Study of the Microstructure Evolution of Low-pH Cements Based on Ordinary Portland Cement (OPC) by Mid- and Near-Infrared Spectroscopy, and Their Influence on Corrosion of Steel Reinforcement
Abstract
:1. Introduction
2. Experimental Section
2.1. Studies Made in Cement Pastes
| Raw material | LI | IR | SiO2 | Al2O3 | Fe2O3 | CaO (total) | MgO | SO3 | Na2O | K2O | CaO (free) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 4.19 | 1.09 | 17.4 | 4.68 | 5.08 | 60.3 | 1.78 | 3.17 | 0.18 | 0.34 | 1.85 |
| SF | 0.09 | 0.06 | 92.7 | 0.60 | 3.78 | 1.31 | 0.93 | – | 0.15 | 0.37 | 0.01 |
| FA | 2.19 | 0.52 | 54.3 | 26.9 | 5.38 | 4.52 | 2.24 | – | 0.63 | 3.17 | 0.15 |
| Sample | OPC (%) | SF (%) | FA (%) | SiO2 (%) total | pH (90 d) |
|---|---|---|---|---|---|
| Ref | 100 | – | – | 18 | 12.9 |
| B-1 | 60 | 40 | – | 47 | 12.2 |
| B-2 | 50 | 50 | – | 55 | 11.2 |
| T-1 | 35 | 35 | 30 | 51 | 11.2 |
2.2. Studies Made in Mortars
3. Results and Discussion
3.1. Evolution of the Microstructure of Low-pH Cement Pastes
3.1.1. FTMIR Results
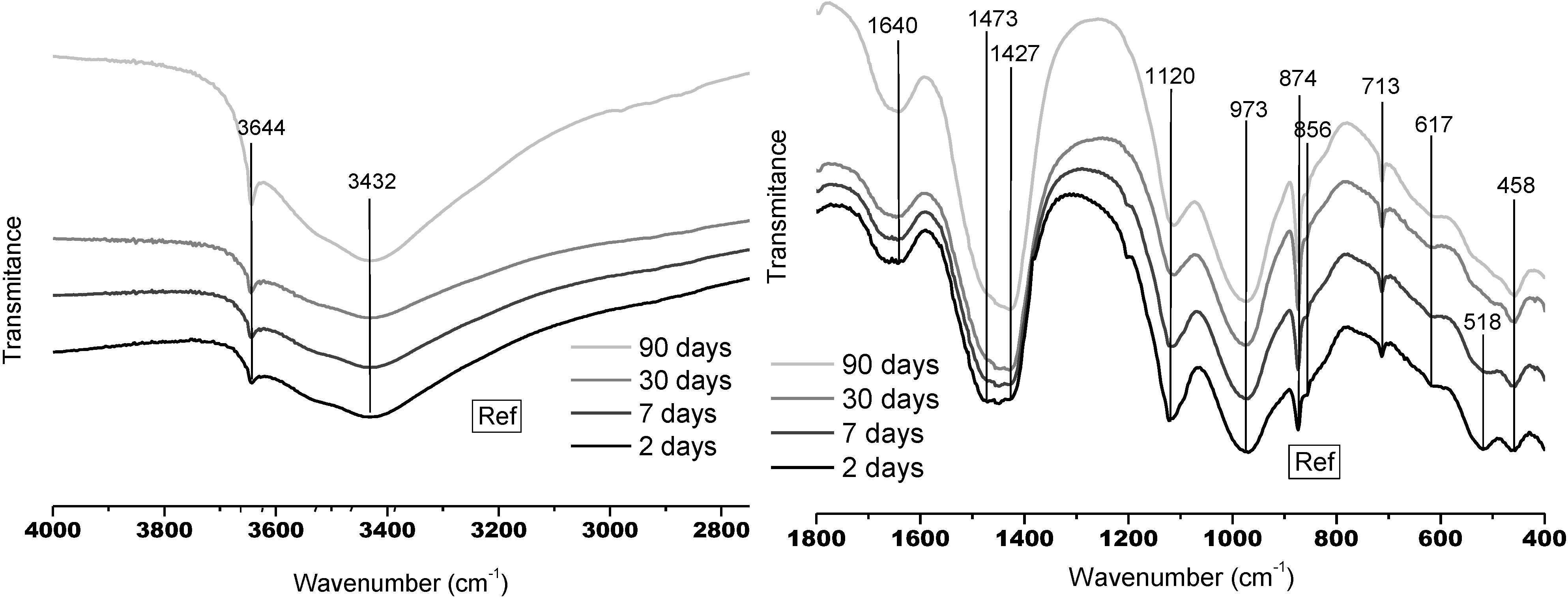
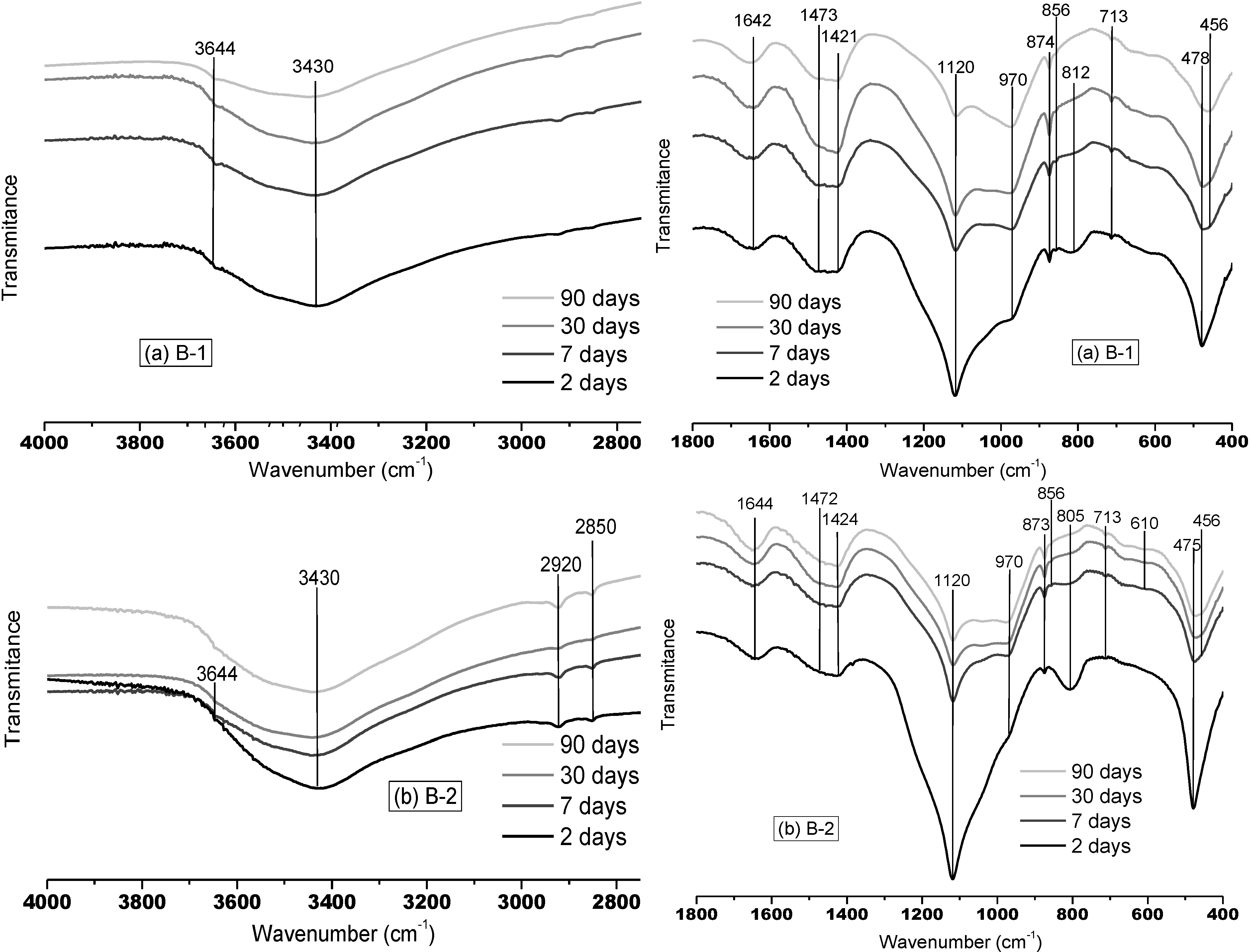
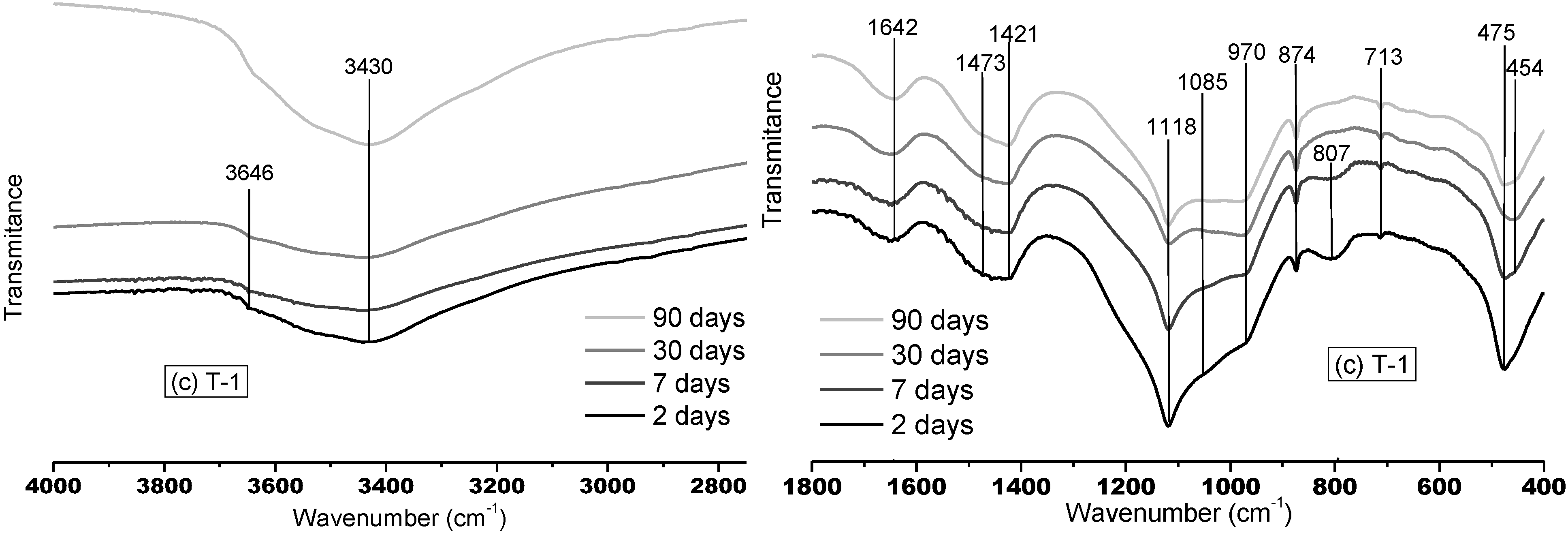
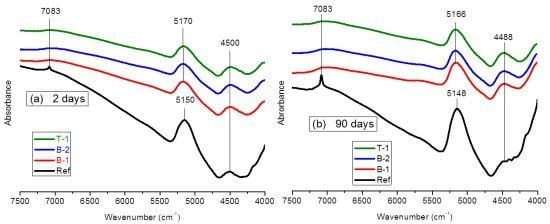
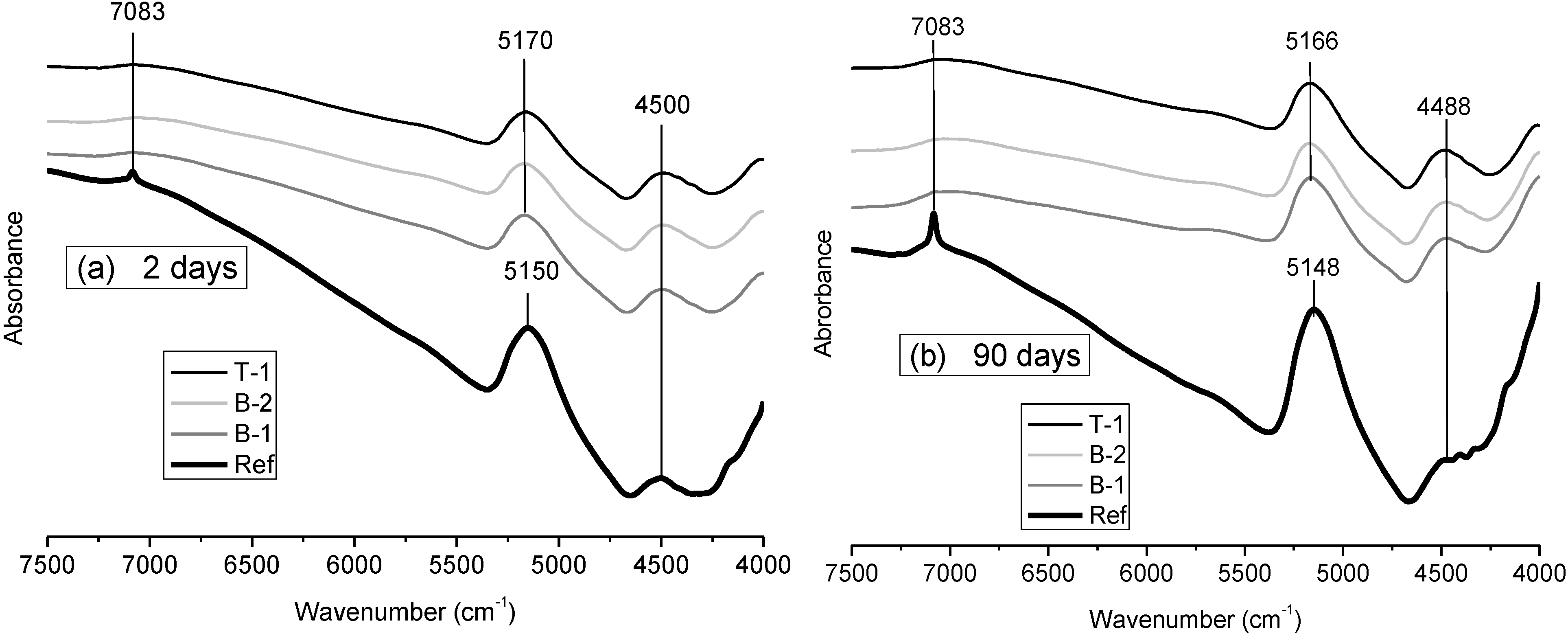
3.1.2. FTNIR Results
3.2. In-Situ Monitoring of the Pore Solution pH Evolution of Low-pH Mortars Using Embedded Metallic Sensors
