Consolidation of Hierarchy-Structured Nanopowder Agglomerates and Its Application to Net-Shaping Nanopowder Materials
Abstract
:1. Introduction
2. Optimal Processing of Iron Base Nanopowder Agglomerates
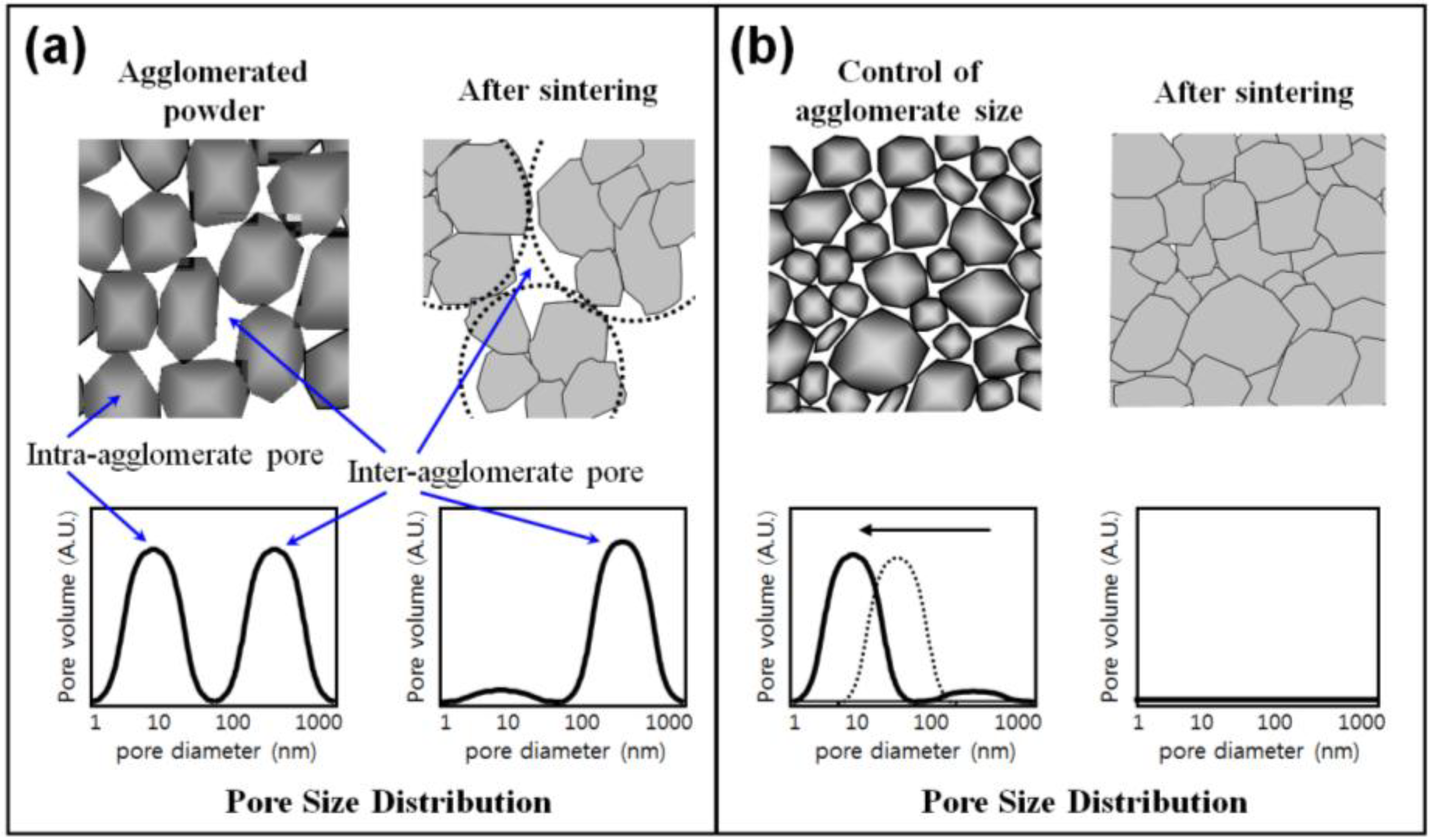

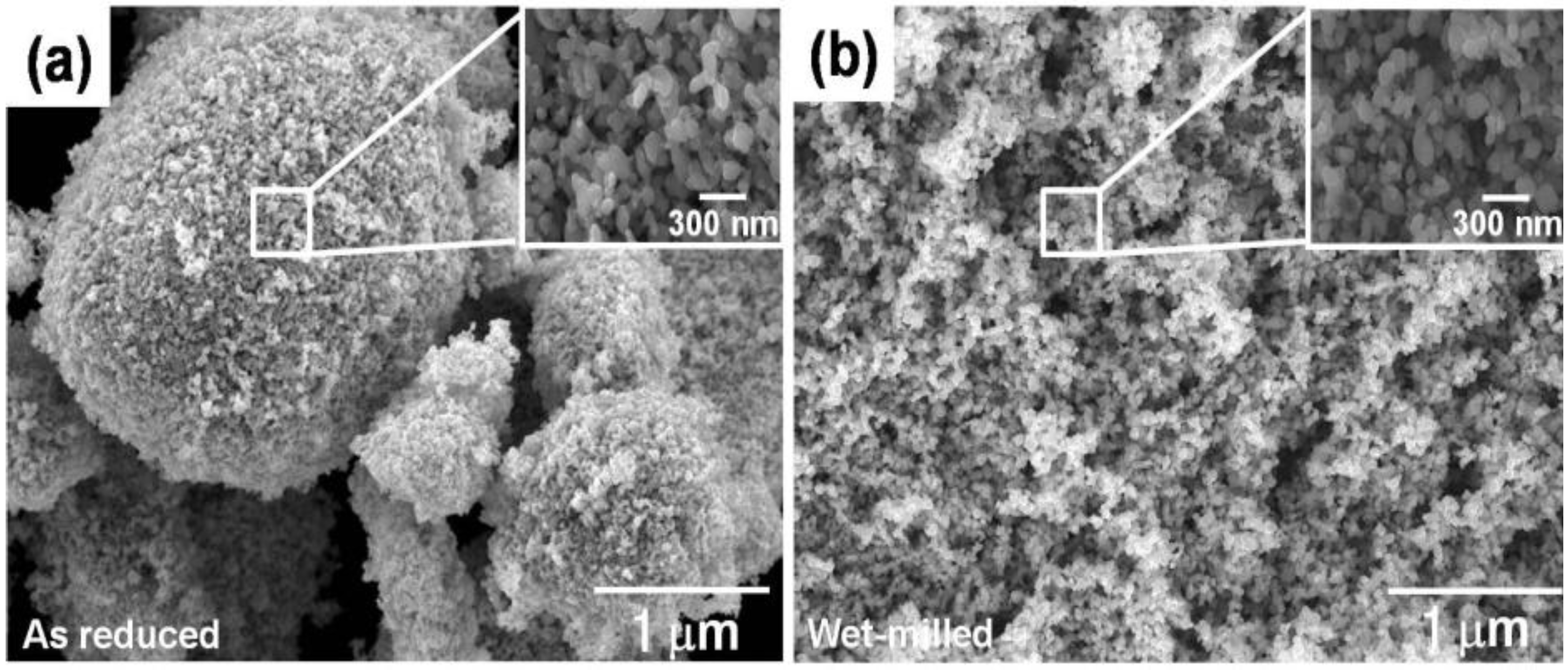

3. Effect of Hierarchical Gb Structure of Nanopowder Agglomerates on Densification Kinetics and Microstructure
