Synthesis of Isothianaphthene (ITN) and 3,4-Ethylenedioxy-Thiophene (EDOT)-Based Low-Bandgap Liquid Crystalline Conjugated Polymers
Abstract
:1. Introduction
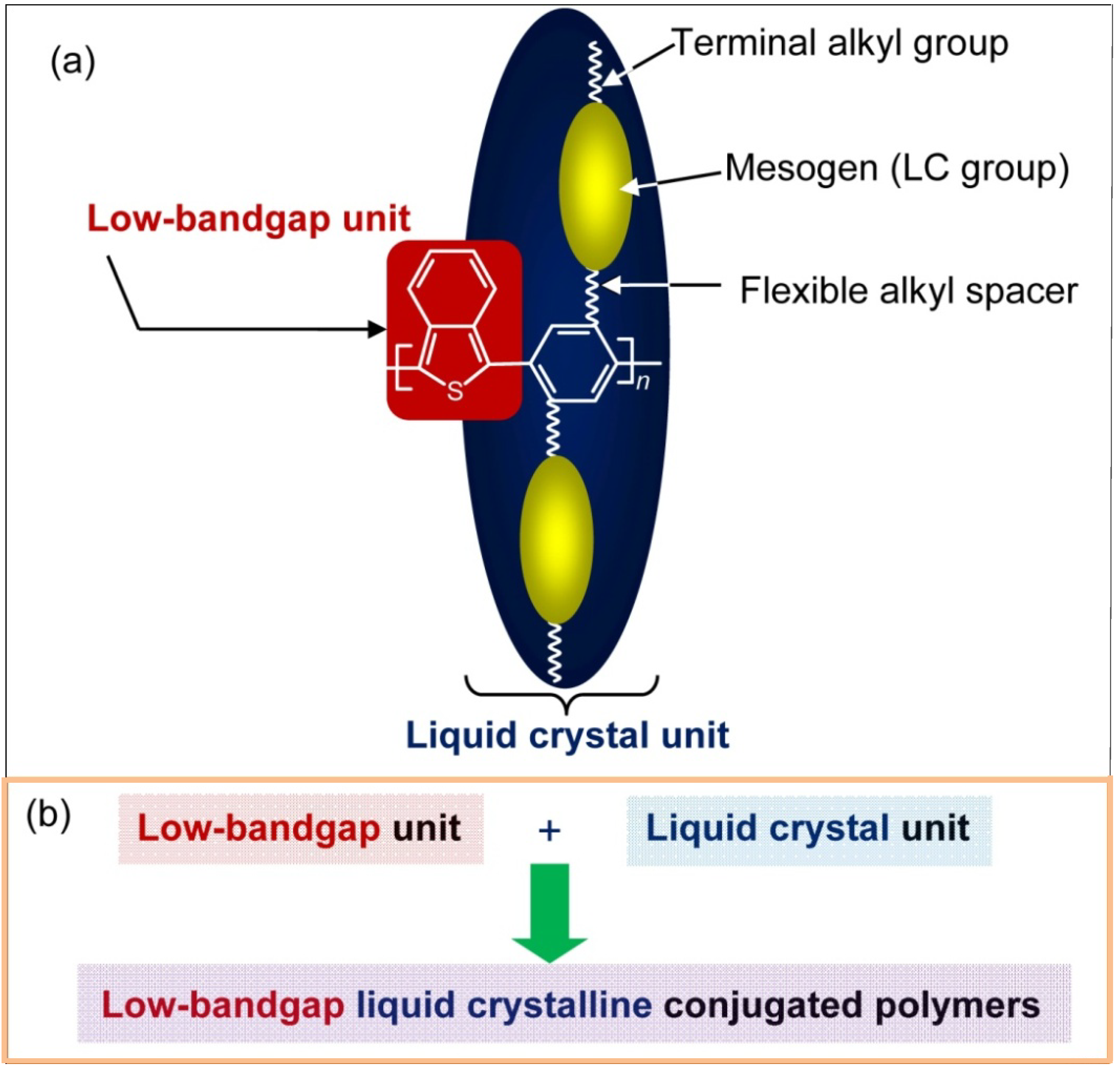
2. Experimental Section
2.1. Materials and Methods
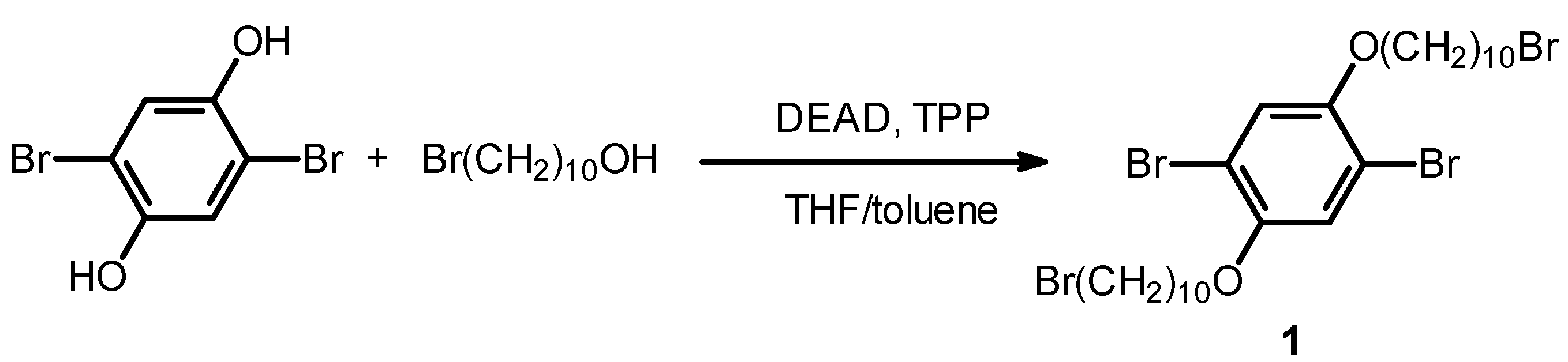
2.2. Monomer Synthesis
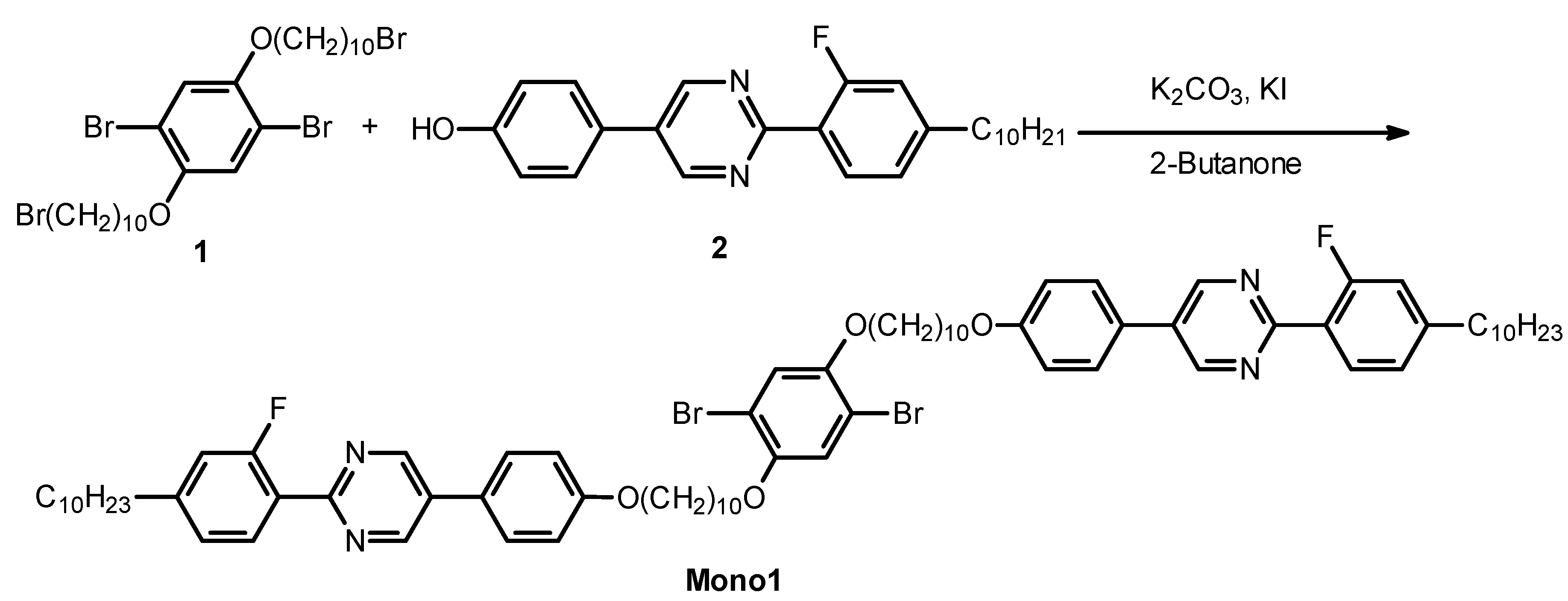
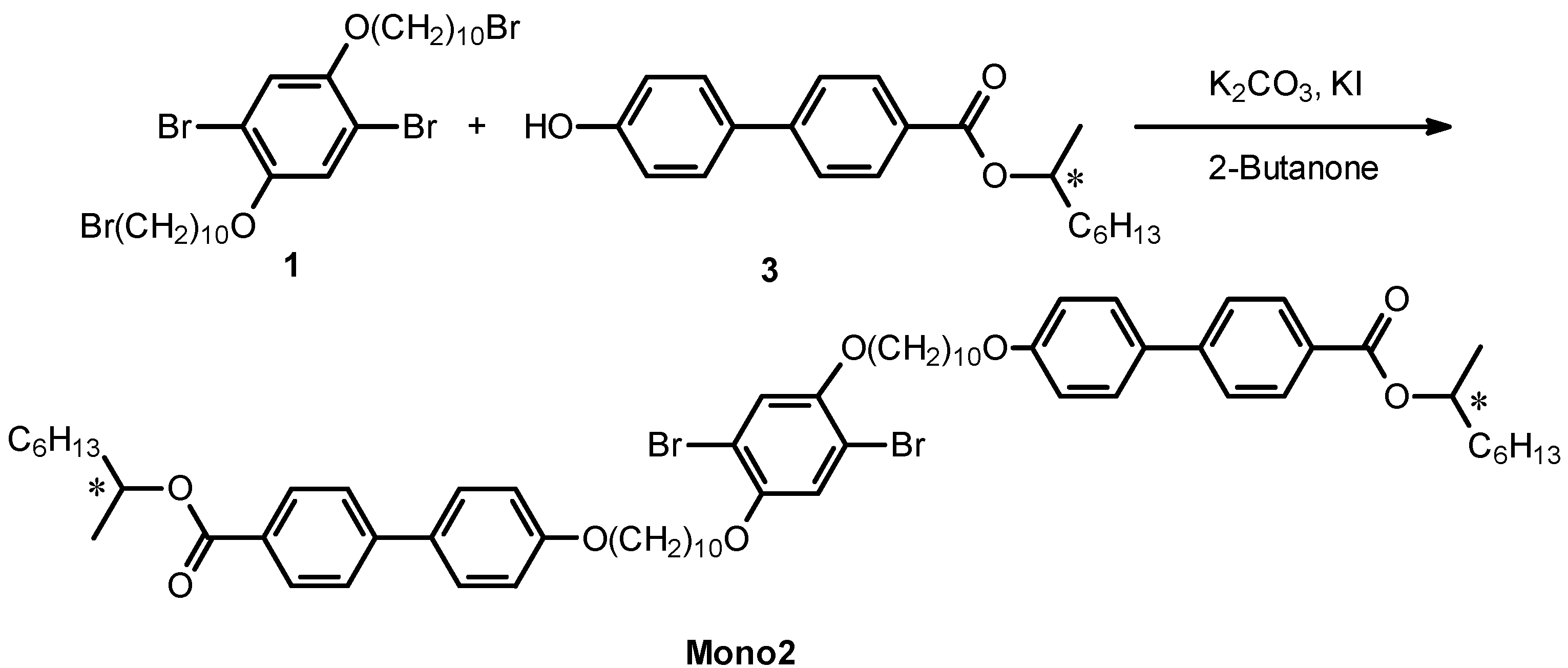
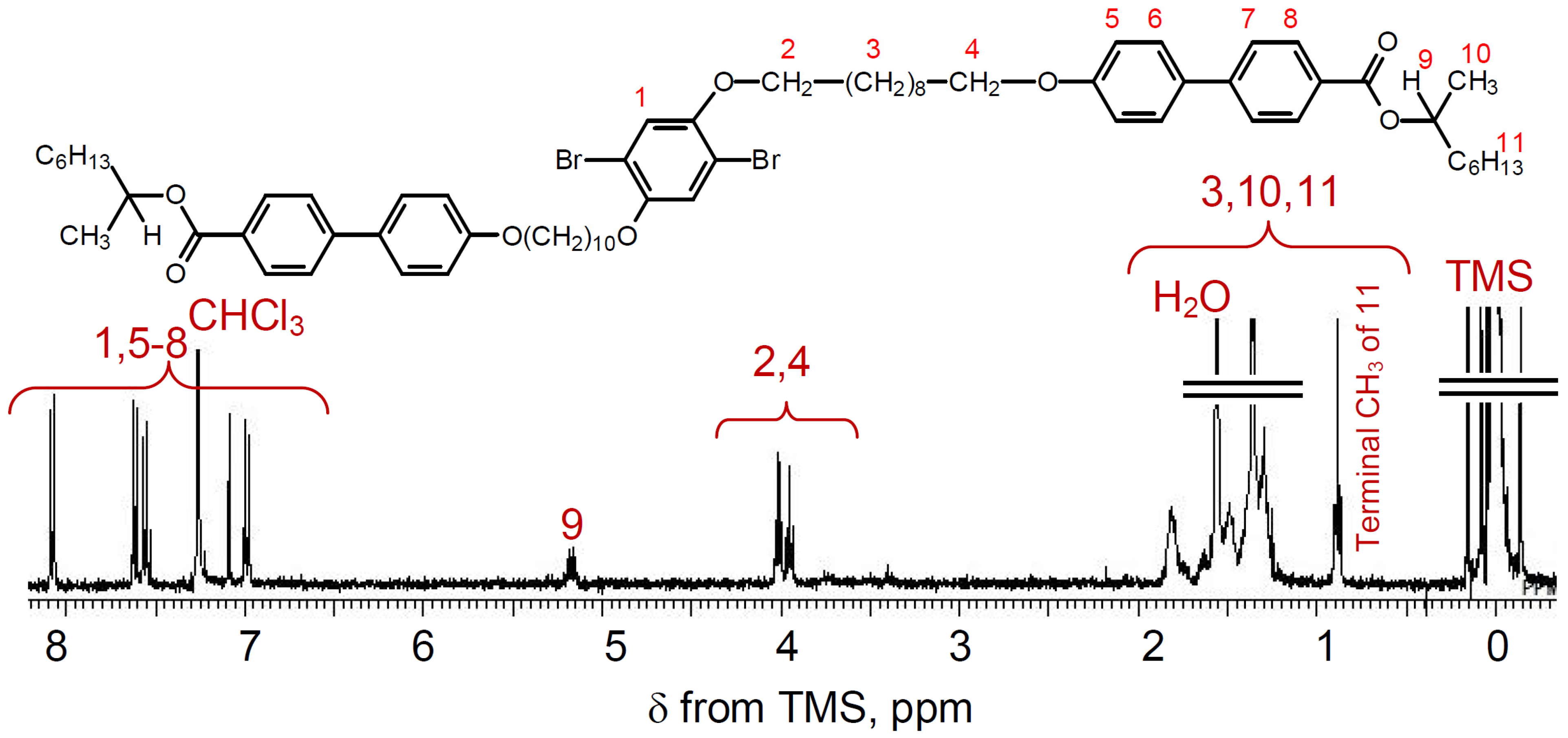
2.3. Polymerization
