Synthesis of Zeolites Na-P1 from South African Coal Fly Ash: Effect of Impeller Design and Agitation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fly Ash Characterization
2.1.1. X-ray Fluorescence
| Major oxides | Trace elements | ||
|---|---|---|---|
| Oxides | (Mean mass %) | Elements | Concentration (ppm) |
| SiO2 | 55.66 | As | 49.10 |
| Al2O3 | 27.95 | Ba | 708.93 |
| Fe2O3 | 3.22 | Ce | 128.45 |
| MnO | 0.04 | Co | 43.87 |
| MgO | 1.91 | Cu | 46.88 |
| CaO | 4.38 | Nb | 58.10 |
| Na2O | 0.31 | Ni | 24.40 |
| K2O | 0.45 | Pb | 56.56 |
| TiO2 | 1.13 | Rb | 29.29 |
| P2O5 | 0.26 | Sr | 1011.45 |
| SO3 | 0.03 | V | 113.49 |
| Loss On Ignition | 4.74 | Y | 84.78 |
| Sum (%) | 100.07 | Zn | 42.09 |
| SiO2/Al2O3 | 1.99 | Zr | 442.60 |
| - | - | Mo | 11.28 |
| - | - | Th | 384.21 |
2.1.2. X-ray Powder Diffraction
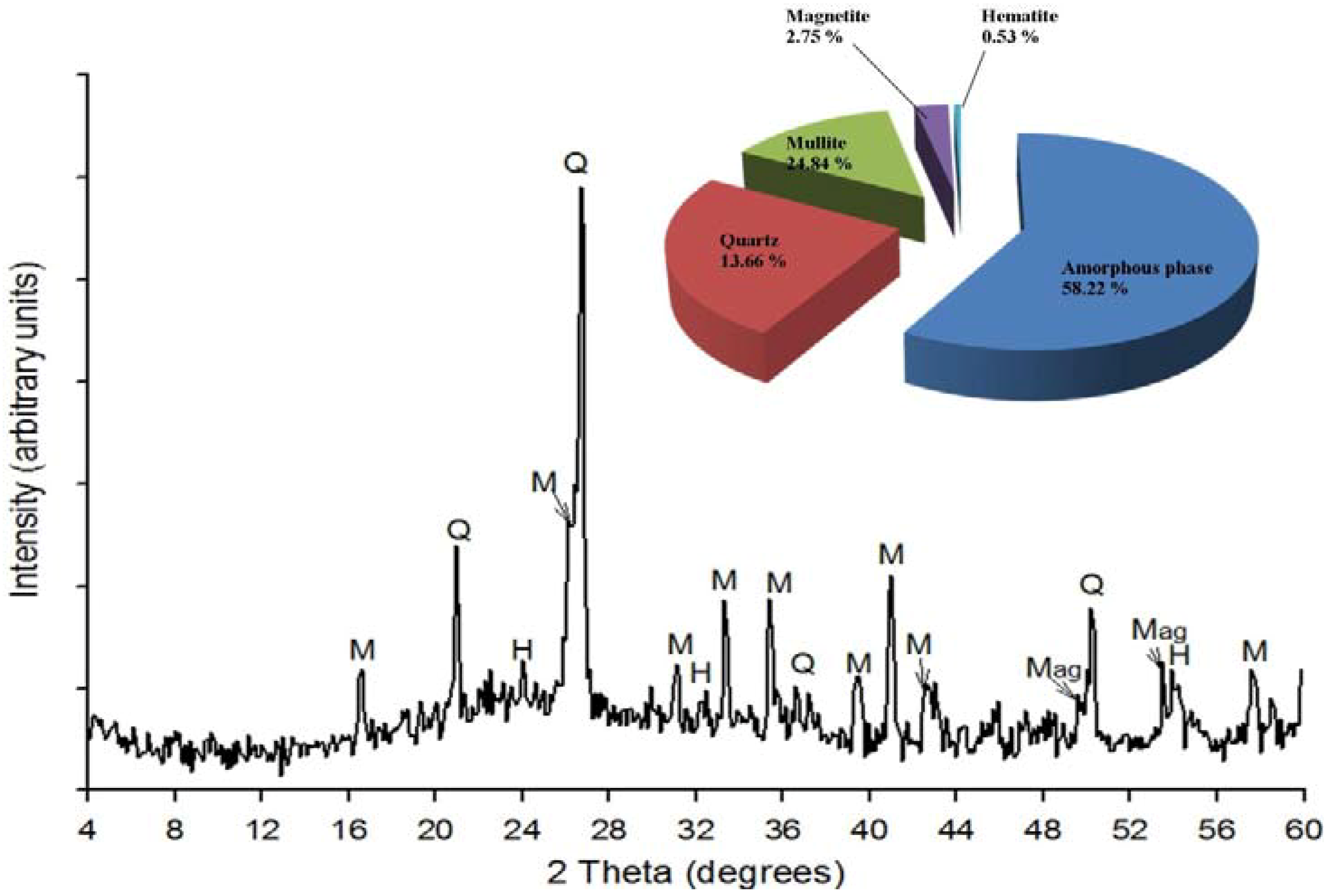
2.1.3. Morphology by SEM

2.2. Product Characterization
2.2.1. Mineralogical Composition
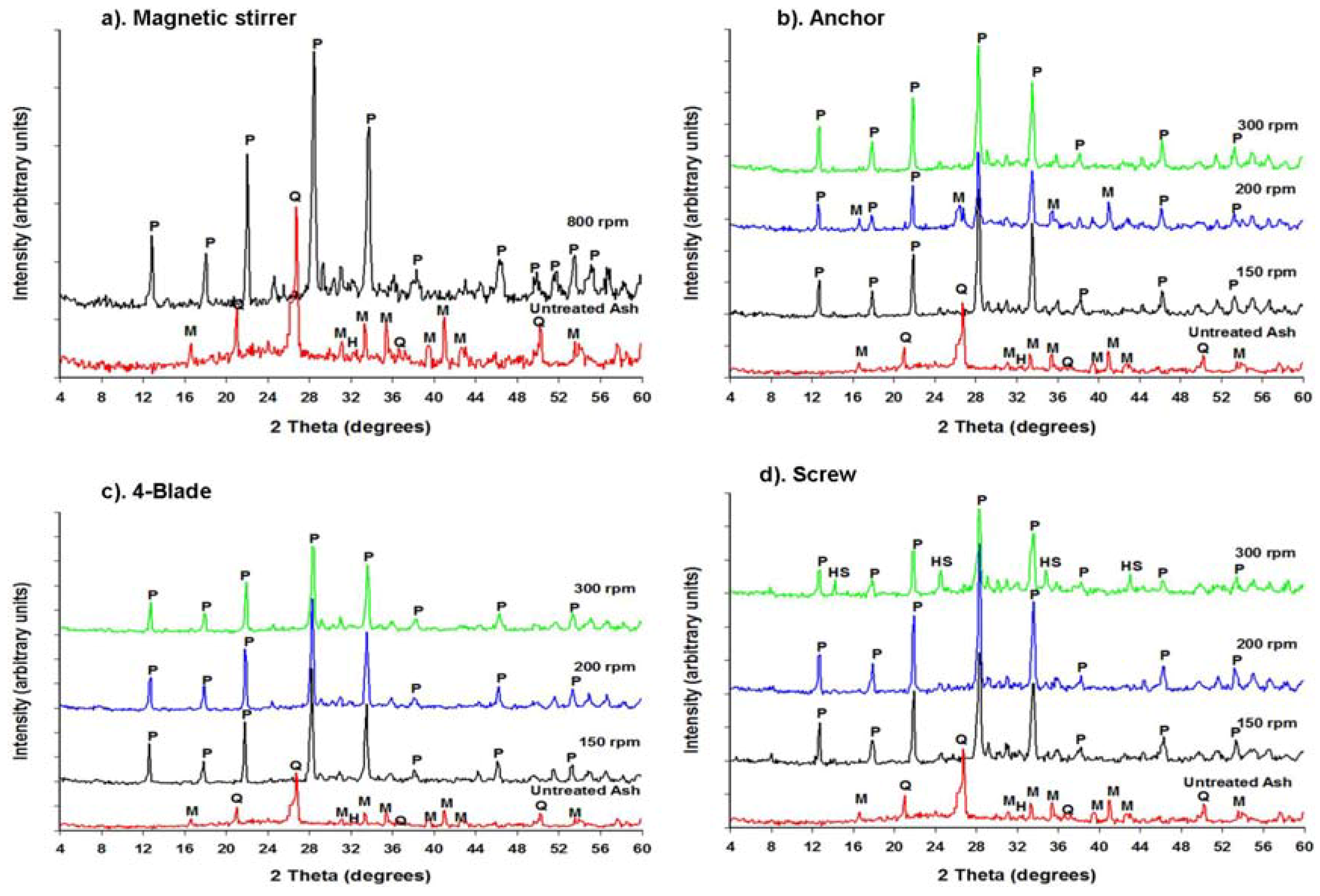
| Impeller | Speed (rpm) | Crystallinity (%) | Impurities |
|---|---|---|---|
| 4-Flat Blade | 150 | 100 | - |
| 200 | 100 | - | |
| 300 | 94.9 | - | |
| Screw | 150 | 92.1 | - |
| 200 | 94.7 | - | |
| 300 | 69.4 | ±30% hydroxysodalite | |
| Anchor | 150 | 91.0 | - |
| 200 | 56.6 | ±40% mullite | |
| 300 | 96.1 | - | |
| Magnetic Stirrer | 800 | 93.0 | - |
2.2.2. Structural Analysis of the Synthesized Zeolites
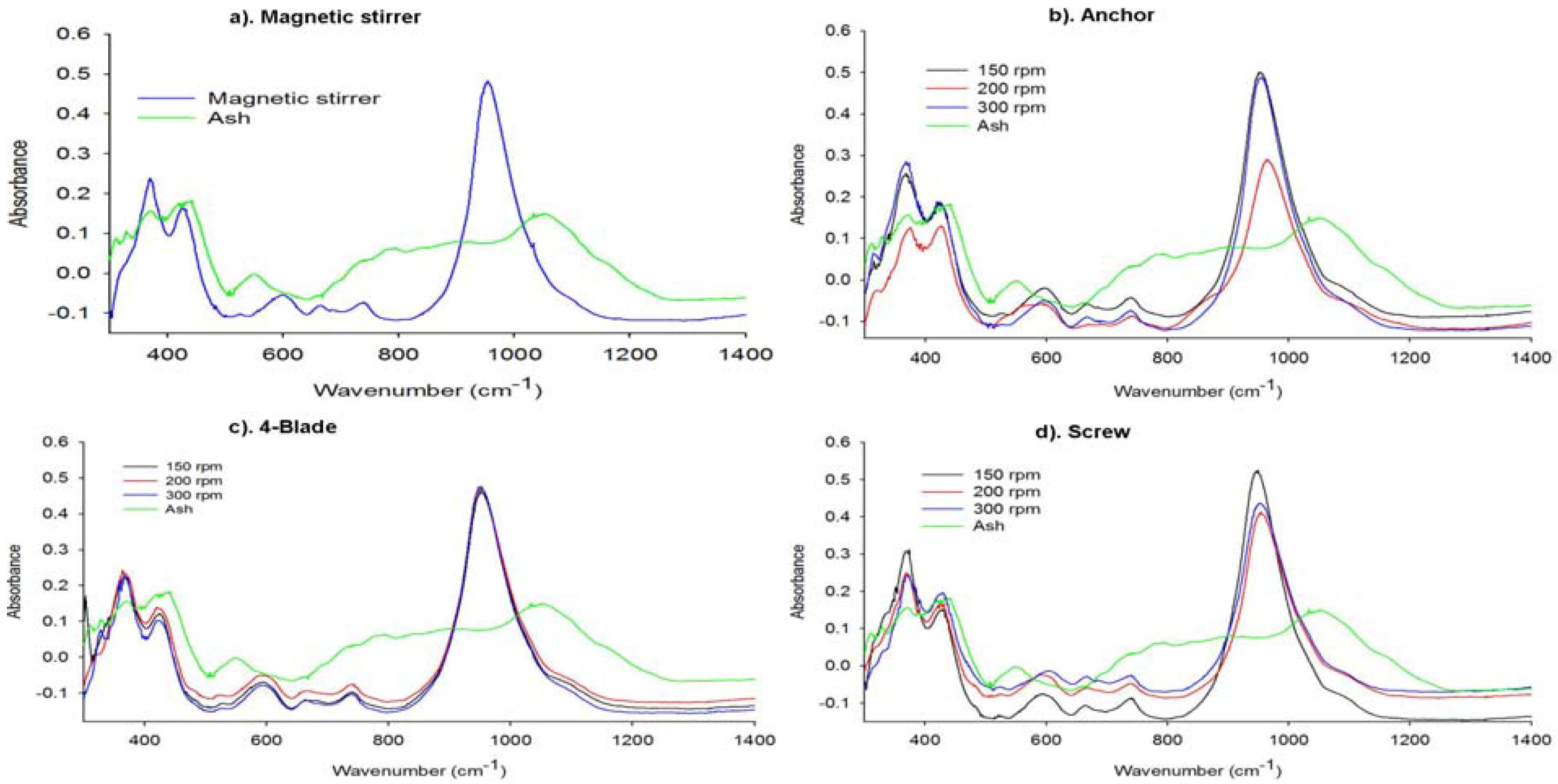
2.2.3. Elemental Composition of the Synthesis Product
| Major oxides (Mass %) | Trace elements (ppm) | ||||
|---|---|---|---|---|---|