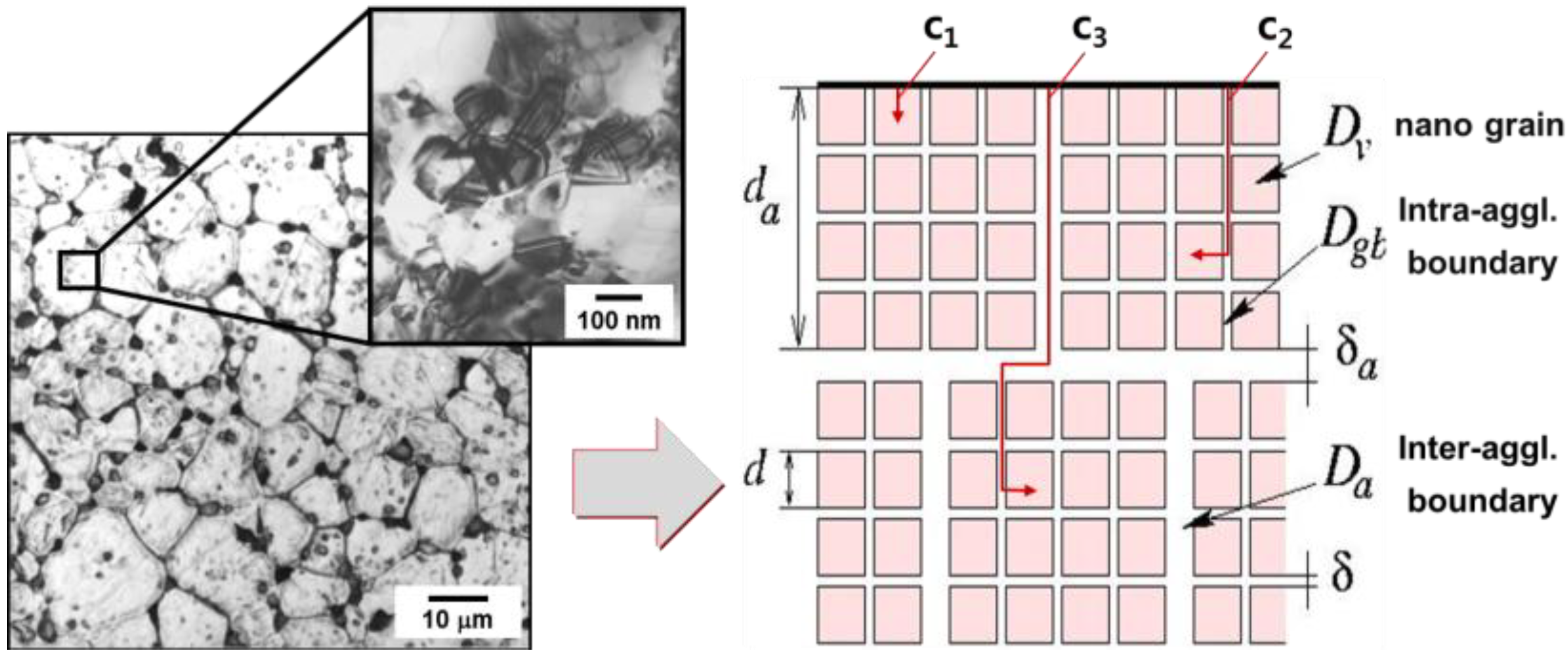
3.1. Diffusion-Controlled Densification Process
| Element | nano-GBs | aggl. boundaries | ||
|---|---|---|---|---|
| D0 (m2/s) | Qgb (kJ/mol) | D0 (m2/s) | Qa(kJ/mol) | |
| Fe | 4.2 × 10−3 | 187 | 3.4 × 10−3 | 148 |
| Ni | 9.4 × 10−4 | 177 | 1.9 × 10−3 | 134 |
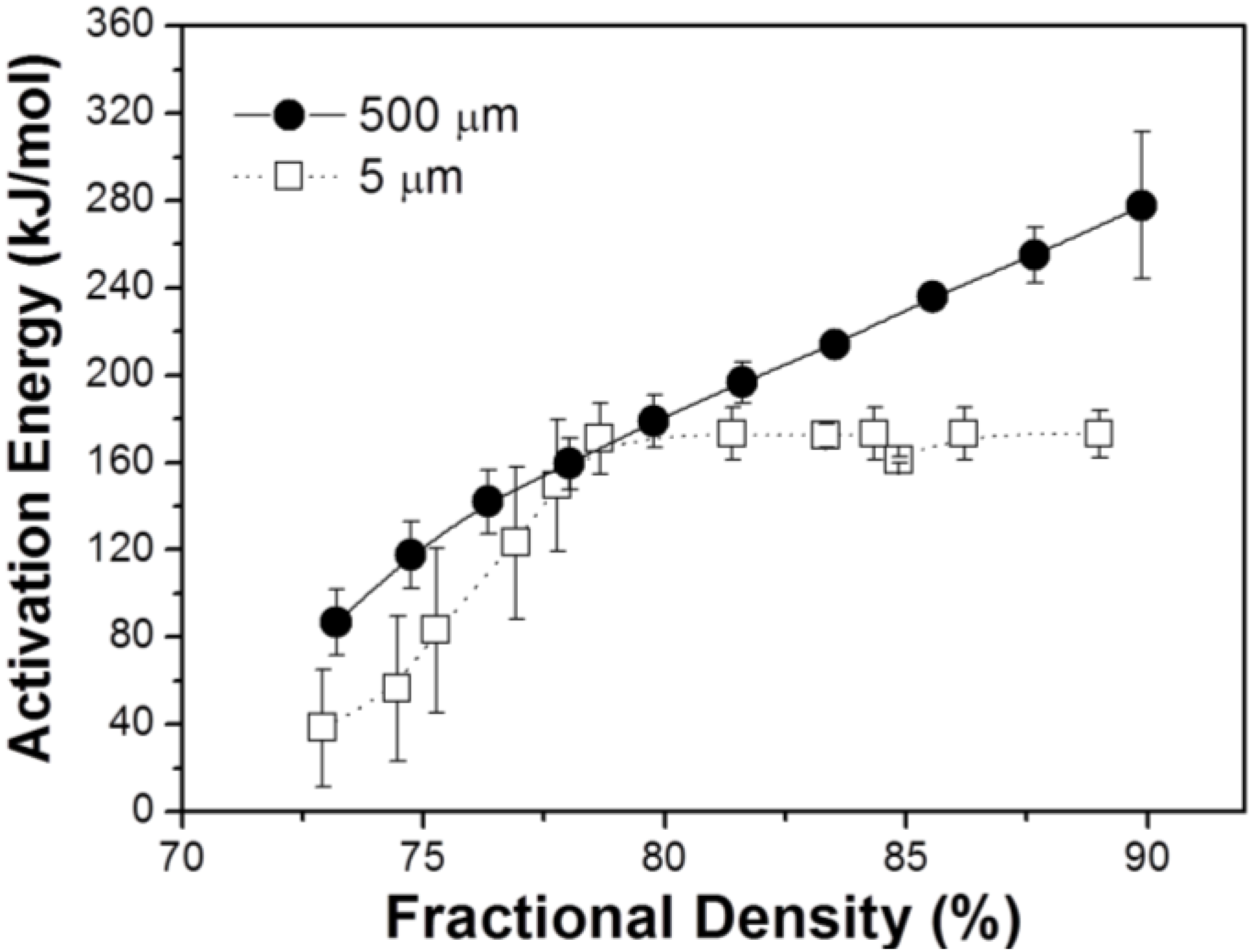
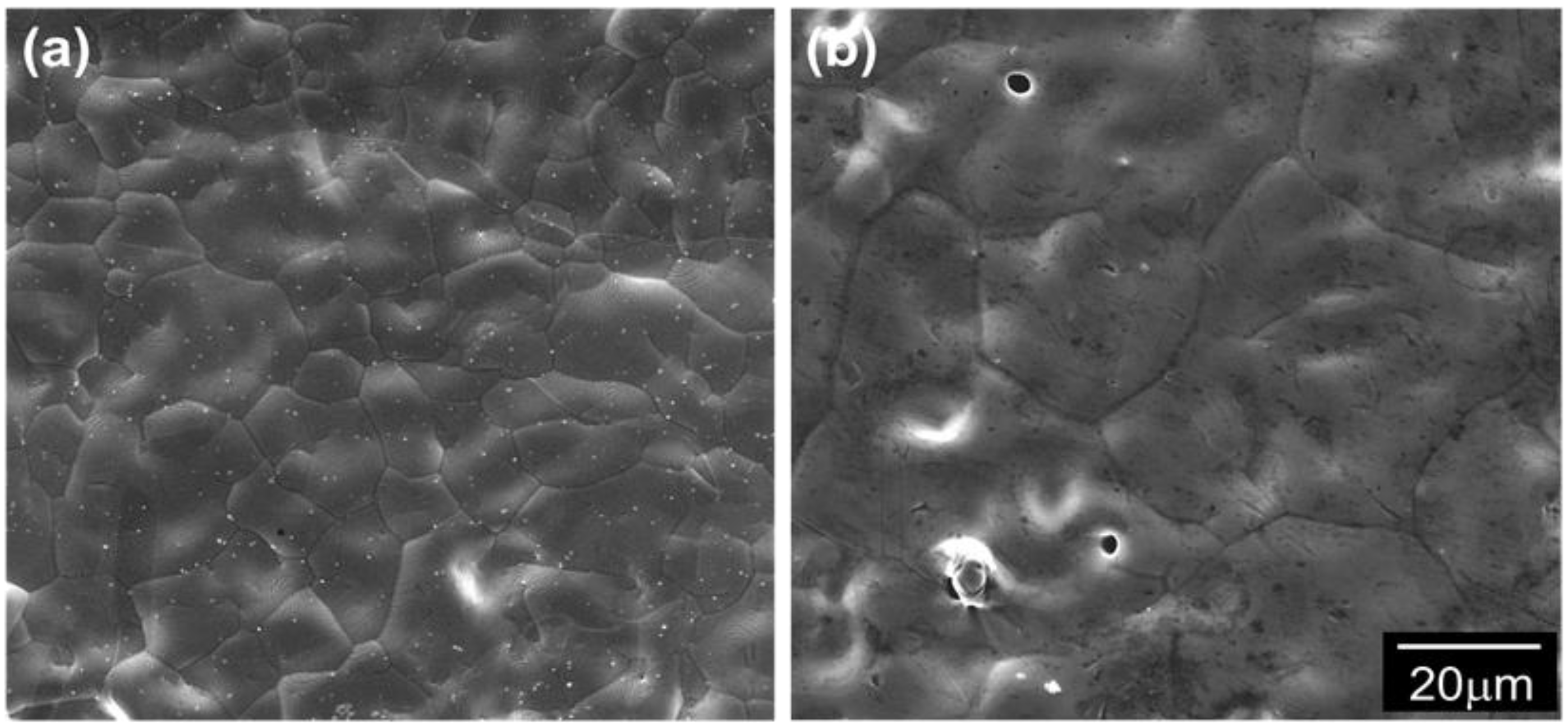
3.2. Densification and Microstructure


