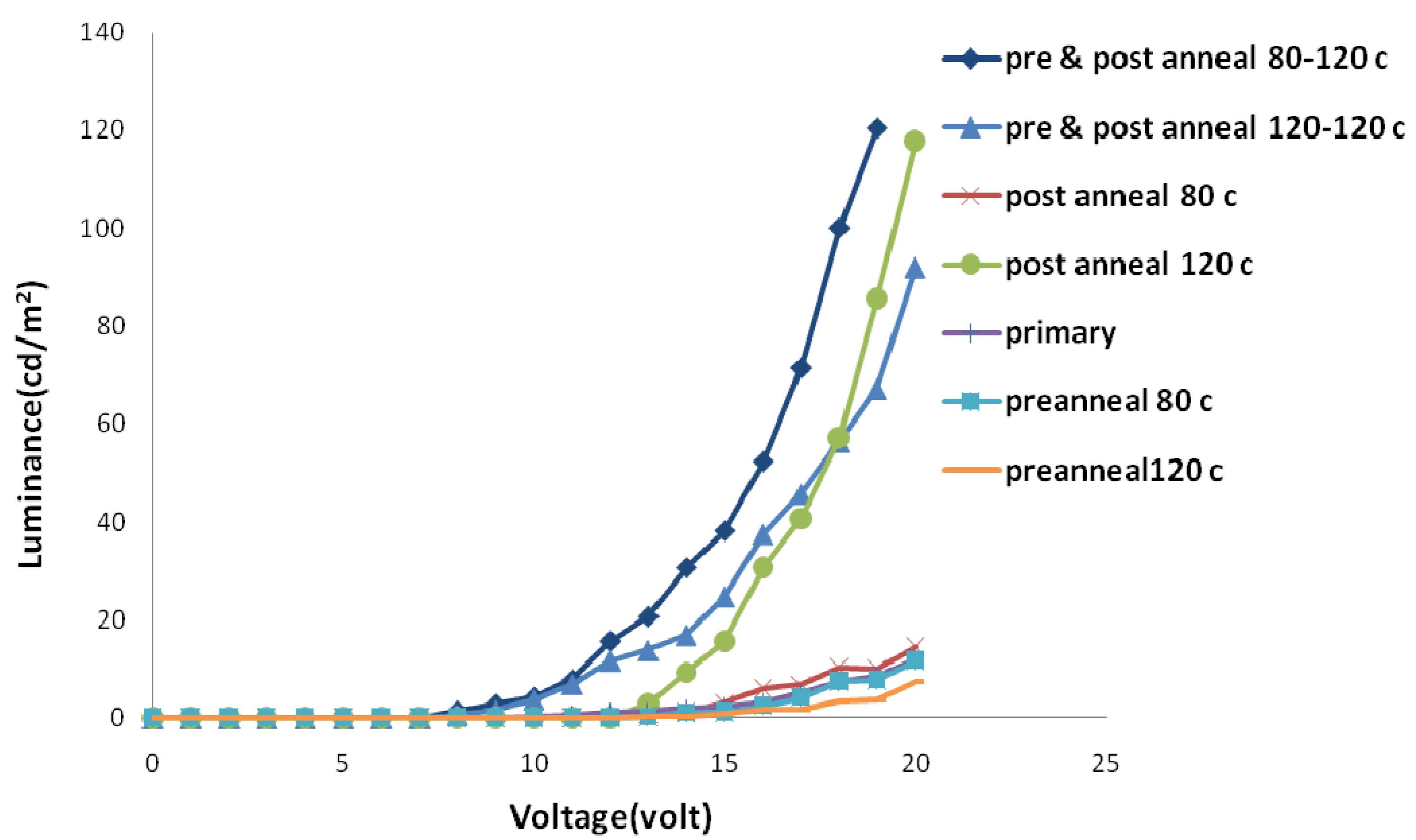
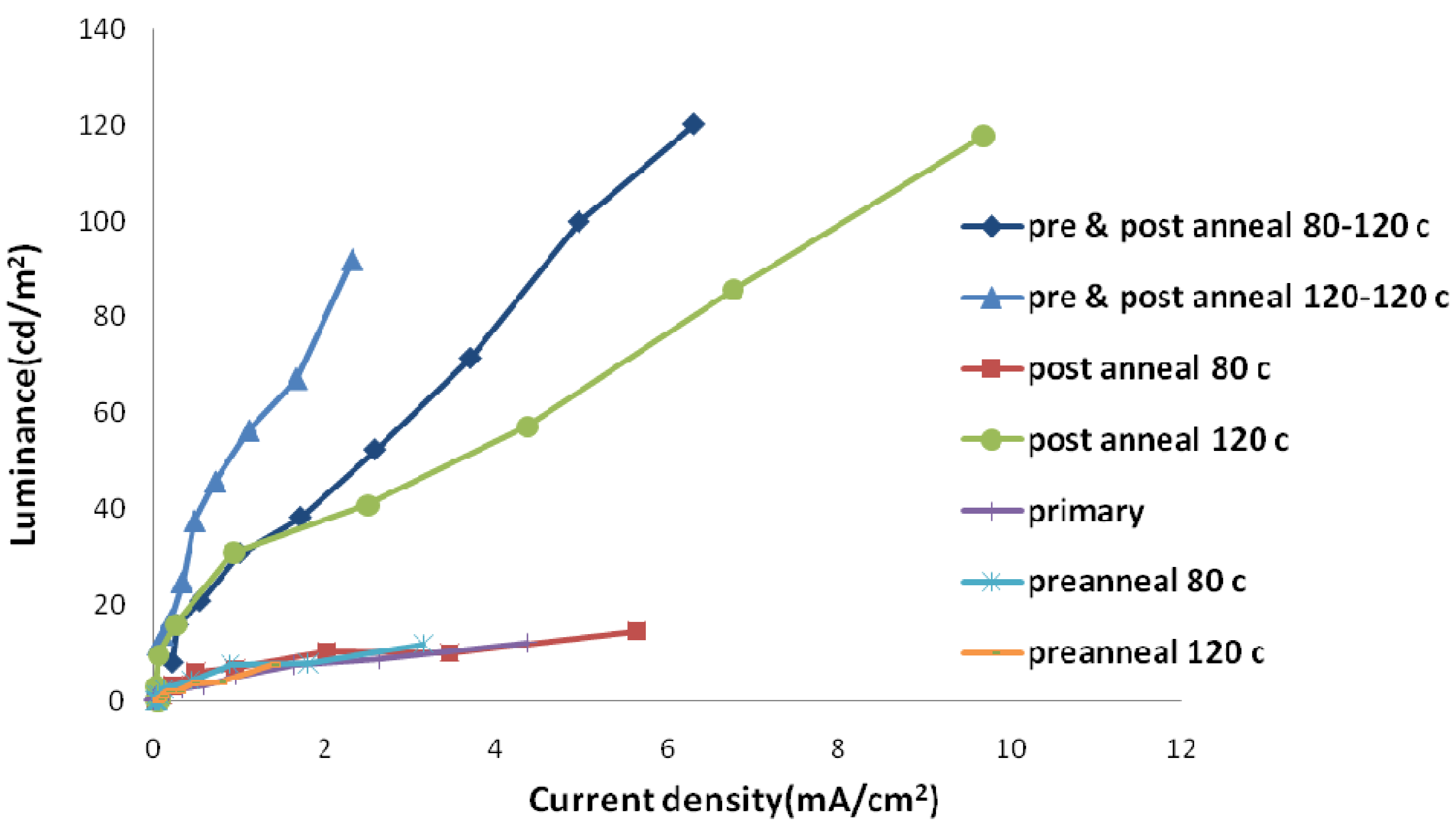
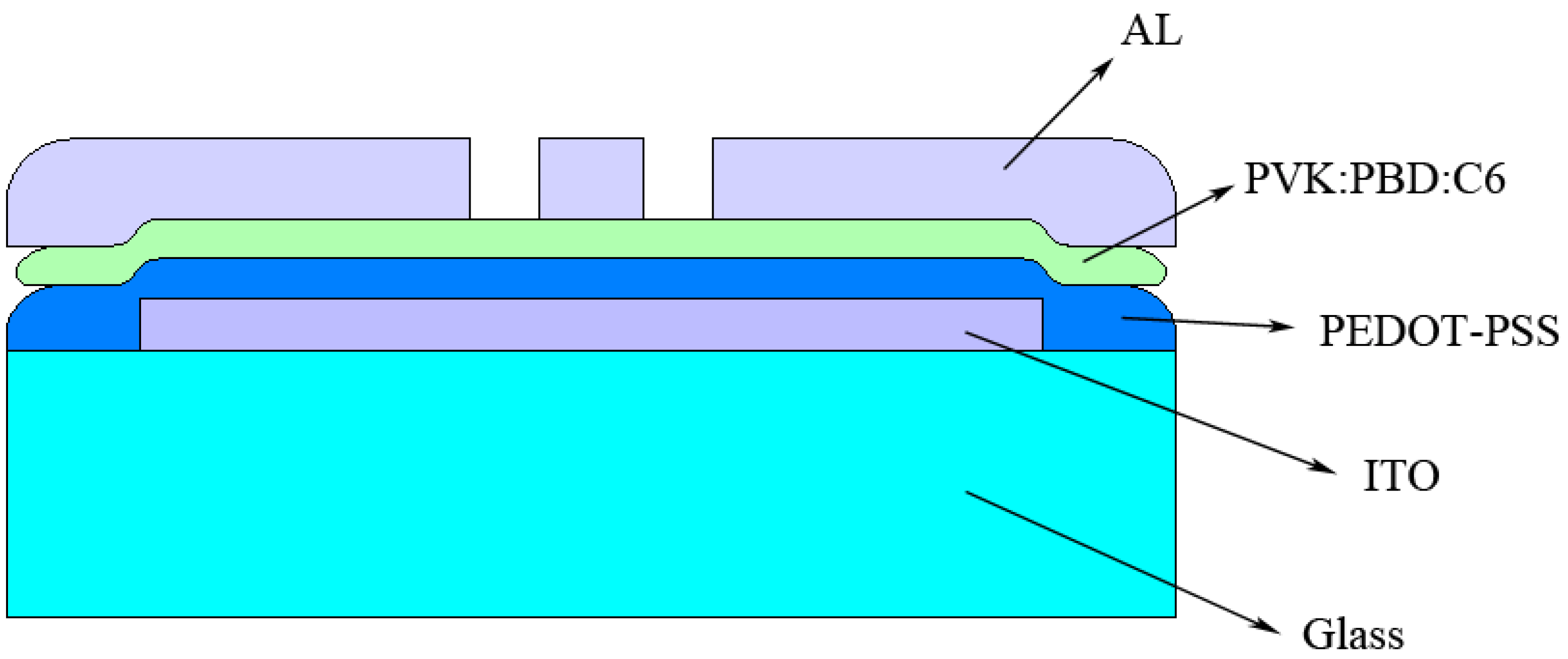
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Tessler, N.; Denton, G.; Friend, R. Lasing from conjugated-polymer microcavities. Nature 1996, 382, 695–697. [Google Scholar] [CrossRef]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Galatsis, K.; Wang, K.; Botros, Y.; Yang, Y.; Xie, Y.H.; Stoddart, J.; Kaner, R.B.; Ozhan, C.; Liu, J.; Ozkan, M. Emerging memory devices. Circuits Devices Mag. IEEE 2006, 22, 12–21. [Google Scholar] [CrossRef]
- Muccini, M. A bright future for organic field-effect transistors. Nat. Mater. 2006, 5, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Chen, Y.; Elshobaki, M.; Mahadevapuram, R.C.; Chaudhary, S. Controlling nanomorphology in plastic solar cells. Nanomater. Energy 2012, 1, 18–26. [Google Scholar] [CrossRef]
- Burroughes, J.; Bradley, D.D.C.; Brown, A.; Marks, R.; Mackay, K.; Friend, R.; Burns, P.; Holmes, A. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Friend, R.; Gymer, R.; Holmes, A.; Burroughes, J.; Marks, R.; Taliani, C.; Bradley, D.; Dos Santos, D.; Bredas, J.; Lögdlun, M. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Bernius, M.T.; Inbasekaran, M.; O’Brien, J.; Wu, W. Progress with light-emitting polymers. Adv. Mater. 2000, 12, 1737–1750. [Google Scholar] [CrossRef]
- Capelli, R.; Toffanin, S.; Generali, G.; Usta, H.; Facchetti, A.; Muccini, M. Organic light-emitting transistors with an efficiency that outperforms the equivalent light-emitting diodes. Nat. Mater. 2010, 9, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y. Light-emitting devices with conjugated polymers. Int. J. Mol. Sci. 2011, 12, 1575–1594. [Google Scholar] [CrossRef] [PubMed]