| Oxides | Average* | Standard deviation* | Element | Average * | Standard deviation* |
| SiO2 | 42.60 | 1.90 | As | 47.15 | 2.25 |
| TiO2 | 1.51 | 0.05 | Ba | 679.13 | 25.55 |
| Al2O3 | 27.10 | 1.35 | Ce | 137.34 | 9.35 |
| Fe2O3 | 4.53 | 0.17 | Co | 42.11 | 2.14 |
| MnO | 0.05 | 0.00 | Cu | 46.12 | 1.40 |
| MgO | 1.33 | 0.04 | Nb | 57.15 | 1.30 |
| CaO | 4.05 | 0.08 | Ni | 24.05 | 2.50 |
| Na2O | 10.45 | 0.70 | Pb | 51.35 | 3.22 |
| K2O | 0.26 | 0.05 | Rb | 32.23 | 3.85 |
| P2O5 | 0.06 | 0.00 | Sr | 981.44 | 36.00 |
| Cr2O3 | 0.03 | 0.00 | V | 111.35 | 2.65 |
| NiO | 0.01 | 0.00 | Y | 81.12 | 3.04 |
| V2O5 | 0.01 | 0.00 | Zn | 39.00 | 3.00 |
| ZrO2 | 0.05 | 0.00 | Zr | 420.55 | 14.5 |
| CuO | <0.01 | <0.01 | Mo | 10.25 | 0.80 |
| LOI | 7.62 | 4.19 | Th | 350.05 | 18.25 |
| TOTAL | 99.66 | 0.29 | - | - | - |
| SiO2/Al2O3 | 1.57 | 0.01 | - | - | - |
2.2.4. Morphological Characterization by SEM
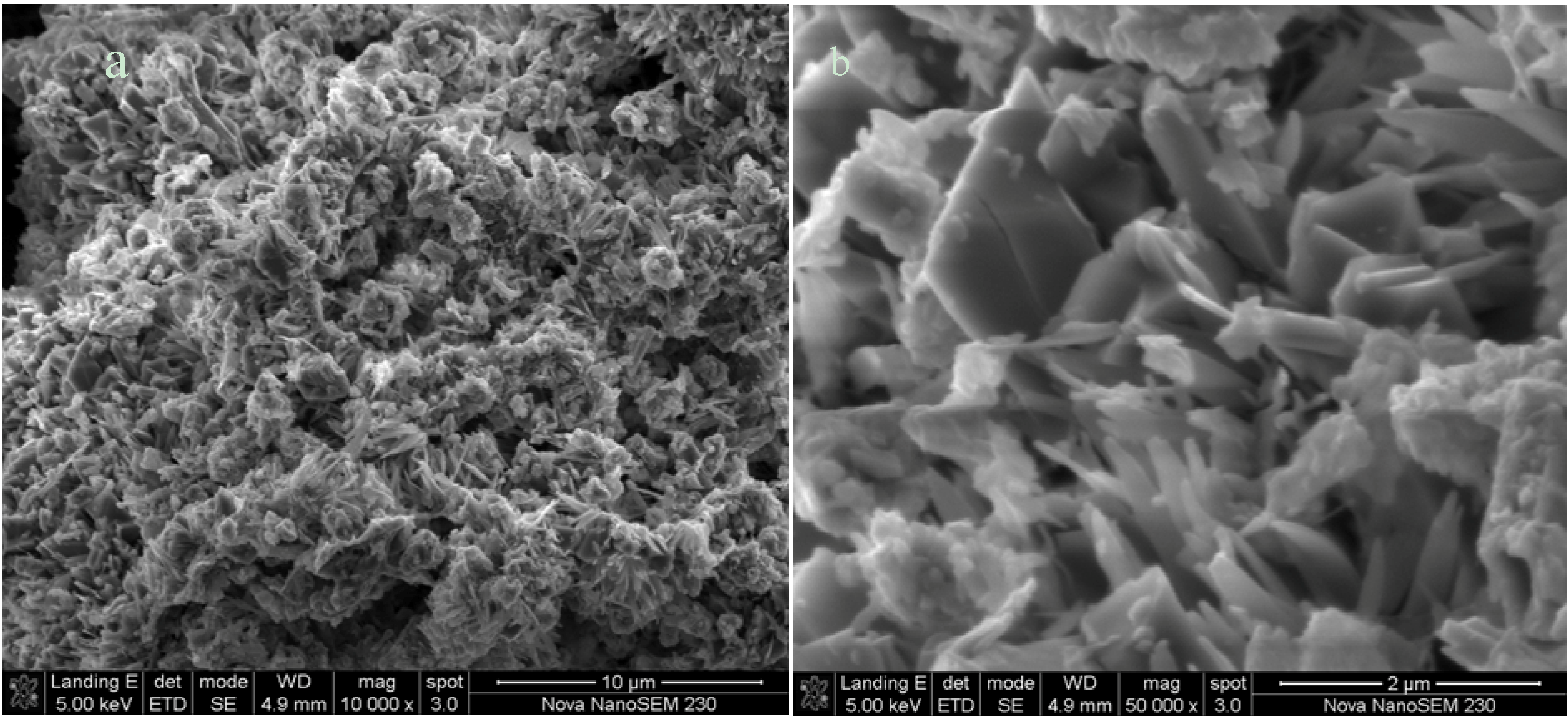
3. Experimental Section
3.1. Materials
3.2. Preparation of Zeolites

3.3. Characterization Techniques
4. Conclusions
Acknowledgments
References
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Eskom Annual Report 2011. Available online: http://financialresults.co.za/2011/eskom_ar2011/index.php (accessed on 11 September 2012).
- Iyer, R.S.; Scott, J.A. Power station fly ash-a review of value added utilization outside of the construction industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Petrik, L.F.; White, R.A.; Klink, M.J.; Somerset, S.; Burgers, L.; Fey, V. Utilization of South African Fly Ash to Treat Acid Coal Mine Drainage, and Production of High Quality Zeolites from the Residual Solids. In Proceedings of the Ash Utilization Symposium, Lexington, KY, USA, 20–22 October 2003.