4. Application of NAS to Fabrication of Iron Base Powder Material Components
4.1. Full Density Processing of Fe Nanopowder by NAS Process
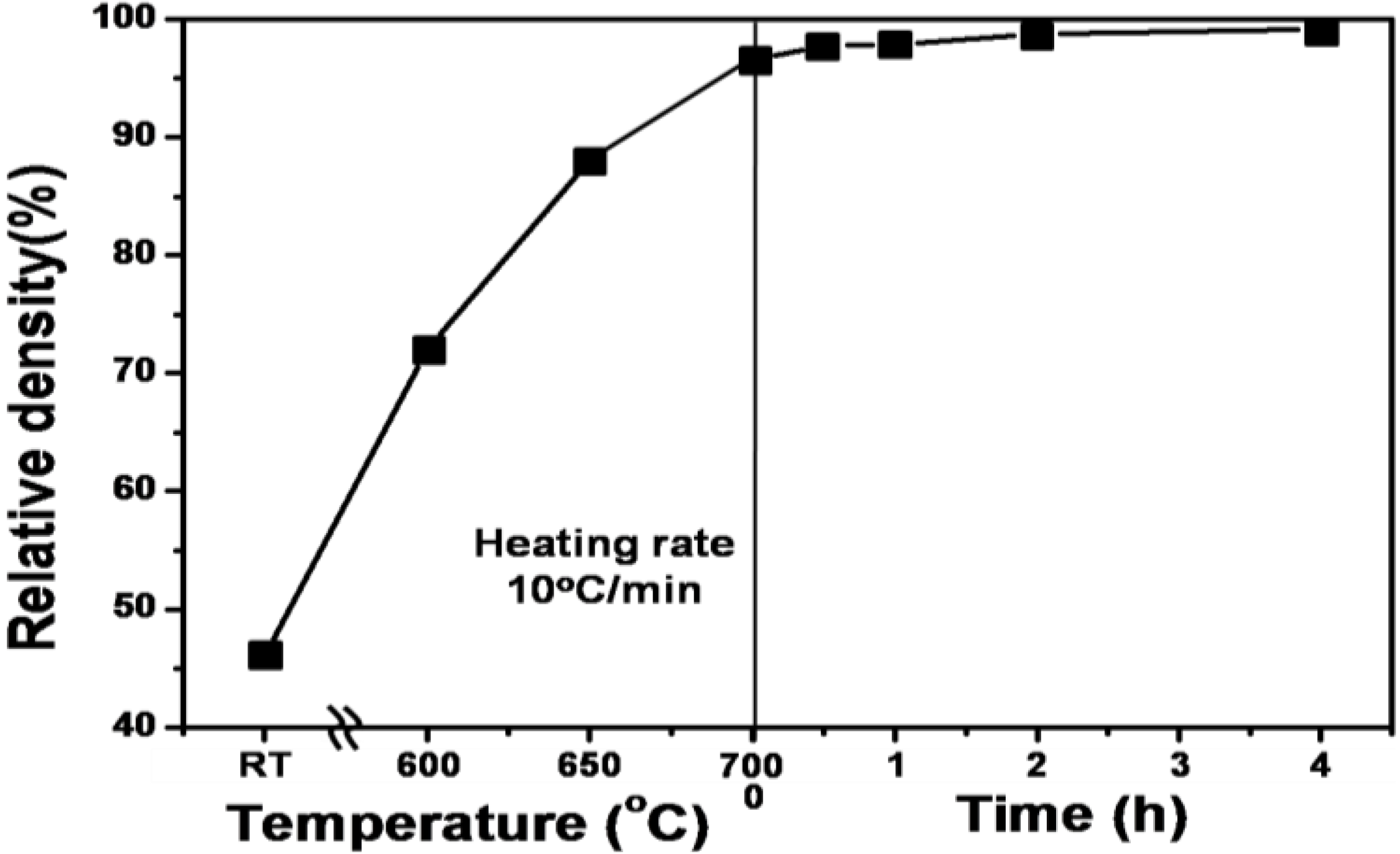
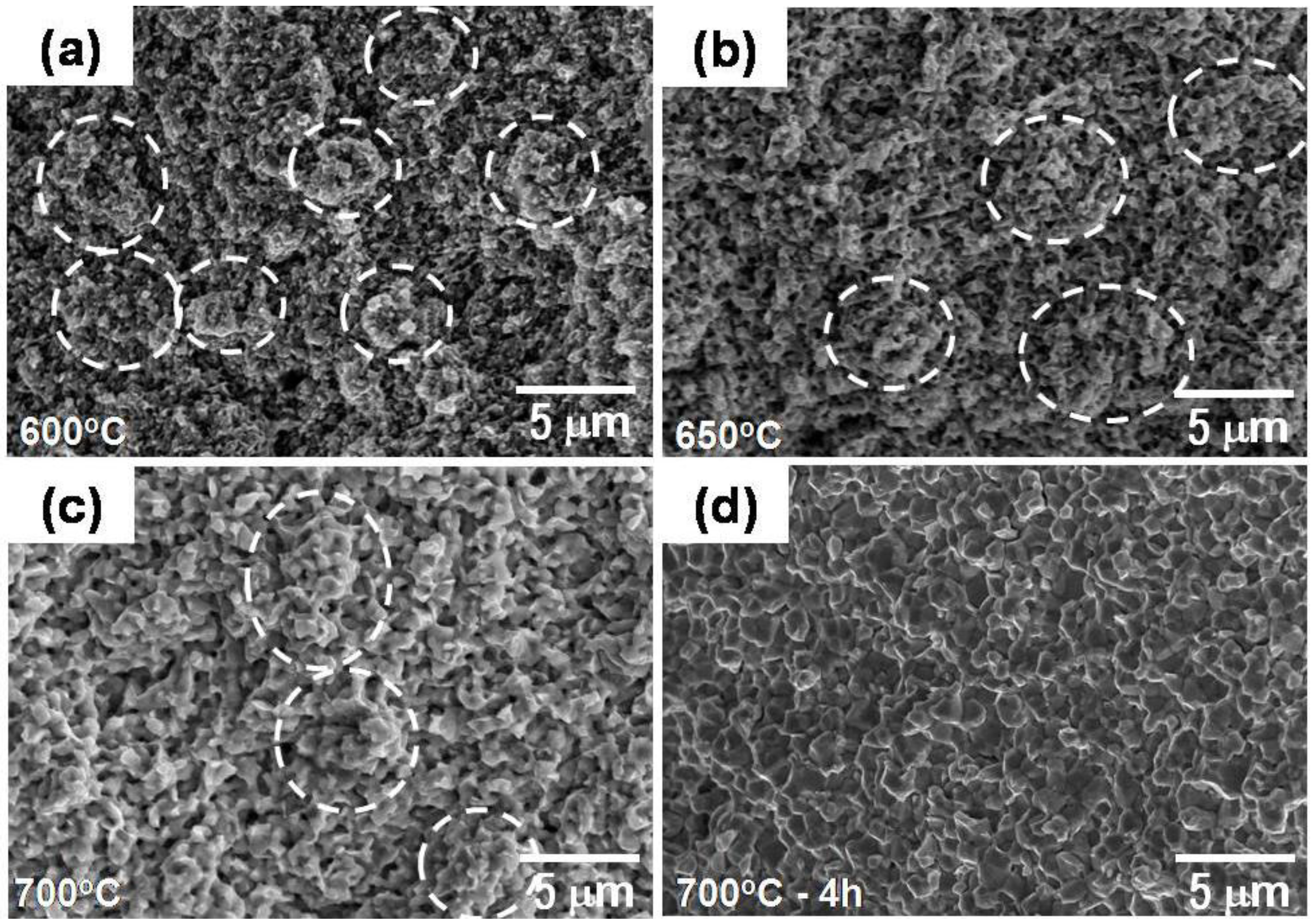
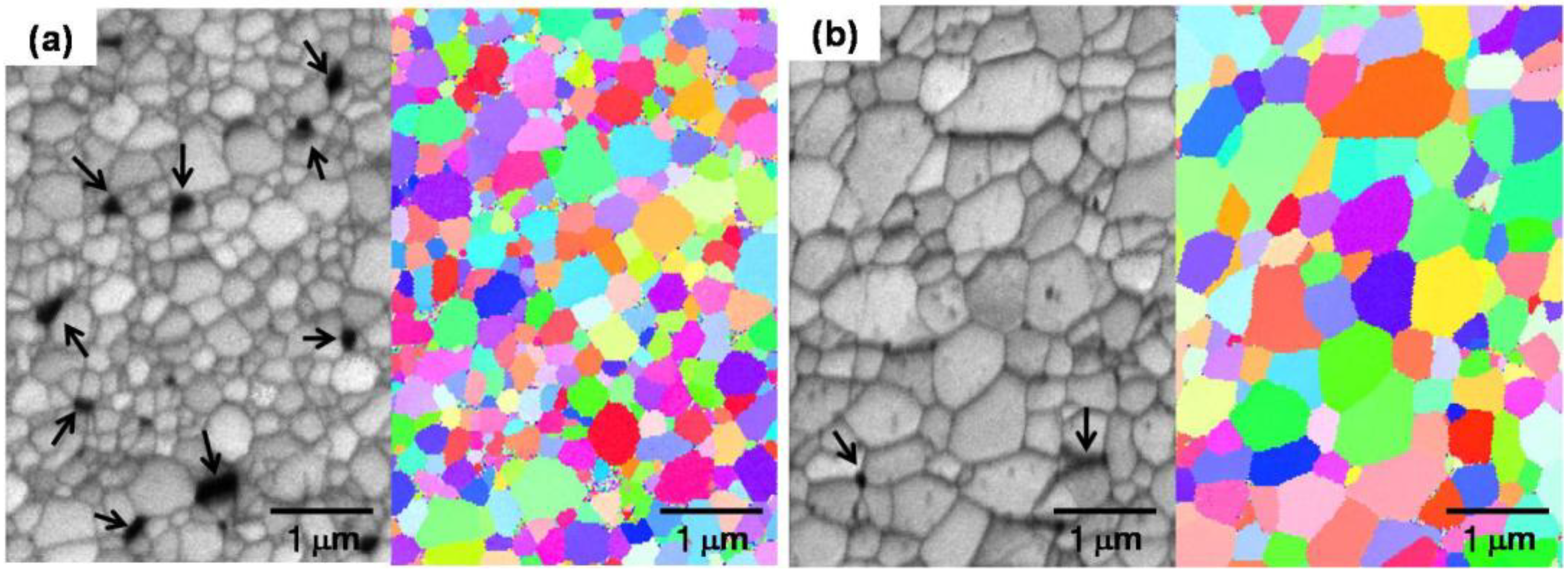
| Composition | Heating time (h) | Relative density (%) | Hardness (Hv) | Grain size (μm) |
|---|---|---|---|---|
