Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vesicle Preparation
2.2. Dynamic Light Scattering (DLS)
2.3. Quartz Crystal Microbalance with Dissipation (QCM-D)
2.4. Fluorescence Recovery after Photobleaching (FRAP)
2.5. Atomic Force Microscopy
3. Results and Discussion
3.1. Size Distribution of Vesicle Suspensions

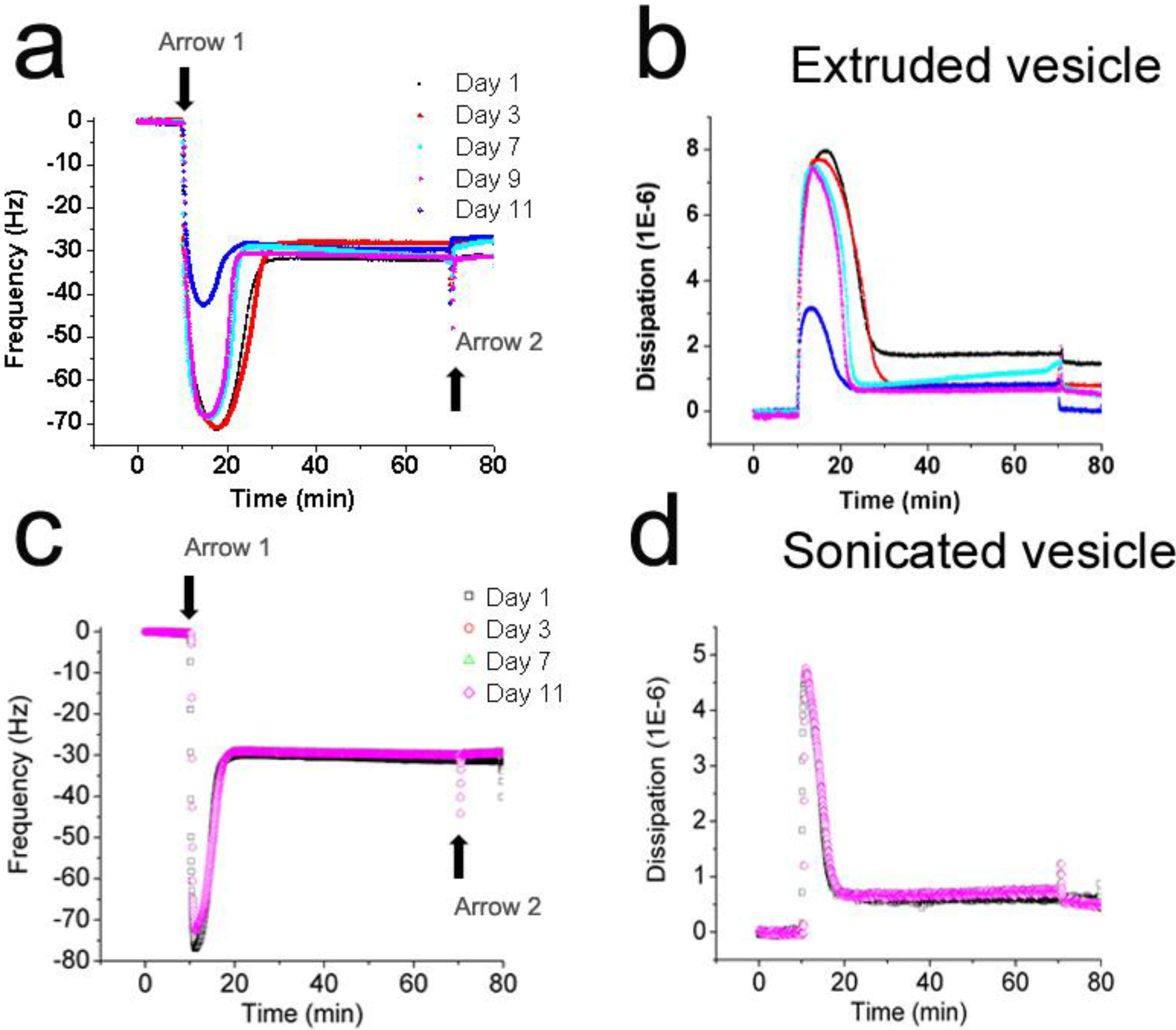
3.2. Interaction Kinetics of Vesicles with Silicon Oxide
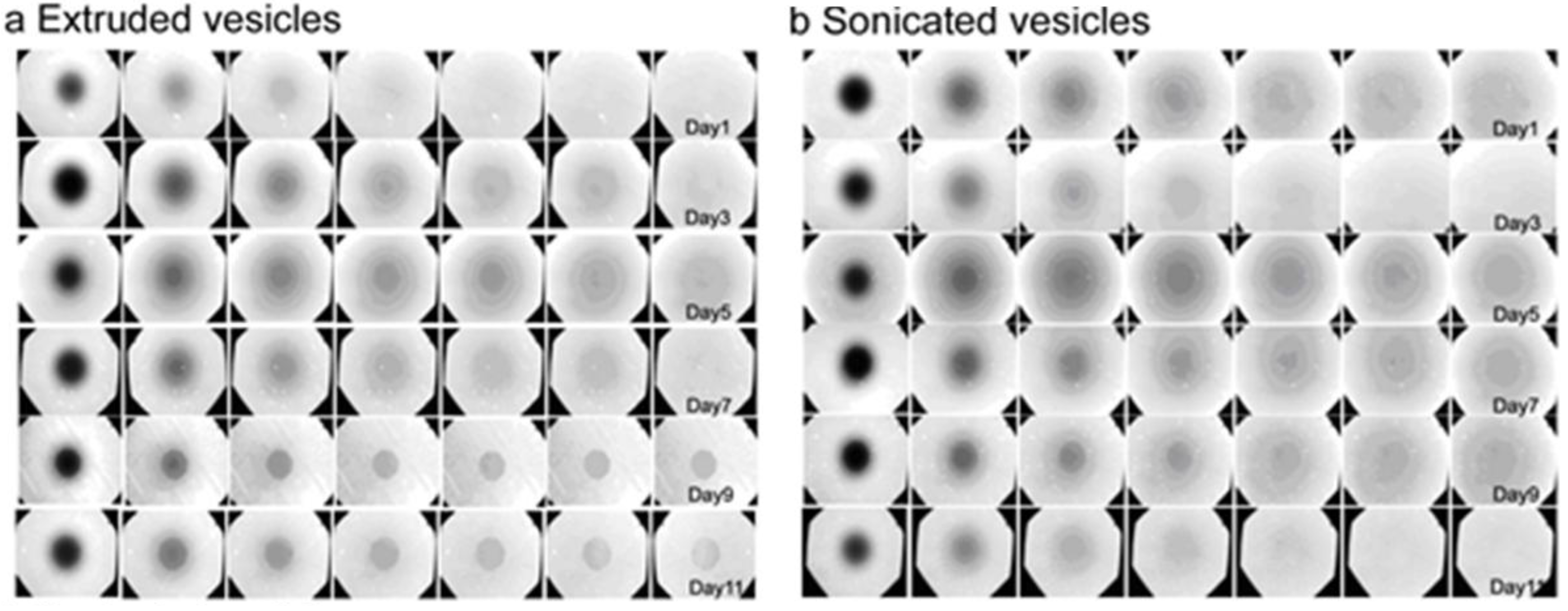
3.3. Lateral Mobility of Planar Lipid Bilayer
| Vesicle Aging | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | |
|---|---|---|---|---|---|---|---|
| Extrusion | Diffusion coefficient (μm2 s−1) | 2.12 ± 0.32 | 2.02 ± 0.22 | 1.97 ± 0.72 | 2.01 ± 0.13 | 0.89 ± 0.64 | 0.96 ± 0.29 |
| Mobile fraction (%) | 83 ± 9 | 81 ± 6 | 79 ± 13 | 82 ± 9 | 48 ± 13 | 53 ± 9 | |
| Sonication | Diffusion coefficient (μm2 s−1) | 1.89 ± 0.28 | 1.93 ± 0.35 | 1.71 ± 0.67 | 1.98 ± 0.42 | 1.47 ± 0.62 | 1.85 ± 0.56 |
| Mobile fraction (%) | 75 ± 5 | 83 ± 7 | 73 ± 12 | 75 ± 15 | 67 ± 11 | 79 ± 5 |
3.4. Morphology of Lipid Assemblies on Silicon Oxide
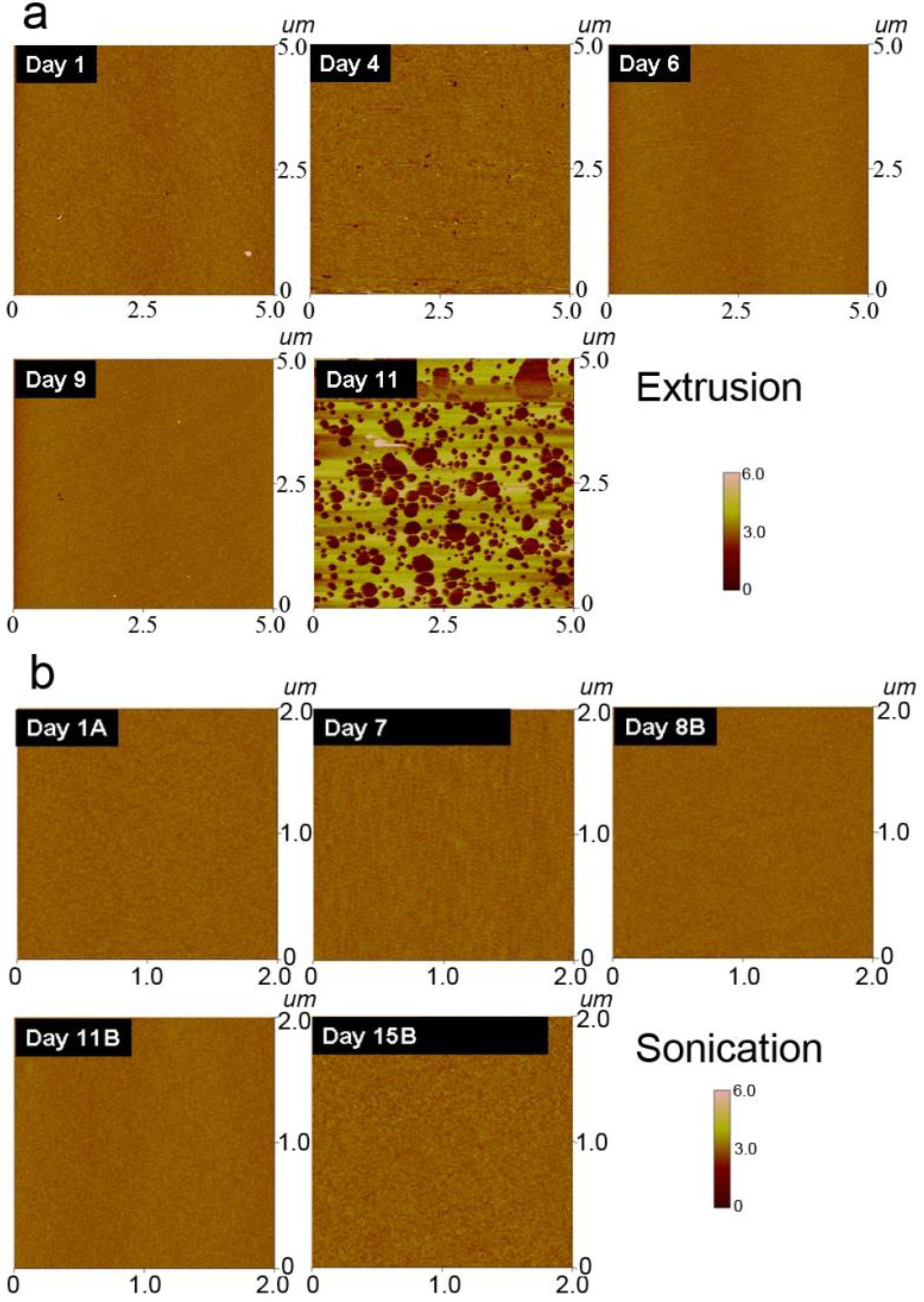
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [PubMed]
- Lasic, D.D. Applications of Liposomes. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: Amsterdam, the Netherlands, 1995; Volume 1, pp. 491–529. [Google Scholar]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Naeff, R. Feasibility of topical liposome drugs produced on an industrial scale. Adv. Drug Deliv. Rev. 1996, 18, 343–347. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2010, 2011, 1–9. [Google Scholar] [CrossRef]