- Gitari, W.M.; Petrik, L.F.; Etchebers, O.; Key, D.L.; Okujeni, C. Utilization of fly ash for treatment of coal mines wastewater: Solubility controls on major inorganic contaminants. Fuel 2008, 87, 2450–2462. [Google Scholar] [CrossRef]
- Vadapalli, V.R.K.; Klink, M.J.; Etchebers, O.; Petrik, L.F.; Gitari, W.; White, R.A.; Key, D.; Iwuoha, E. Neutralization of acid mine drainage using fly ash, and strength development of the resulting solid residues. S. Afr. J. Sci. 2008, 104, 317–322. [Google Scholar]
- Madzivire, G.; Petrik, L.F.; Gitari, W.M.; Ojumu, T.V.; Balfour, G. Application of coal fly ash to circumneutral mine waters for the removal of sulphates as gypsum and ettringite. Miner. Eng. 2010, 23, 252–257. [Google Scholar] [CrossRef]
- Madzivire, G.; Gitari, W.M.; Vadapalli, V.; Ojumu, T.V.; Petrik, L.F. Fate of sulphate removed during the treatment of circumneutral mine water and acid mine drainage with coal fly ash: Modelling and experimental approach. Miner. Eng. 2011, 24, 1467–1477. [Google Scholar] [CrossRef]
- Querol, X.; Umana, J.C.; Plana, F.; Alastuey, A.; Lopez-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Garcia-Rojo, E. Synthesis of zeolites from fly ash in a pilot plant scale:Examples of potential environmental applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umana, J.C.; Alastuey, A.; Hernandez, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Somerset, V.; Petrik, L.; White, R.; Klink, M.; Key, D.; Iwuoha, E. The