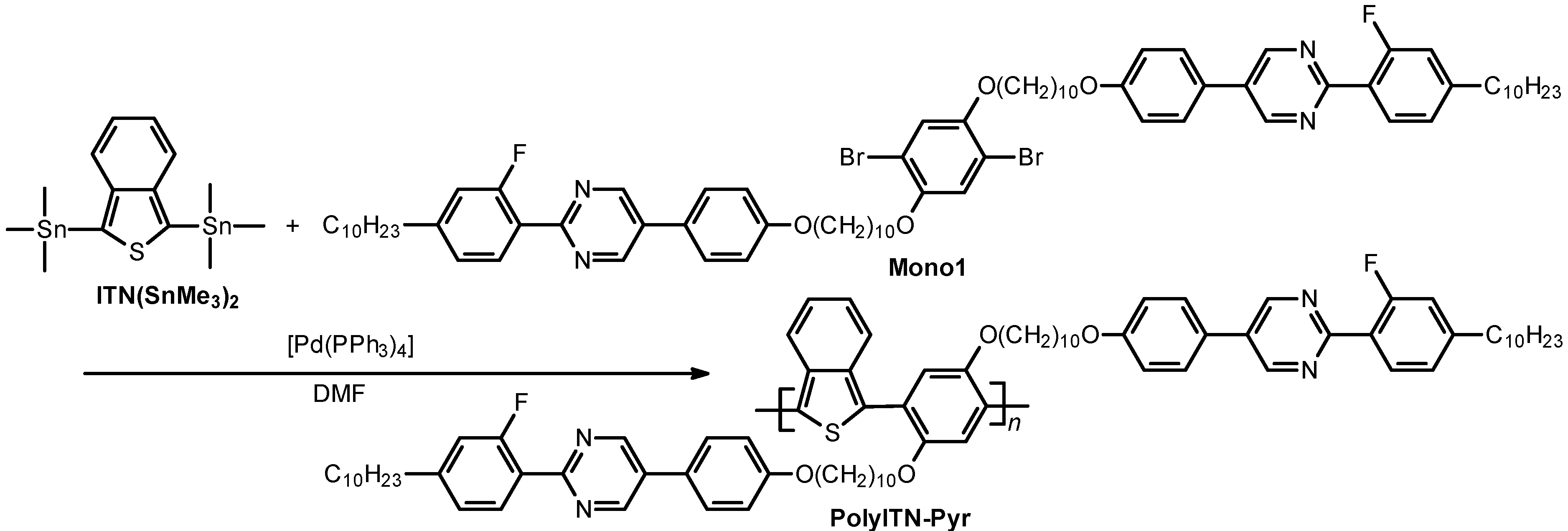
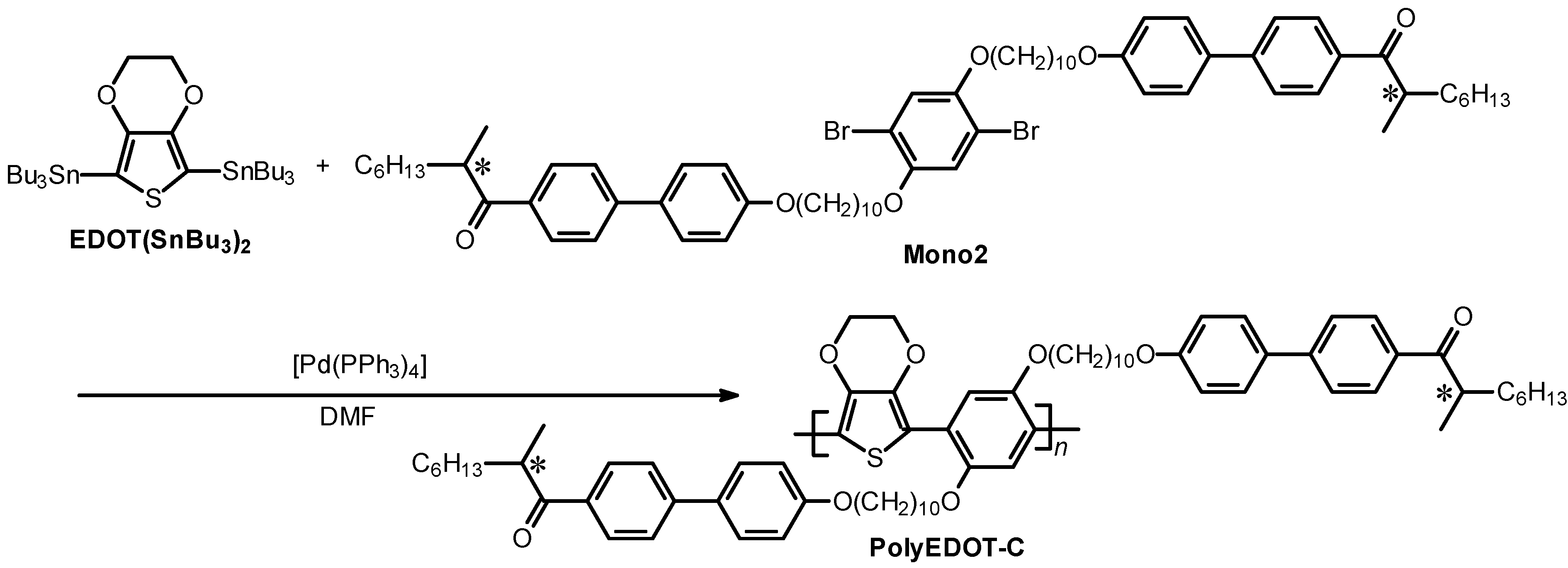
| Polymer | Mn (g/mol) a | Mw (g/mol) a | Mw/Mn |
|---|---|---|---|
| PolyITN-Pyr | 5150 | 6500 | 1.3 |
| PolyEDOT-C | 5060 | 6160 | 1.1 |
3. Results and Discussion
3.1. IR
3.2. Ultraviolet-Visible Absorption Spectra
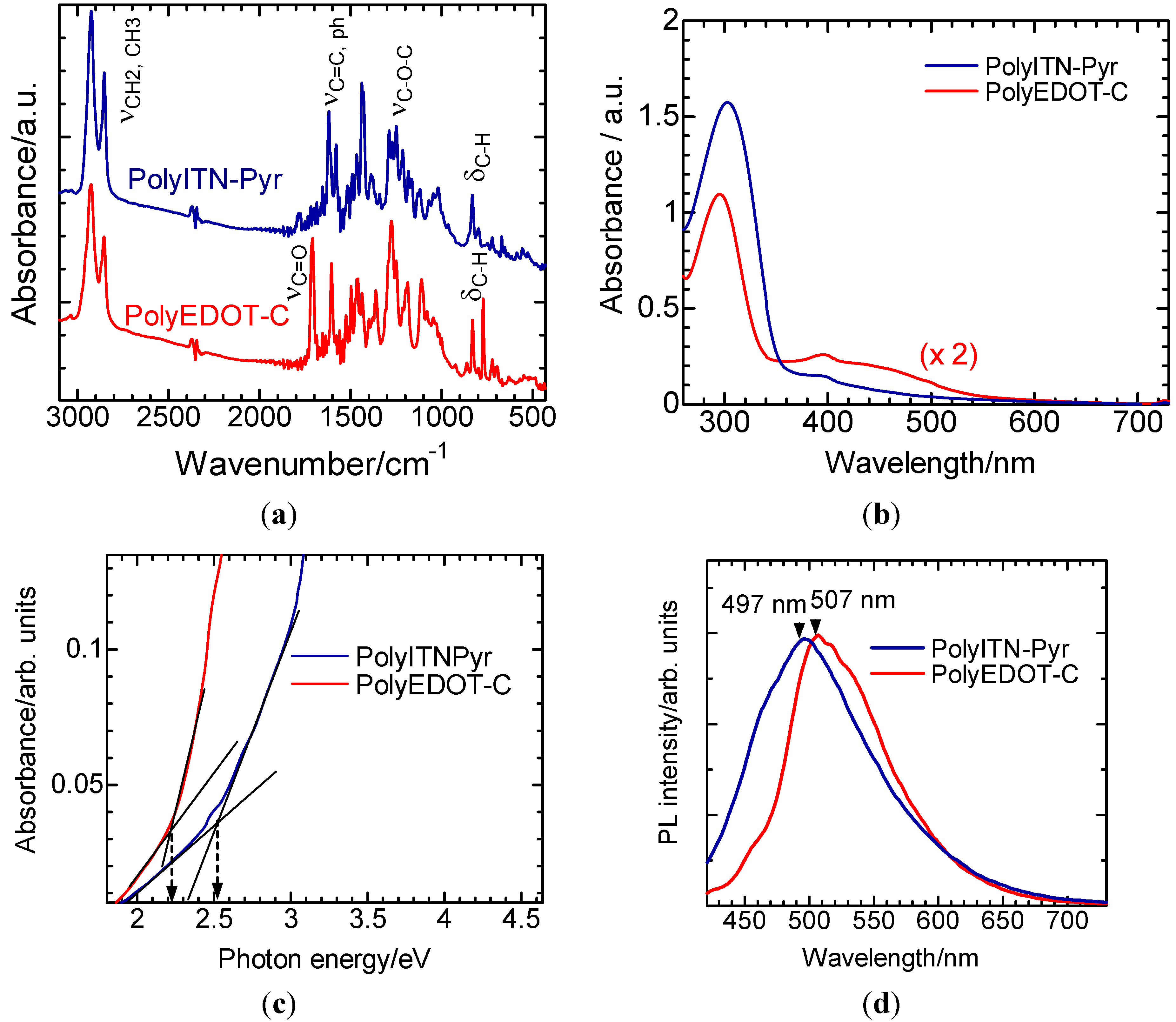
3.3. Photoluminescence
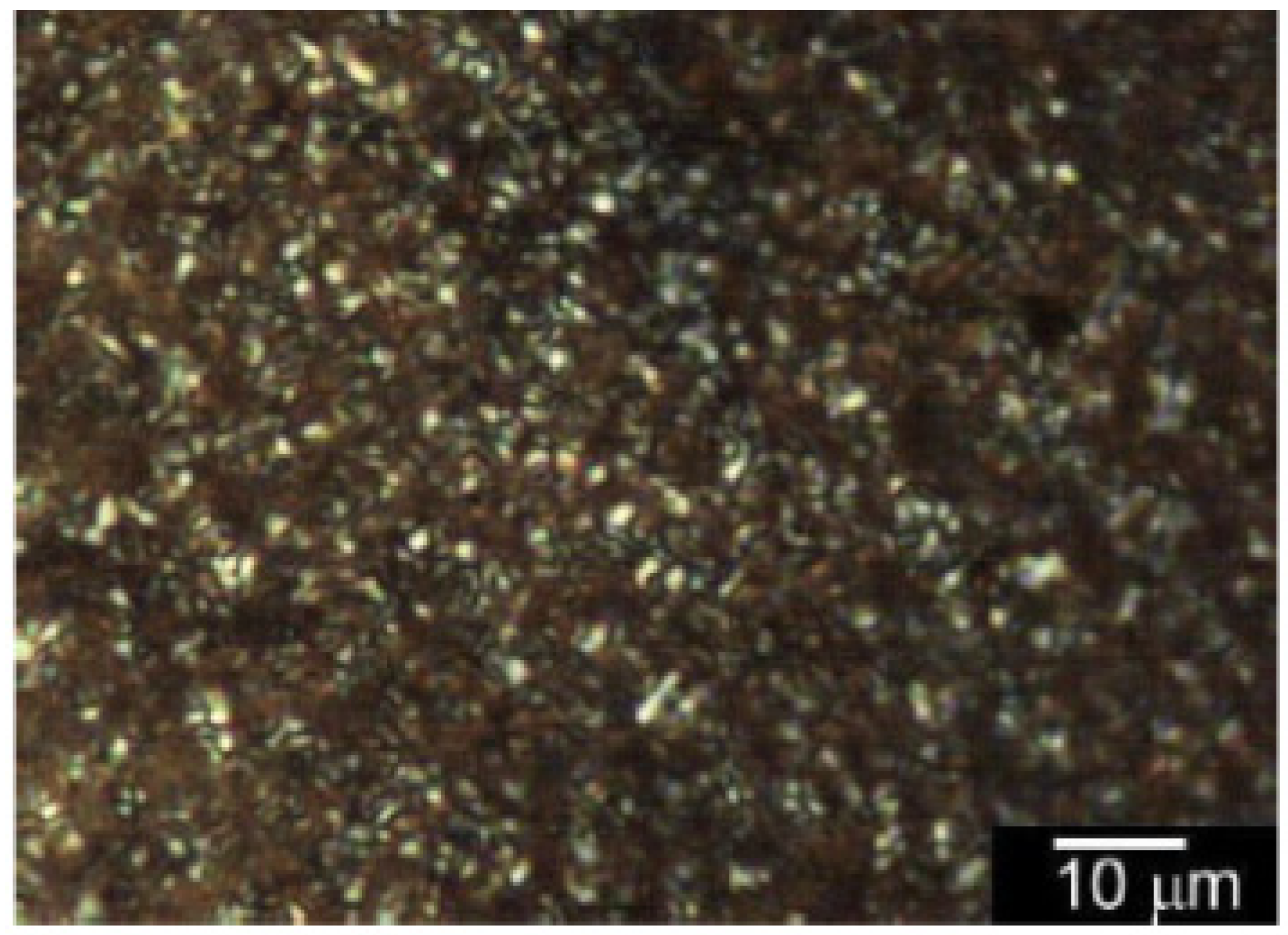
3.4. Polarizing Optical Microscopy Observation