| Fe | 0 | 96.3 | 210 | 0.57 |
| 0.5 | 97.6 | 191 | 0.64 | |
| 1 | 97.7 | 176 | 0.77 | |
| 2 | 98.6 | 167 | 1.1 | |
| 4 | 99 | 160 | 1.3 |
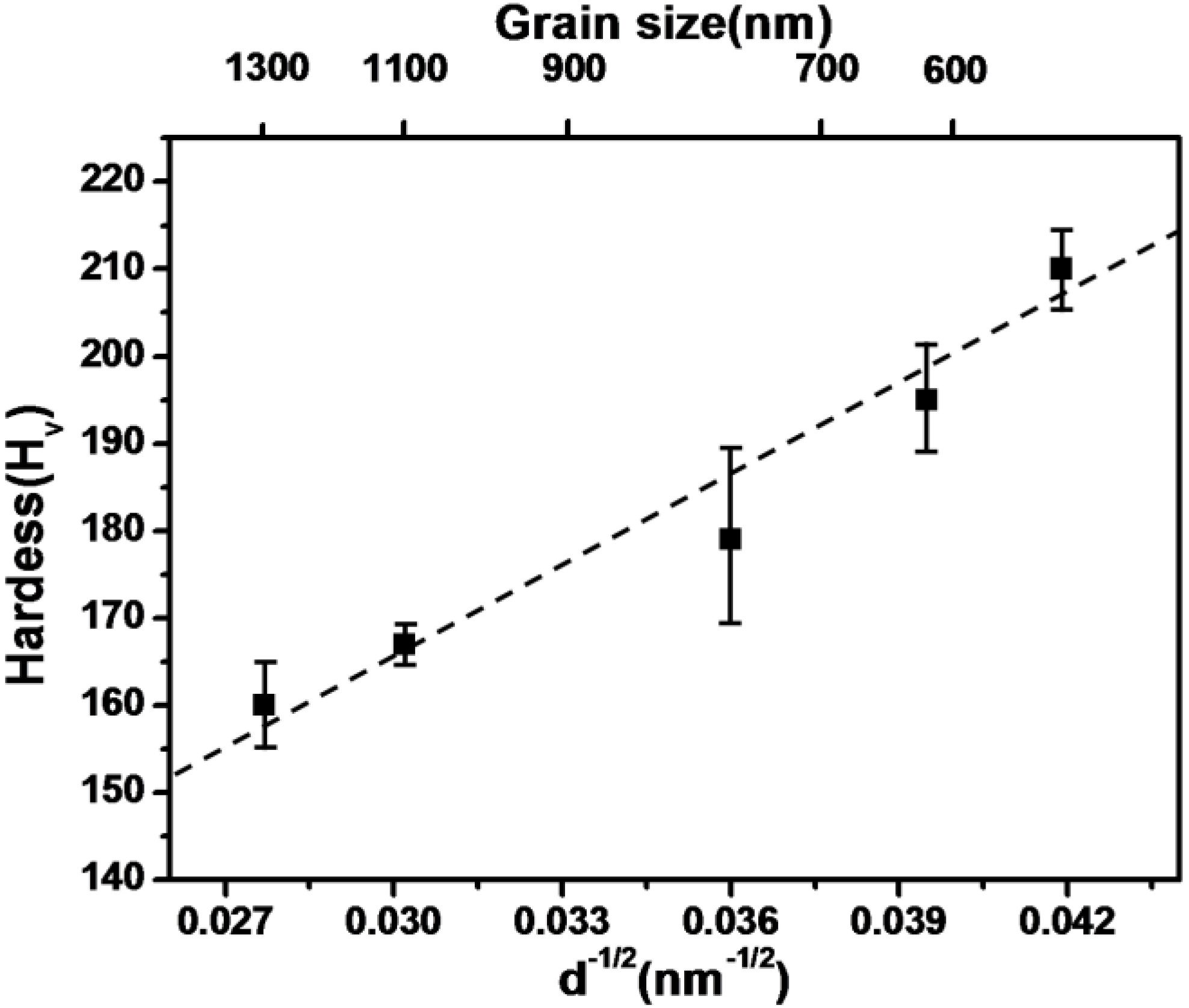
4.2. Powder Injection Molding of Fe Base Nanopowder Agglomerates
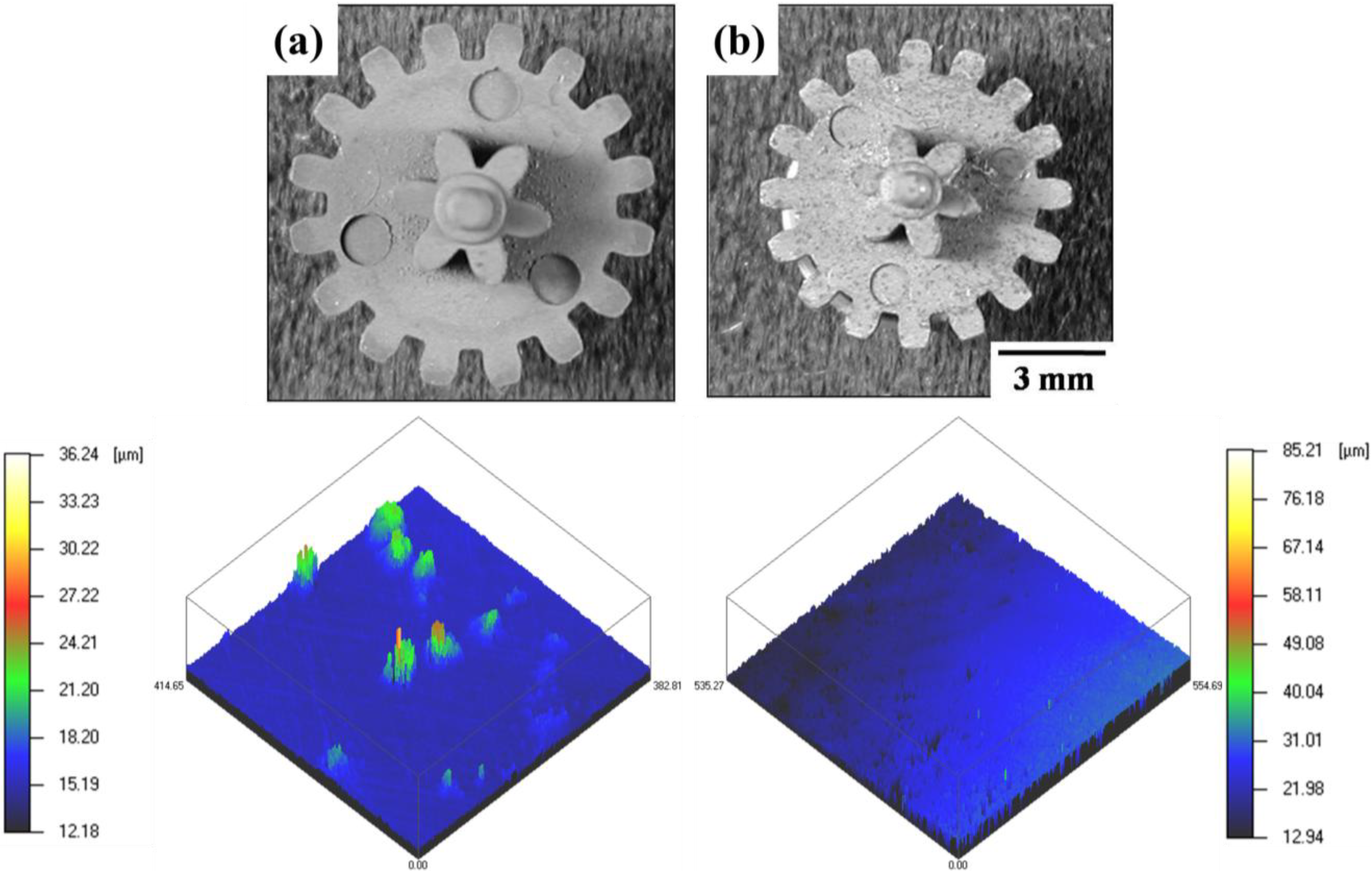
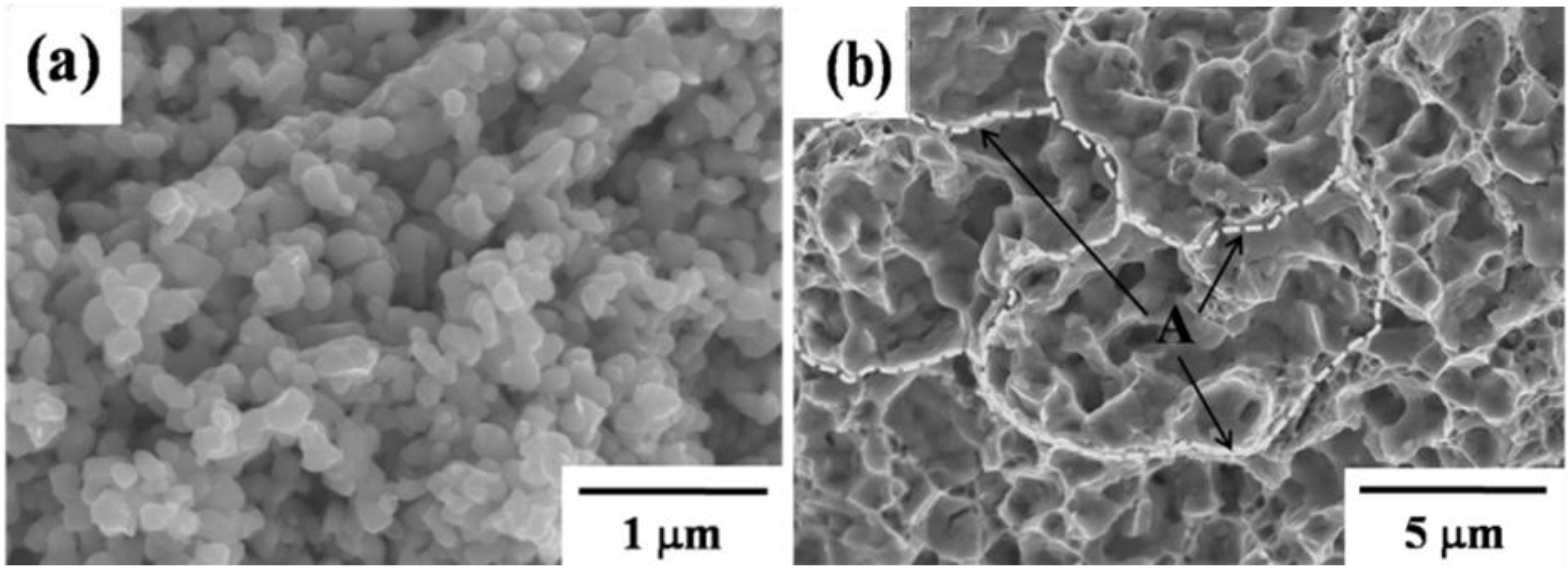
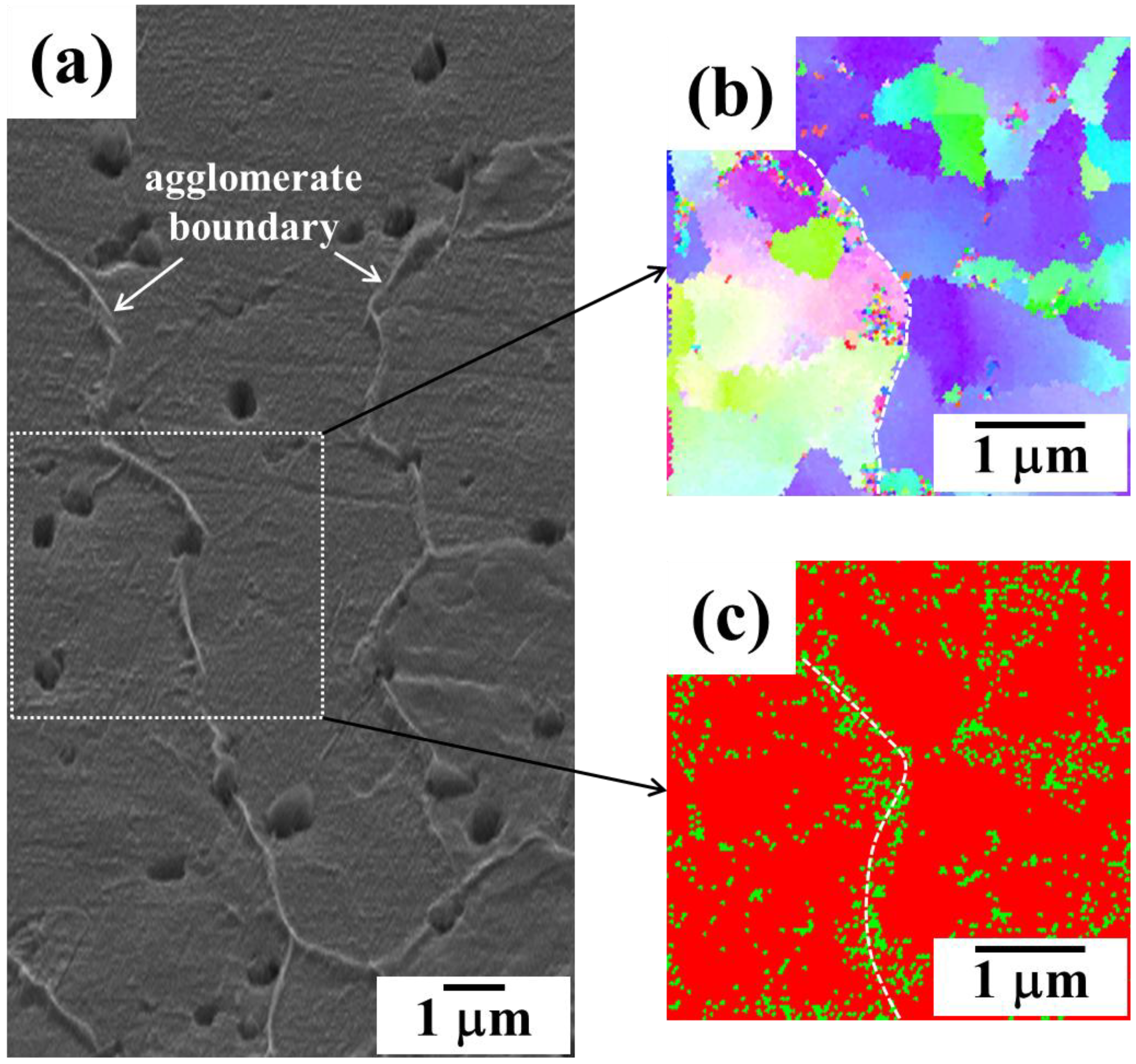
4.3. Powder Injection Molding of Bimodal Type Micro-Nano Powder