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N. Studies of spin-coated polymer films. Annu. Rep. Prog. Chem. C Phys. Chem. 2005, 101, 174–201. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Solar Energy Mater. Solar Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Zhang, F.; Jespersen, K.G.; Bjoerstroem, C.; Svensson, M.; Andersson, M.R.; Sundström, V.; Magnusson, K.; Moons, E.; Yartsev, A.; Inganaes, O. Influence of solvent mixing on the morphology and performance of solar cells based on polyfluorene copolymer/fullerene blends. Adv. Funct. Mater. 2006, 16, 667–674. [Google Scholar] [CrossRef]
- Yao, Y.; Hou, J.; Xu, Z.; Li, G.; Yang, Y. Effects of solvent mixtures on the nanoscale phase separation in polymer solar cells. Adv. Funct. Mater. 2008, 18, 1783–1789. [Google Scholar] [CrossRef]
- Fahlman, M.; Salaneck, W.R. Surfaces and interfaces in polymer-based electronics. Surf. Sci. 2002, 500, 904–922. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Liu, J.; Guo, T.F. The Control of Morphology and the Morphological Dependence of Device Electrical and Optical Properties in Polymer Electronics. In Electronic and Optical Properties of Conjugated Molecular Systems in Condensed Phases; Hotta, S., Ed.; Research Signpost: Kerala, India, 2003; pp. 307–354. [Google Scholar]
- Müllen, K.; Scherf, U. Organic Light Emitting Devices: Synthesis, Properties and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Padinger, F.; Rittberger, R.S.; Sariciftci, N.S. Effects of postproduction treatment on plastic solar cells. Adv. Funct. Mater. 2003, 13, 85–88. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Yao, Y.; Yang, Y. Investigation of annealing effects and film thickness dependence of polymer solar cells based on poly (3-hexylthiophene). J. Appl. Phys. 2005, 98, 043704:1–043704:5. [Google Scholar]