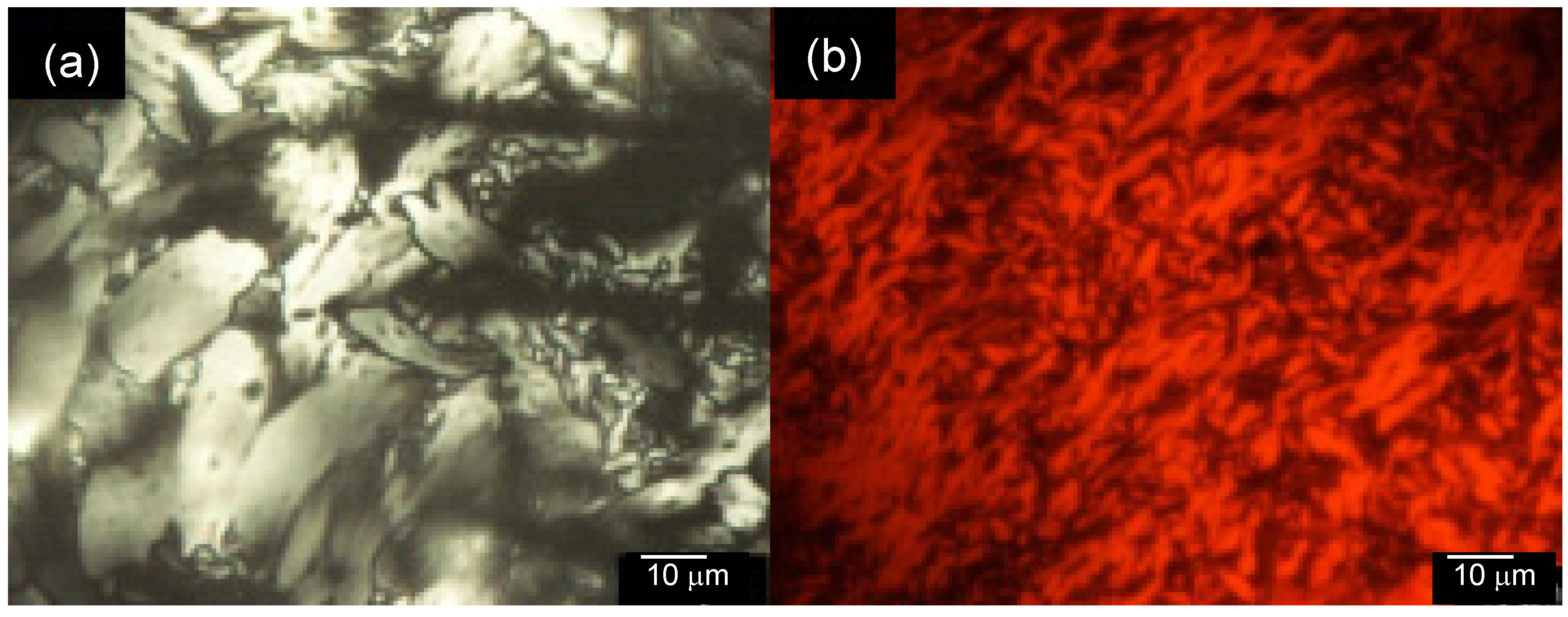
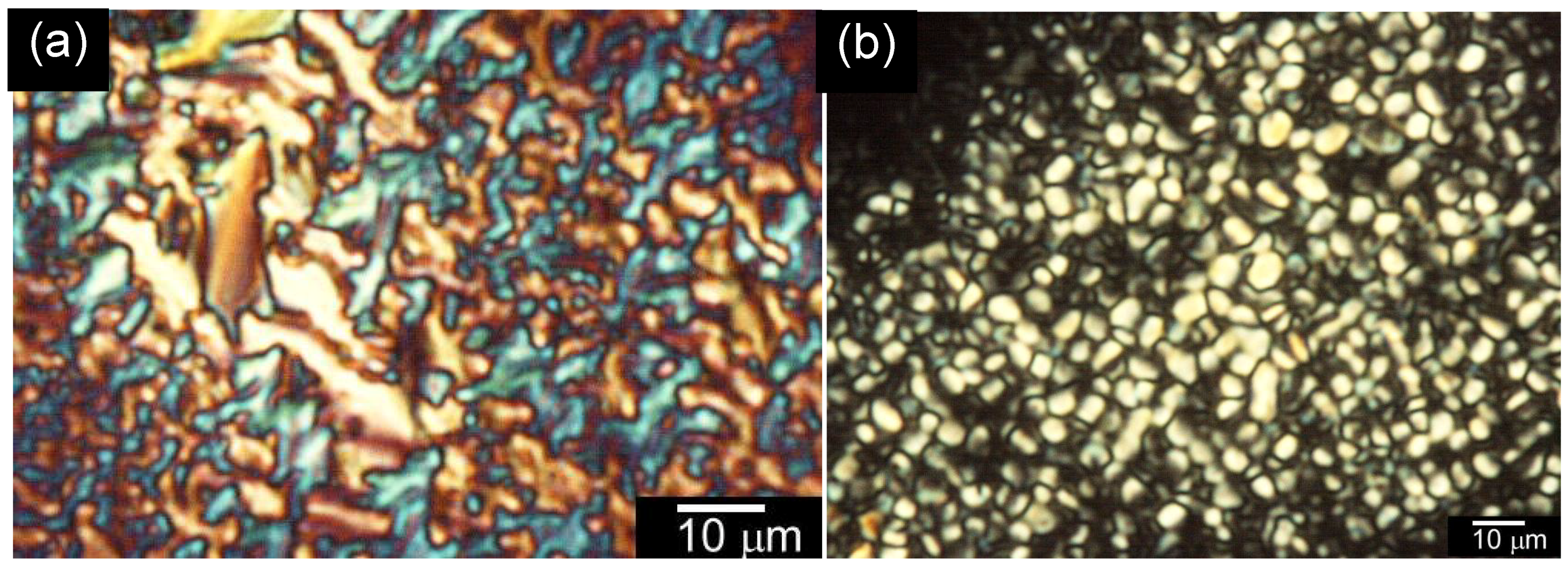
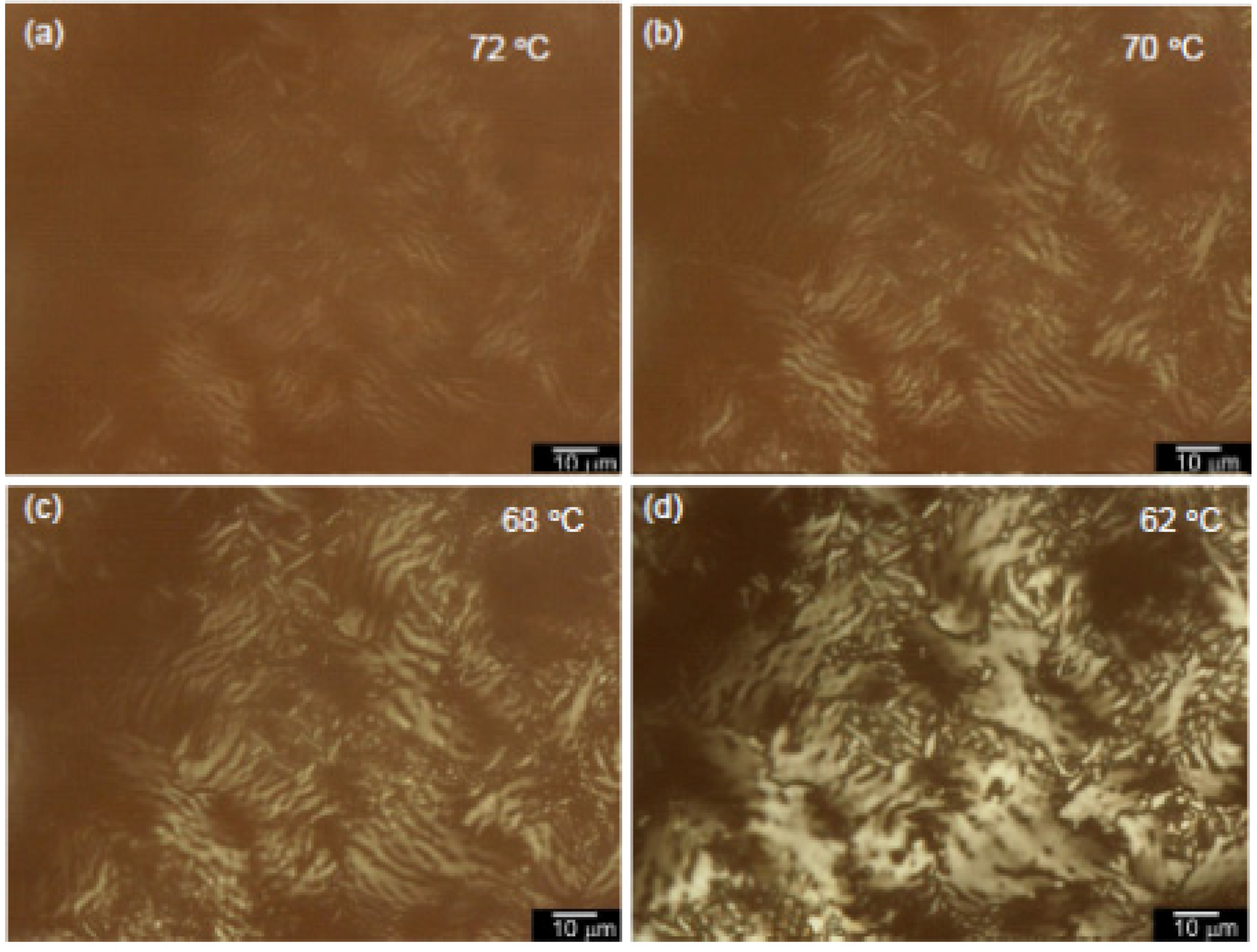
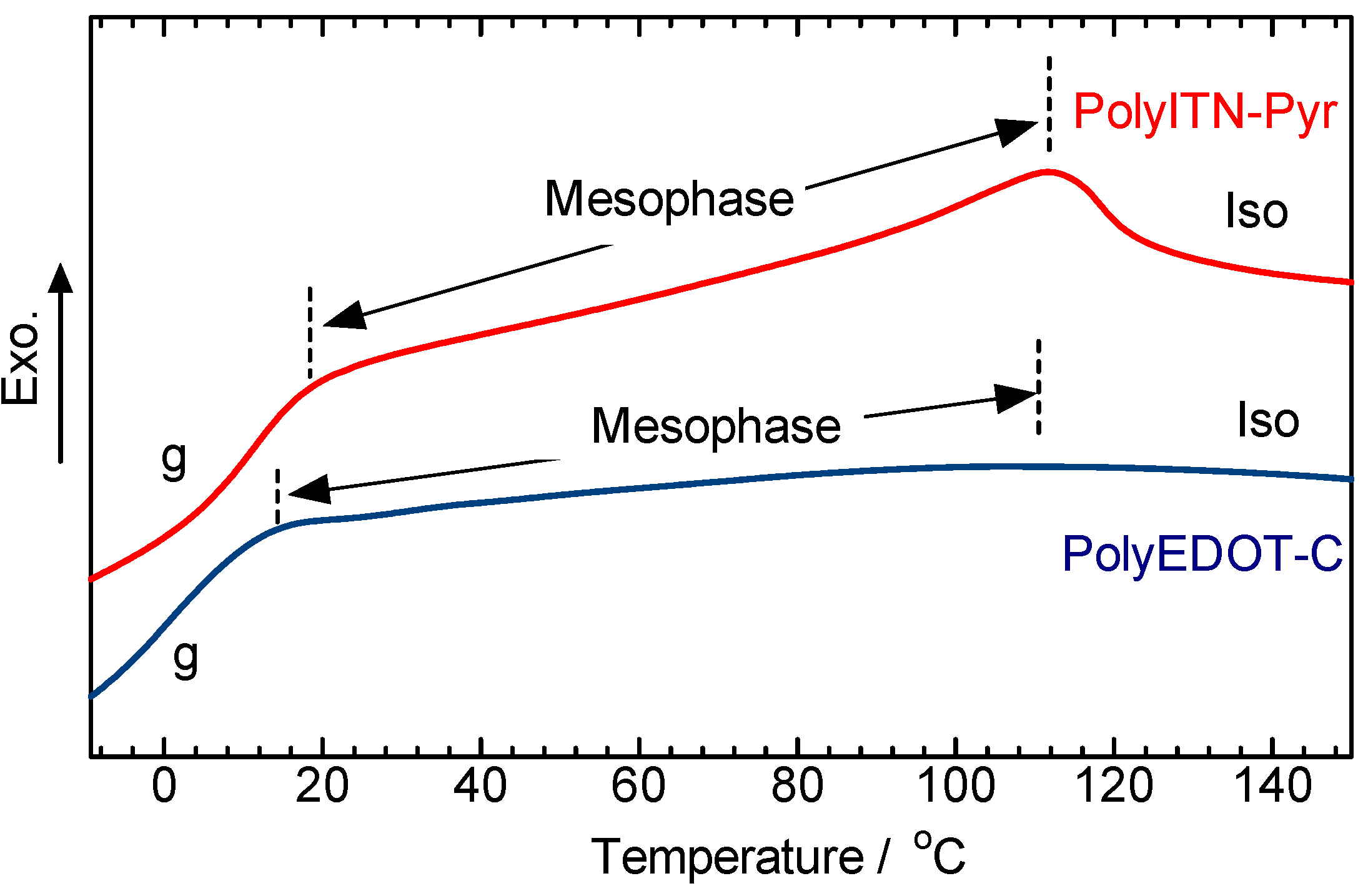
4. Conclusions
Acknowledgments
References
- Adias, A.B.; Hall, H.K., Jr. Mechanism studies of LCP synthesis. Polymers 2011, 3, 833–845. [Google Scholar] [CrossRef]
- Noirez, L.; Mendil-Jakani, H.; Baroni, P.; Wendorff, J.H. Richness of side-chain liquid-crystal polymers: From isotropic