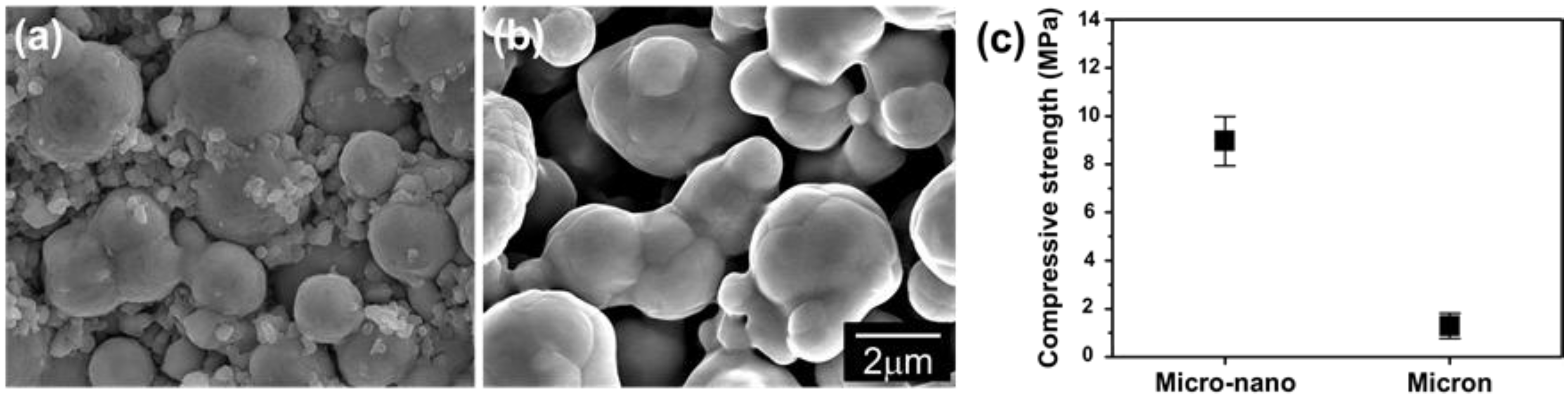

5. Conclusions
Acknowledgements
Conflicts of interest
References
- Suryanarayana, C. Nanocrystalline materials. Int. Mater. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- Knorr, P.; Nam, J.G.; Lee, J.S. Sintering behavior of nanocrystalline γ-Ni-Fe powders. Metall. Mater. Trans. 2000, 31, 503–510. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, B.H.; Kang, Y.S. Processing of net-shaped nanocrystalline Fe–Ni material. Adv. Eng. Mater. 2005, 7, 467–473. [Google Scholar] [CrossRef]
- Cha, B.H.; Lee, J.S. Surface roughness and sintering characteristics of Fe–8 wt % Ni component fabricated by PIM. J. Kor. Powder Metall. Inst. 2009, 16, 342–350. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, B.H.; You, W.K. Full-density nanopowder agglomerate sintering of injection molded iron-nickel. Int. J. Powder Metall. 2010, 46, 27–34. [Google Scholar]
- Lee, J.S.; Kang, H.G.; Kang, Y.S. The Importance of Agglomerate-Size Control in Full-Density Processing of Nanopowder by Pressureless Sintering. In Proceedings of the World Congress on Powder Metallrugy & Particulate Materials 2008, Washington, DC, USA, 8–12 June 2008.