use of X-ray fluorescence (XRF) analysis in predicting the alkaline hydrothermal conversion of fly ash precipitates into zeolites. Talanta 2004, 64, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Musyoka, N.M.; Petrik, L.F.; Gitari, W.M.; Balfour, G.; Hums, E. Optimization of hydrothermal synthesis of pure phase zeolite Na-P1 from South African coal fly ashes. J. Environ. Sci. Health Part A 2012, 47, 337–350. [Google Scholar] [CrossRef]
- Musyoka, N.; Petrik, L.; Balfour, G.; Ndungu, P.; Gitari, W.; Hums, E. Synthesis of zeolites from coal fly ash: Application of a statistical experimental design. Res. Chem. Intermed. 2012, 38, 471–486. [Google Scholar] [CrossRef]
- Casci, J.L. Zeolite molecular sieves: Preparation and scale-up. Microporous Mesoporous Mater. 2005, 82, 217–226. [Google Scholar] [CrossRef]
- Marrot, B.; Bebon, C.; Colson, D.; Klein, J. Influence of the shear rate during the synthesis of zeolites. Cryst. Res. Technol. 2001, 36, 269–281. [Google Scholar] [CrossRef]
- Weitkamp, J.; Puppe, L. Catalysis and Zeolites: Fundamentals and Applications; Springer: Berlin, German, 1999; p. 564. [Google Scholar]
- Musyoka, N.M. Hydrothermal Synthesis and Optimisation of Zeolite Na-P1 from South African Coal Fly Ash; University of the Western Cape: Cape Town, South Africa, 2009. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley & Sons, Inc.: New York, NY, USA, 1974; p. 771. [Google Scholar]
- Querol, X.; Plana, F.; Alastuey, A.; López-Soler, A. Synthesis of Na-zeolites from fly ash. Fuel 1997, 76, 793–799. [Google Scholar] [CrossRef]