phase towards the identification of neglected solid-like properties in liquids. Polymers 2012, 4, 1109–1124. [Google Scholar]
- Sonmez, G.; Meng, H.; Zhang, Q.; Wudl, F. A highly stable, new electrochromic polymer: Poly(1,4-bis(2-(3′,4′-ethylenedioxy) thienyl)-2-methoxy-5-2′′-ethylhexyloxybenzene). Adv. Funct. Mater. 2003, 13, 726–731. [Google Scholar] [CrossRef]
- Kawabata, K.; Goto, H. Liquid crystalline π-conjugated copolymers bearing a pyrimidine type mesogenic group. Materials 2009, 2, 22–37. [Google Scholar] [CrossRef]
- Ohkawa, S.; Ohta, R.; Kawabata, K.; Goto, H. Polymerization in liquid crystal medium: Preparation of polythiophene derivatives bearing a bulky pyrimidine substituent. Polymers 2010, 2, 393–406. [Google Scholar]
- Yang, Q.; Kim, J.; Frisbie, C.; Hillmyer, M. Novel distannylated isothianaphthene as a versatile building block for low band-gap polymers. Macromolecules 2008, 41, 5563–5570. [Google Scholar] [CrossRef]
- Kawabata, K.; Goto, H. Synthesis and optical properties of 1,1-binaphthyl–thiophene alternating copolymers with main-chain chirality. J. Mater. Chem. 2012, 22, 23514–23524. [Google Scholar] [CrossRef] [Green Version]
- Booth, C.J.; Dummur, D.A.; Goodby, J.W.; Kang, J.S.; Toyne, K.J. Effect of the position of lateral fluoro substituents on the phase behaviour and ferroelectric properties of chiral 1-methylheptyl 4′-[(2- or 3-fluoro-4-tetradecyloxyphenyl)propioloyloxy]biphenyl-4-carboxylates. J. Mater. Chem. 1994, 4, 747–759. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, A.; Kawabata, K.; Goto, H. Synthesis of Isothianaphthene (ITN) and 3,4-Ethylenedioxy-Thiophene (EDOT)-Based Low-Bandgap Liquid Crystalline Conjugated Polymers. Materials 2013, 6, 2218-2228. https://doi.org/10.3390/ma6062218
Wang A, Kawabata K, Goto H. Synthesis of Isothianaphthene (ITN) and 3,4-Ethylenedioxy-Thiophene (EDOT)-Based Low-Bandgap Liquid Crystalline Conjugated Polymers. Materials. 2013; 6(6):2218-2228. https://doi.org/10.3390/ma6062218
Chicago/Turabian StyleWang, Aohan, Kohsuke Kawabata, and Hiromasa Goto. 2013. "Synthesis of Isothianaphthene (ITN) and 3,4-Ethylenedioxy-Thiophene (EDOT)-Based Low-Bandgap Liquid Crystalline Conjugated Polymers" Materials 6, no. 6: 2218-2228. https://doi.org/10.3390/ma6062218