- Lee, J.S.; You, W.K.; Cha, B.H. Hierarchical structured nanomaterial fabricated by nanopowder agglomerate sintering. Mater. Sci. Forum 2010, 638–642, 93–97. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, Y.S. Processing of bulk nanostructure Ni-Fe materials. Scr. Mater. 2001, 44, 1591–1594. [Google Scholar] [CrossRef]
- Divinski, S.V.; Hisker, F.; Kang, Y.-S.; Lee, J.S.; Herzig, Chr. 59Fe grain boundary diffusion in nanostructured γ–Fe–Ni: Part I: Radiotracer experiments and Monte-Carlo simulation in the type-A and B kinetic regimes. Z. Metallkd. 2002, 93, 256–264. [Google Scholar] [CrossRef]
- Divinski, S.V.; Hisker, F.; Kang, Y.-S.; Lee, J.S.; Herzig, Chr. 59Fe grain boundary diffusion in nanostructured γ-Fe-Ni part: Effect of bimodal microstructure on diffusion behavior in type-C kinetic regime. Z. Metallkd. 2002, 93, 265–272. [Google Scholar]
- Divinski, S.V.; Hisker, F.; Kang, Y.S.; Lee, J.S.; Herzig, Chr. Tracer diffusion of 63Ni in nano-γ-FeNi produced by powder metallurgical method: Systematic investigations in the C, B, and A diffusion regimes. Interface Sci. 2003, 11, 67–80. [Google Scholar] [CrossRef]
- Lee, J.S.; Yun, J.C.; Choi, J.P.; Lee, G.Y. Consolidation of iron nanopowder by nanopowder-agglomerate sintering at elevated sintering. J. Kor. Powder Metall. Inst. 2013, 20, 1–6. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, Y.S.; Kwon, S.K.; Cha, B.H.; Qin, X.Y. A new processing route for net-shaped nanoparticulate materials. Adv. Powder Technol. 2004, 15, 639–655. [Google Scholar] [CrossRef]
- Qin, X.Y.; Lee, J.S.; Nam, J.G.; Kim, B.S. Synthesis and microstructural characterization of nanostructured γ-Ni-Fe powder. Nanostr. Mater. 1995, 11, 383–397. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.S.; Sekino, T.; Choa, Y.H.; Niihara, K. Hydrogen reduction behavior of NiO dispersoid during processing of Al2O3-Ni nanocomposites. Scr. Mater. 2001, 44, 2121–2125. [Google Scholar] [CrossRef]
- Jung, S.S.; Kang, Y.S.; Lee, J.S. The optimization of hydrogen reduction process for mass production of Fe–8 wt % Ni nanoalloy powder. Mater. Sci. Forum 2007, 534–536, 153–156. [Google Scholar] [CrossRef]
- Jung, S.S.; Lee, J.S. In-situ kinetic study of hydrogen reduction of Fe2O3 for the production of Fe nanopowder. Mater. Trans. 2009, 50, 2270–2276. [Google Scholar] [CrossRef]
- Lee, J.S.; Yun, J.C.; You, W.K. Full Density Processing of Pure Iron Nanomaterial by Nanopowder Agglomerate Sintering and Related Properties. In Proceeding of the World Congress PM 2010, Florence, Italy, 10–14 October 2010.
- Nam, H.Y.; Kwon, S.K.; Kang, Y.S.; Lee, J.S. Sintering behavior of the PIMed Fe–50 wt % Ni nanoalloy powder. Mater. Sci. Forum 2004, 449–452, 1141–1144. [Google Scholar] [CrossRef]
- Rahaman, M.N. Ceramic Processing and Sintering; Marcel Dekker, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Cha, B.H. Sintering Behavior of Net-shaped Nanocrystalline Fe–Ni Powder Material and Its Mechanical Properties. Ph.D. Thesis, Hanyang University, Hanyang, Korea, 2009. [Google Scholar]
- Groza, J.R. Nanosintering. Nanostr. Mater. 1999, 12, 987–992. [Google Scholar] [CrossRef]
- Kang, Y.S.; Lee, J.S.; Divinski, S.V.; Herzig, Chr. Ni grain boundary diffusion in coarse-grained Fe–40% Ni alloy and comparison with Ni diffusion in the nanocrystalline alloy. Z. Metallkd. 2004, 95, 76–79. [Google Scholar] [CrossRef]
- Kang, Y.S.; Cha, B.H.; Kang, H.G.; Lee, J.S. Densification behavior and microstructural development of nano-agglomerate powder during sintering. Mater. Sci. Forum 2007, 534–536, 505–508. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy and Particulate Materials Processing; Metal Powder Industries Federation: Princeton, NJ, USA, 2005. [Google Scholar]
- Cha, B.H.; Kang, Y.S.; Lee, J.S. Processing of net-shaped Fe–Ni nanomaterials by powder injection molding. J. Jpn. Soc. Powder Powder Metall. 2006, 53, 769–775. [Google Scholar] [CrossRef]
- You, W.K.; Lee, J.S.; Ko, S.H.; Lee, W.S. Mixing behavior and microstructural development during fabrication of Fe micro-nano powder feedstock for micro-PIM. Kor. J. Met. Mater. 2010, 48, 630–638. [Google Scholar]
- You, W.K.; Choi, J.P.; Lee, J.S. Die compaction and sintering behavior of Fe micro-nano powder feedstock for micro-PIM. Kor. J. Met. Mater. 2010, 10, 32–39. [Google Scholar]
- German, R.M.; Bose, A. Injection Molding of Metals and Ceramics; Metal Powder Industries Federation: Princeton, NJ, USA, 1997. [Google Scholar]
- You, W.K.; Choi, J.P.; Yoon, S.M.; Lee, J.S. Low temperature powder injection molding of iron micro-nano powder mixture. Powder Technol. 2012, 225, 199–205. [Google Scholar] [CrossRef]
- Gonçalves, A.C. Metallic powder injection molding using low pressure. J. Mater. Process. Technol. 2001, 118, 193–198. [Google Scholar] [CrossRef]
- Amorós, J.L.; Cantavella, V.; Jarque, J.C.; Felíu, C. Green strength tensile of pressed compacts: An analysis of the different methods. J. Eur. Ceram. Soc. 2008, 28, 701–710. [Google Scholar] [CrossRef]
- Ruprecht, R.; Gietzelt, T.; Müller, M.; Piotter, V.; Haußelt, J. Injection molding of microstructured components from plastics, metals and ceramics. Microsyst. Tecnol. 2002, 8, 351–358. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys; Champman & Hall: London, UK, 1992. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, J.-S.; Choi, J.-P.; Lee, G.-Y. Consolidation of Hierarchy-Structured Nanopowder Agglomerates and Its Application to Net-Shaping Nanopowder Materials. Materials 2013, 6, 4046-4063. https://doi.org/10.3390/ma6094046
Lee J-S, Choi J-P, Lee G-Y. Consolidation of Hierarchy-Structured Nanopowder Agglomerates and Its Application to Net-Shaping Nanopowder Materials. Materials. 2013; 6(9):4046-4063. https://doi.org/10.3390/ma6094046
Chicago/Turabian StyleLee, Jai-Sung, Joon-Phil Choi, and Geon-Yong Lee. 2013. "Consolidation of Hierarchy-Structured Nanopowder Agglomerates and Its Application to Net-Shaping Nanopowder Materials" Materials 6, no. 9: 4046-4063. https://doi.org/10.3390/ma6094046