- Inada, M.; Eguchi, Y.; Enomoto, N.; Hojo, J. Synthesis of zeolite from coal fly ashes with different silica–alumina composition. Fuel 2005, 84, 299–304. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Fisher, G.L.; Chang, D.; Brummer, M. Fly ash collected from electrostatic precipitators: Microcrystalline structures and the mystery of the spheres. Science 1976, 192, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.A.R. Synthesis of Zeolites from Geological Materials and Industrial Wastes for Potential Application in Environmental Problems; University of Wolverhampton: Wolverhampton, UK, 2008. [Google Scholar]
- Moreno, N.; Querol, X.; Plana, F.; Andres, J.M.; Janssen, M.; Nugteren, H. Pure zeolite synthesis from silica extracted from coal fly ashes. J. Chem. Technol. Biotechnol. 2002, 77, 274–279. [Google Scholar] [CrossRef]
- Inada, M.; Tsujimoto, H.; Eguchi, Y.; Enomoto, N.; Hojo, J. Microwave-assisted zeolite synthesis from coal fly ash in hydrothermal process. Fuel 2005, 84, 1482–1486. [Google Scholar] [CrossRef]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Zeolite from fly ash: Synthesis and characterisation. Mater. Sci. 2004, 27, 555–564. [Google Scholar]
- Gosh, B.; Agrawal, D.C.; Bhatia, S. Synthesis of zeolite A from calcined diatomaceous clay: Optimization studies. Ind. Eng. Chem. Res. 1994, 33, 2107–2110. [Google Scholar] [CrossRef]
- Hemrajani, R.R.; Tatterson, G.B. Mechanically stirred vessels. In Handbook of Industrial Mixing; Paul, E.L., Atiemo-Obeng, V.A., Kresta, S.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 345–389. [Google Scholar]