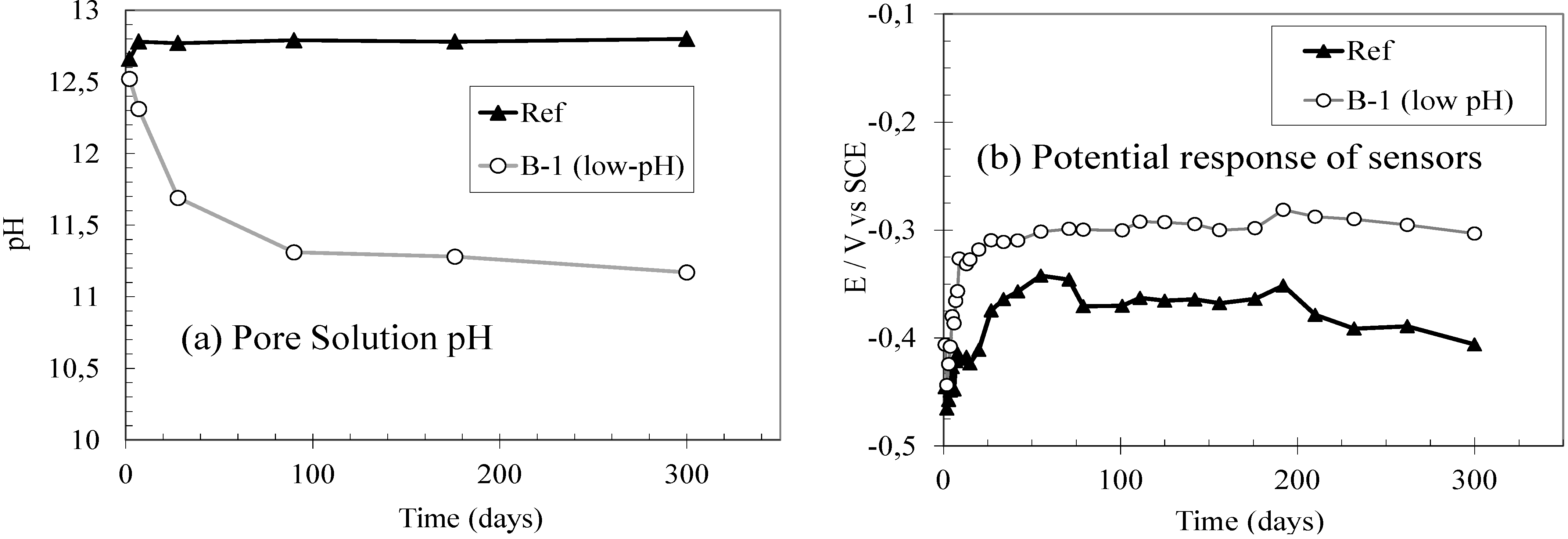
3.3. Influence of the Use of Low-pH Cements in the Corrosion Performance of Steel Reinforcements

4. Conclusions
- Mid- and near-infrared spectroscopy techniques were used in the study of the hydration of low-pH cement pastes based on OPC plus mineral admixtures, enabling the identification of their main solid phases. Whereas the Ref paste is mainly composed of portlandite and C–S–H gels with a jennite type structure and high C/S ratios, the low-pH cement pastes are mainly composed of high polymerized C–S–H or C–A–S–H gels with a tobermorite type structure (with C/S ratios below 0.95 according to the EDAX microanalyses made). In the low pH cement pastes the portlandite is totally consumed at 90 d of curing, or even before.
- The feasibility of monitoring the pore solution pH of low-pH mortars by using embedded metallic sensors was preliminary proven. The different stable pH values measured for conventional or low-pH mortars are expressed as different potential stable values with the embedded sensors: more anodic potentials are registered for the lower pore fluid pH.
- The susceptibility to corrosion when the steel reinforcements are embedded in low-pH cementitious materials was also analysed. In low-pH mortars a significant increase of icorr values is detected after 25 d of exposure, when the pore fluid pH is lower than 11.8. After this time, icorr values above the limit considered for passivation (0.2 µA/cm2) are maintained, as this fact is associated to the initiation of an active corrosion process.
Acknowledgments
Conflict of Interest
References
- Gray, M.N.; Shenton, B.S. For Better Concrete, Take out Some of the Cement. In Proceedings of 6th ACI/CANMET Symposium on the Durability of Concrete, Bangkok, Thailand, 31 May–5 June 1998.
- Iriya, K.; Matsui, A.; Mihara, M. Study on Applicability of HFSC for Radioactive Waste Repositories. In Proceedings of Radioactive Waste Management and Environmental Remediation, ASME Conference, Nagoya, Japan, 26–30 September 1999.