- Tengstedt, C.; Kanciurzewska, A.; de Jong, M.P.; Braun, S.; Salaneck, W.R.; Fahlman, M. Ultraviolet light–ozone treatment of poly (3,4-ethylenedioxy-thiophene)-based materials resulting in increased work functions. Thin Solid Films 2006, 515, 2085–2090. [Google Scholar] [CrossRef]
- Jin, H.; Hou, Y.; Meng, X.; Li, Y.; Shi, Q.; Teng, F. Enhanced photovoltaic properties of polymer–fullerene bulk heterojunction solar cells by thermal annealing. Solid State Commun. 2007, 142, 181–184. [Google Scholar] [CrossRef]
- Ray, B.; Nair, P.R.; Alam, M.A. Annealing dependent performance of organic bulk-heterojunction solar cells: A theoretical perspective. Solar Energy Mater. Solar Cells 2011, 95, 3287–3294. [Google Scholar] [CrossRef]
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.; Janssen, R.A.J. Nanoscale morphology of high-performance polymer solar cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Loos, J. Toward high-performance polymer solar cells: The importance of morphology control. Macromolecules 2007, 40, 1353–1362. [Google Scholar] [CrossRef]
- Pivrikas, A. Relation between Nanomorphology and Performance of Polymer-based Solar Cells. In Solar Cells—New Aspects and Solutions; Kosyachenko, L.A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 121–148. [Google Scholar]
- Brown, T.; Kim, J.; Friend, R.; Cacialli, F.; Daik, R.; Feast, W. Built-in field electroabsorption spectroscopy of polymer light-emitting diodes incorporating a doped poly (3,4-ethylene dioxythiophene) hole injection layer. Appl. Phys. Lett. 1999, 75, 1679–1681. [Google Scholar] [CrossRef]
- Skotheim, T.A.; Reynolds, J.R. Handbook of Conducting Polymers: Theory, Synthesis Properties and Characterization, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Smallwood, I.M. Handbook of Organic Solvent Properties, 1st ed.; Arnold: London, UK, 1996. [Google Scholar]