- Szoka, F., Jr.; Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Hope, M.; Cullis, P. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta (BBA) Biomembr. 1986, 858, 161–168. [Google Scholar] [CrossRef]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.M.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biomembranes 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Maulucci, G.; De Spirito, M.; Arcovito, G.; Boffi, F.; Castellano, A.C.; Briganti, G. Particle size distribution in DMPC vesicles solutions undergoing different sonication times. Biophys. J. 2005, 88, 3545–3550. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.S.; Pitt, W.G.; Woodbury, D.J. The role of cavitation in liposome formation. Biophys. J. 2007, 93, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Blume, G.; Cevc, G. Liposomes for the sustained drug release in vivo. Biochim. Biophys. Acta (BBA) Biomembr. 1990, 1029, 91–97. [Google Scholar] [CrossRef]
- Terrell, J.; Yadava, P.; Castro, C.; Hughes, J. Liposome fluidity alters interactions between the ganglioside GM1 and cholera toxin B subunit. J. Liposome Res. 2008, 18, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, M.M.; Castro-Forero, A.; Greiner, A.J.; Ofoli, R.Y.; Blanchard, G.J. Comparison of liposomes formed by sonication and extrusion: Rotational and translational diffusion of an embedded chromophore. Langmuir 2007, 23, 11677–11683. [Google Scholar] [CrossRef] [PubMed]
- Brian, A.A.; McConnell, H.M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc. Natl. Acad. Sci. 1984, 81, 6159–6163. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar] [CrossRef]
- Boni, L.; Minchey, S.; Perkins, W.; Ahl, P.; Slater, J.; Tate, M.; Gruner, S.; Janoff, A. Curvature dependent induction of the interdigitated gel phase in DPPC vesicles. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1146, 247–257. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 2002, 19, 1681–1691. [Google Scholar] [CrossRef]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Boudard, S.; Seantier, B.; Breffa, C.; Decher, G.; Felix, O. Controlling the pathway of formation of supported lipid bilayers of DMPC by varying the sodium chloride concentration. Thin Solid Films 2006, 495, 246–251. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid-supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2007, 61, 429–444. [Google Scholar] [CrossRef]
- Chan, Y.-H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Supported membranes: scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.; Cho, N.-J. Model membrane platforms for biomedicine: Case study on antiviral drug development. Biointerphases 2012, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Maury, N.; Brisson, A. On the effect of the solid support on the interleaflet distribution of lipids in supported lipid bilayers. Langmuir 2004, 21, 299–304. [Google Scholar] [CrossRef]
- Rossetti, F.F.; Textor, M.; Reviakine, I. Asymmetric distribution of phosphatidyl serine in supported phospholipid bilayers on titanium dioxide. Langmuir 2006, 22, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Zasadzinski, J. Transmission electron microscopy observations of sonication-induced changes in liposome structure. Biophys. J. 1986, 49, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.J.; Richardson, E.S.; Grigg, A.W.; Welling, R.D.; Knudson, B.H. Reducing liposome size with ultrasound: Bimodal size distributions. J. Liposome Res. 2006, 16, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Bernt, W.; Polosukhina, K.; Weiner, B.; Tscharnuter, W.; Highsmith, S. Active site control of myosin cross-bridge zeta potential. Biochemistry 2002, 41, 11308–11314. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Kaler, E.W.; Herrington, K.L.; Murthy, A.K.; Zasadzinski, J.A.N. Phase behavior and structures of mixtures of anionic and cationic surfactants. J. Phys. Chem. 1992, 96, 6698–6707. [Google Scholar] [CrossRef]