- Pera, J.; Ambroise, J. Fiber reinforced magnesia phosphate cement composites for rapid repair. Cem. Concr. Compos. 1998, 20, 31–39. [Google Scholar] [CrossRef]
- Andac, O.; Glasser, F.P. Pore solution composition of calcium sulfoaluminate cement. Adv. Cem. Res. 1999, 11, 23–26. [Google Scholar] [CrossRef]
- Chow, L.C. Calcium phosphate cements: Chemistry, properties and applications. Mater. Res. Soc. Symp. Proc. 2000, 599, 27–37. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Courtois, S.; Nectoux, D.; Leclerq, S.; Bourbon, X. Formulating a low-alkalinity, high-resistance and low-heat concrete for radioactive waste repositories. Cem. Concr. Res. 2006, 36, 2152–2163. [Google Scholar] [CrossRef]
- Nakayama, M.; Iriya, K.; Fujishima, A.; Mihara, M.; Hatanaka, K.; Kurihara, Y.; Yui, M. Development of low alkaline cement considering pozzolanic reaction for support system. HLW repository construction. Mater. Res. Soc. Symp. Proc. 2006, 932, 159–166. [Google Scholar]
- Codina, M.; Cau Dit Coumes, C.; Le Bescop, P.; Verdier, J.; Ollivier, J.P. Design and characterization of low-heat and low-alkalinity cements. Cem. Concr. Res. 2008, 38, 437–448. [Google Scholar] [CrossRef]
- García Calvo, J.L.; Hidalgo, A.; Alonso, C.; Fernández Luco, L. Low pH concretes based on CAC for underground repositories of HLRW. Resistance respect to ground water aggression. In NUCPERF 2009—Long Term Performance of Cementitious Barriers and Reinforced Concrete in Nuclear Power Plants and Waste Management; RILEM Publications SARL.: Bagneux, France, 2009; pp. 99–108. [Google Scholar]
- García Calvo, J.L.; Hidalgo, A.; Alonso, C.; Fernández Luco, L. Development of low-pH cementitious materials for HLRW repositories. Resistance against ground waters aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef] [Green Version]
- Savage, D.; Noy, D.; Mihara, M. Modeling the interaction of bentonite with hyperalkaline fluids. Appl. Geochem. 2002, 17, 207–223. [Google Scholar]
- Durning, T.G.; Hicks, M.C. Using microsilica to increase concrete’s resistance to aggressive chemicals. Concr. Int. 1991, 13, 42–48. [Google Scholar]
- Saeki, T.; Monteiro, P.J.M. A model to predict the amount of calcium hydroxide in concrete containing mineral admixtures. Cem. Concr. Res. 2005, 35, 1914–1921. [Google Scholar] [CrossRef]
- Greenberg, S.A. Reaction between silica and calcium hydroxide solutions. I. Kinetics in the temperature range 30 °C to 85 °C. J. Phys. Chem. 1961, 65, 12–16. [Google Scholar] [CrossRef]
- Blumentritt, M.; Melhorn, K.; Flachsbarth, J.; Kroener, M.; Kowalsky, W.; Johannes, H. A novel fabrication method of fiber-optical planar transmission sensors for monitoring pH in concrete structures. Sens. Actuators B 2008, 131, 504–508. [Google Scholar] [CrossRef]
- García-Siñeriz, J.L.; Alonso, M.C.; Blümling, P.; Pettersson, S.; Salo, J.-P.; Huertas, F. Input Data and Functional Requirements; Deliverable 1 Module 4 WP-1, EC Contract FI6W-CT-2004-508851; European Commission: Strasbourg, France, 2004. [Google Scholar]
- Alonso, M.C.; García Calvo, J.L.; Sánchez, M.; Fernández, A. Ternary mixes with high mineral additions contents and corrosion related properties. Mater. Corros. 2012, 63, 1078–1086. [Google Scholar]
- Longuet, P.; Burglen, L.; Zelwer, A. The liquid phase of hydrated cement. Rev. Matér. Constr. Trav. Publics. 1973, 676, 35–41. [Google Scholar]
- Barneyback, R.S.; Diamond, S. Expression and analysis of pore fluids from hardened cement pastes and mortars. Cem. Concr. Res. 1981, 11, 279–285. [Google Scholar] [CrossRef]
- Alonso, M.C.; García Calvo, J.L.; Walker, C.; Naito, M.; Pettersson, S.; Puigdomenech, I.; Cuñado, M.A.; Vuorio, M.; Weber, H.; et al. Development of an Accurate pH Measurement Methodology for the Pore Fluids of Low pH Cementitious Materials. In Proceedings of the 13th International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011.