- Chang, M.Y.; Yen, H.B.; Hung, C.Y.; Chen, Y.F.; Lin, S.H.; Huang, W.Y.; Hanb, Y.K. Effect of solvent-assisted thermal treatment on the performance of polyfluorene-based polymer light emitting diodes. J. Electrochem. Soc. 2010, 157, J116–J119. [Google Scholar] [CrossRef]
- Chen, C.W.; Cho, T.Y.; Wu, C.C.; Yu, H.L.; Luh, T.Y. Fuzzy-junction organic light-emitting devices. Appl. Phys. Lett. 2002, 81, 1570–1572. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, Y. Naturally formed graded junction for organic light-emitting diodes. Appl. Phys. Lett. 2003, 83, 2453–2455. [Google Scholar] [CrossRef]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Treat, N.D.; Brady, M.A.; Smith, G.; Toney, M.F.; Kramer, E.J.; Hawker, C.J.; Chabinyc, M.L. Interdiffusion of PCBM and P3HT reveals miscibility in a photovoltaically active blend. Adv. Funct. Mater. 2011, 1, 82–89. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rezvani, M.H.; Farajollahi, F.; Nikfarjam, A.; Bakhtiarpour, P.; Saydanzad, E. Effect of Solvents, Their Mixture and Thermal Annealing on the Performance of Solution Processed Polymer Light-Emitting Diodes. Materials 2013, 6, 1994-2006. https://doi.org/10.3390/ma6051994
Rezvani MH, Farajollahi F, Nikfarjam A, Bakhtiarpour P, Saydanzad E. Effect of Solvents, Their Mixture and Thermal Annealing on the Performance of Solution Processed Polymer Light-Emitting Diodes. Materials. 2013; 6(5):1994-2006. https://doi.org/10.3390/ma6051994
Chicago/Turabian StyleRezvani, Mohammad Hashem, Farid Farajollahi, Alireza Nikfarjam, Parisa Bakhtiarpour, and Erfan Saydanzad. 2013. "Effect of Solvents, Their Mixture and Thermal Annealing on the Performance of Solution Processed Polymer Light-Emitting Diodes" Materials 6, no. 5: 1994-2006. https://doi.org/10.3390/ma6051994