- Cheng, Z.; Luisi, P.L. Coexistence and mutual competition of vesicles with different size distributions. J. Phys. Chem. B 2003, 107, 10940–10945. [Google Scholar] [CrossRef]
- Mui, B.; Chow, L.; Hope, M.J. Extrusion technique to generate liposomes of defined size. Methods Enzymol. 2003, 367, 3–14. [Google Scholar] [PubMed]
- Mui, B.L.S.; Cullis, P.R.; Evans, E.A.; Madden, T.D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys. J. 1993, 64, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Nollert, P.; Kiefer, H.; Jähnig, F. Lipid vesicle adsorption versus formation of planar bilayers on solid surfaces. Biophys. J. 1995, 69, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Hamai, C.; Cremer, P.S.; Musser, S.M. Single giant vesicle rupture events reveal multiple mechanisms of glass-supported bilayer formation. Biophys. J. 2007, 92, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: a combined QCM-D and AFM study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef] [PubMed]
- Dimitrievski, K. Deformation of adsorbed lipid vesicles as a function of vesicle size. Langmuir 2010, 26, 3008–3011. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.; Kasemo, B. Comments on rupture of adsorbed vesicles. Langmuir 2001, 17, 3518–3521. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cho, N.-J.; Hwang, L.Y.; Solandt, J.J.R.; Frank, C.W. Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly. Materials 2013, 6, 3294-3308. https://doi.org/10.3390/ma6083294
Cho N-J, Hwang LY, Solandt JJR, Frank CW. Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly. Materials. 2013; 6(8):3294-3308. https://doi.org/10.3390/ma6083294
Chicago/Turabian StyleCho, Nam-Joon, Lisa Y. Hwang, Johan J.R. Solandt, and Curtis W. Frank. 2013. "Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly" Materials 6, no. 8: 3294-3308. https://doi.org/10.3390/ma6083294