- Romer, J.; Schwabe, K. Über pH-messungen in der diffusionsschicht an anionenaustaus chermembranen (in German). Electrochim. Acta 1970, 15, 885–897. [Google Scholar] [CrossRef]
- Einerhand, R.E.F.; Visscher, W.H.M.; Barendrecht, E. pH measurement in strong KOH solutions with a bismuth electrode. Electrochim. Acta 1989, 34, 345–353. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974. [Google Scholar]
- Gadsden, J.A. Infrared Spectra of Minerals and Related Inorganic Compounds; Butterworths: London, UK, 1975. [Google Scholar]
- Stepkowska, E.T.; Blanes, J.M.; Real, C.; Perez-Rodríguez, J.L. Hydration products in two aged cement pastes. J. Therm. Anal. Calorim. 2005, 82, 731–739. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C–S–H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar]
- Farcas, F.; Touzé, P. La spectrométrie infrarouge à transformée de Fourier (IRTF). Une méthode intéressante pour la caractérisation des ciments (in French). Bull. Lab. Ponts Chaussées 2001, 230, 77–88. [Google Scholar]
- Stepkowska, E.T. Simultaneous IR/TG study of calcium carbonate in two aged cement pastes. J. Therm. Anal. Calorim. 2006, 84, 175–180. [Google Scholar] [CrossRef]
- Hidalgo, A.; Petit, S.; Domingo, C.; Alonso, C.; Andrade, C. Microstuctural characterization of leaching effects in cement pastes due to neutralisation of their alkaline nature Part I: Portland cement pastes. Cem. Concr. Res. 2007, 37, 63–70. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Hydrated calcium silicates. Part I. Compound formation at ordinary temperatures. J. Chem. Soc. 1950, 30, 3682–3690. [Google Scholar] [CrossRef]
- Langer, K.; Florke, O.W. Near infrared absorption spectra (4000–9000 cm−1) of opals and the role of “water” in these SiO2·nH2O minerals. Fortschr. Mineral. 1974, 52, 17–51. [Google Scholar]
- Gastaldi, D.; Canonico, F.; Irico, S.; Pellerej, D.; Paganini, M.C. Near-infrared spectroscopy investigation on the hydration degree of a cement paste. J. Mater. Sci. 2010, 45, 3169–3174. [Google Scholar] [CrossRef]
- Stronach, S.A.; Glasser, F.P. Modelling the impact of abundant geochemical components on phase stability and solubility of the CaO–SiO2–H2O system at 25 °C: Na+, K+, SO42−, Cl− and CO32−. Adv. Cem. Res. 1997, 9, 167–181. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F.P. Alkali binding in cement pastes Part I. The C–S–H phase. Cem. Concr. Res. 1999, 29, 1893–1903. [Google Scholar] [CrossRef]
- Bach, T.T.H.; Chabas, E.; Pochard, I.; Cau Dit Coumes, C.; Haas, J.; Frizon, F.; Nonat, A. Retention of alkali ions by hydrated low-pH cements: Mechanism and Na+/K+ selectivity. Cem. Concr. Res. 2013, 51, 14–21. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
García Calvo, J.L.; Sánchez Moreno, M.; Alonso Alonso, M.C.; Hidalgo López, A.; García Olmo, J. Study of the Microstructure Evolution of Low-pH Cements Based on Ordinary Portland Cement (OPC) by Mid- and Near-Infrared Spectroscopy, and Their Influence on Corrosion of Steel Reinforcement. Materials 2013, 6, 2508-2521. https://doi.org/10.3390/ma6062508
García Calvo JL, Sánchez Moreno M, Alonso Alonso MC, Hidalgo López A, García Olmo J. Study of the Microstructure Evolution of Low-pH Cements Based on Ordinary Portland Cement (OPC) by Mid- and Near-Infrared Spectroscopy, and Their Influence on Corrosion of Steel Reinforcement. Materials. 2013; 6(6):2508-2521. https://doi.org/10.3390/ma6062508
Chicago/Turabian StyleGarcía Calvo, José Luis, Mercedes Sánchez Moreno, María Cruz Alonso Alonso, Ana Hidalgo López, and Juan García Olmo. 2013. "Study of the Microstructure Evolution of Low-pH Cements Based on Ordinary Portland Cement (OPC) by Mid- and Near-Infrared Spectroscopy, and Their Influence on Corrosion of Steel Reinforcement" Materials 6, no. 6: 2508-2521. https://doi.org/10.3390/ma6062508