- Barrer, R.M. Hydrothermal Chemistry of Zeolites; Academic Press: London, UK, 2007; p. 320. [Google Scholar]
- Singer, A.; Berkgaut, V. Cation exchange properties of hydrothermally treated coal fly ash. Environ. Sci. Technol. 1995, 29, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, P.W.; Ojumu, T.V.; Petrik, L.F. Waste minimization protocols for the process of synthesizing zeolites from South African coal fly ash. Materials 2013, 6, 1688–1703. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated total reflectance fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Fernandez-Jimenez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.; Cazorla-Amoros, D.; Linares-Solano, A. Selective synthesis of zeolite briquettes from conformed ashes. J. Chem. Technol. Biotechnol. 2002, 77, 287–291. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mainganye, D.; Ojumu, T.V.; Petrik, L. Synthesis of Zeolites Na-P1 from South African Coal Fly Ash: Effect of Impeller Design and Agitation. Materials 2013, 6, 2074-2089. https://doi.org/10.3390/ma6052074
Mainganye D, Ojumu TV, Petrik L. Synthesis of Zeolites Na-P1 from South African Coal Fly Ash: Effect of Impeller Design and Agitation. Materials. 2013; 6(5):2074-2089. https://doi.org/10.3390/ma6052074
Chicago/Turabian StyleMainganye, Dakalo, Tunde Victor Ojumu, and Leslie Petrik. 2013. "Synthesis of Zeolites Na-P1 from South African Coal Fly Ash: Effect of Impeller Design and Agitation" Materials 6, no. 5: 2074-2089. https://doi.org/10.3390/ma6052074





