Plasma-Enabled Carbon Nanostructures for Early Diagnosis of Neurodegenerative Diseases
Abstract
:1. Introduction
1.1. Biosensors
1.2. Advanced Materials: Carbon Nanostructures
1.3. From Fabrication to Performance: The Plasma Advantage
2. Graphene
2.1. Graphene-Based Electrical Biosensors
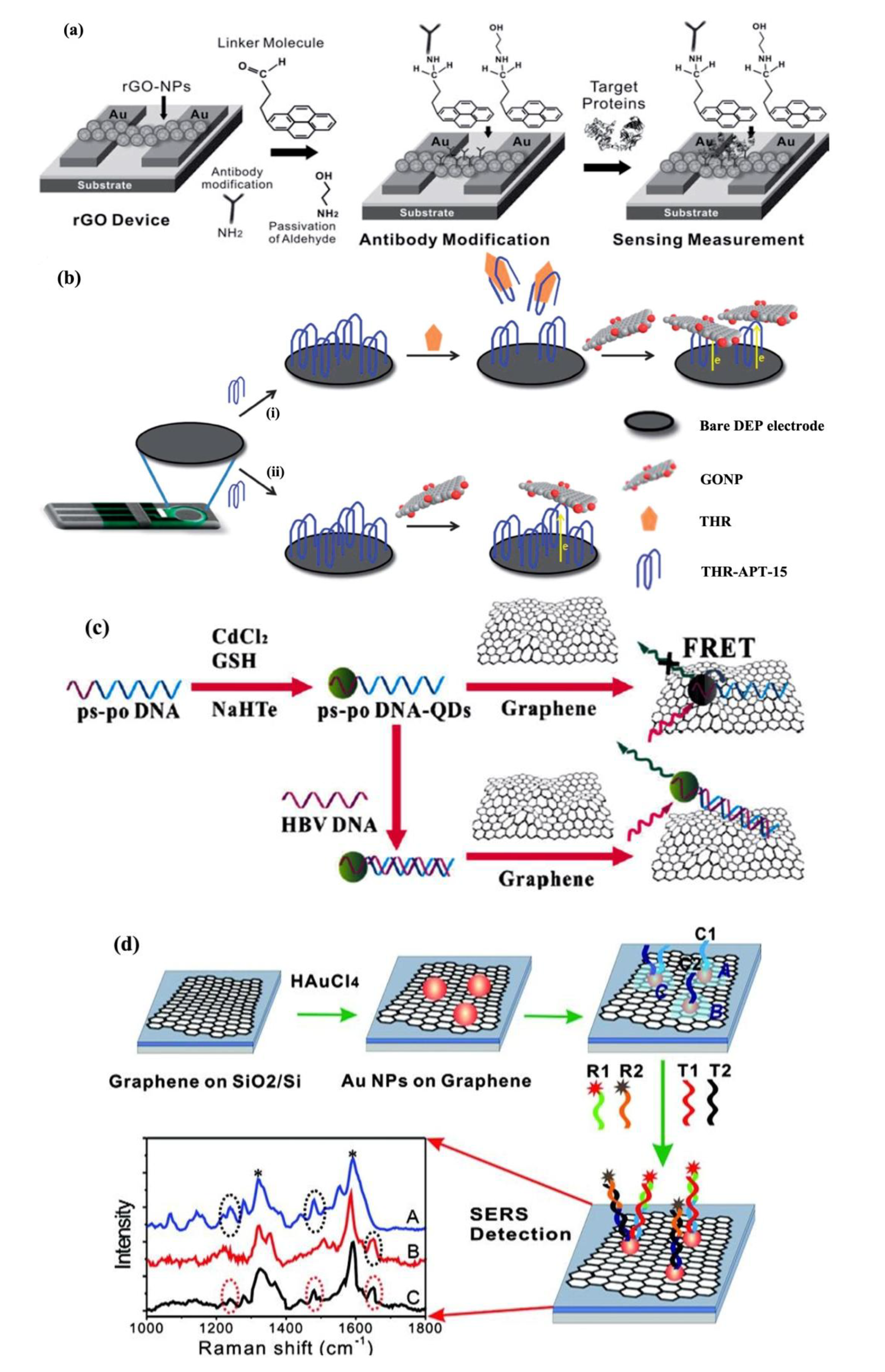
2.2. Graphene-Based Optical Biosensors
2.3. Plasma-Processing: Graphene

3. Carbon Nanotubes
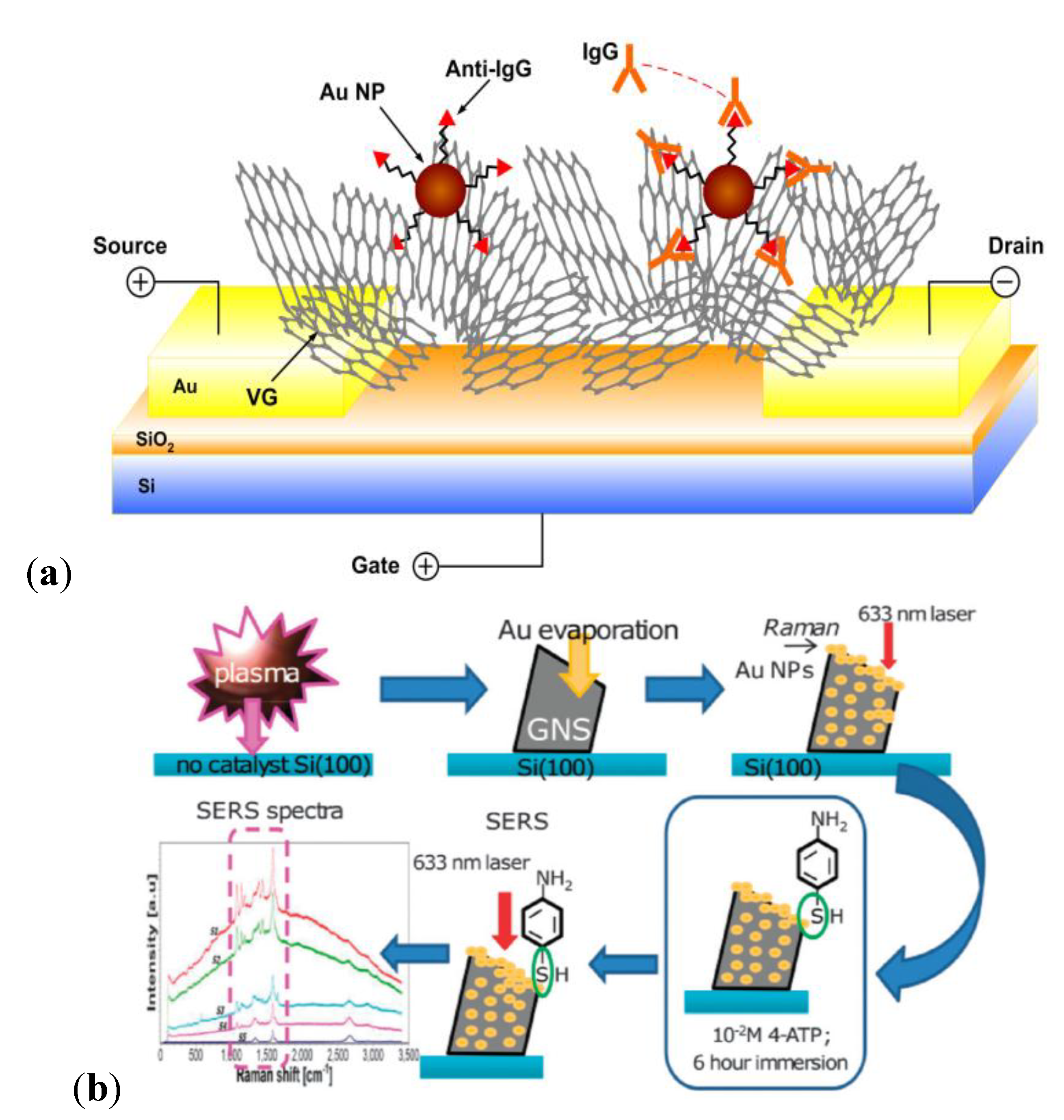
3.1. CNT-Based Electrical Biosensors
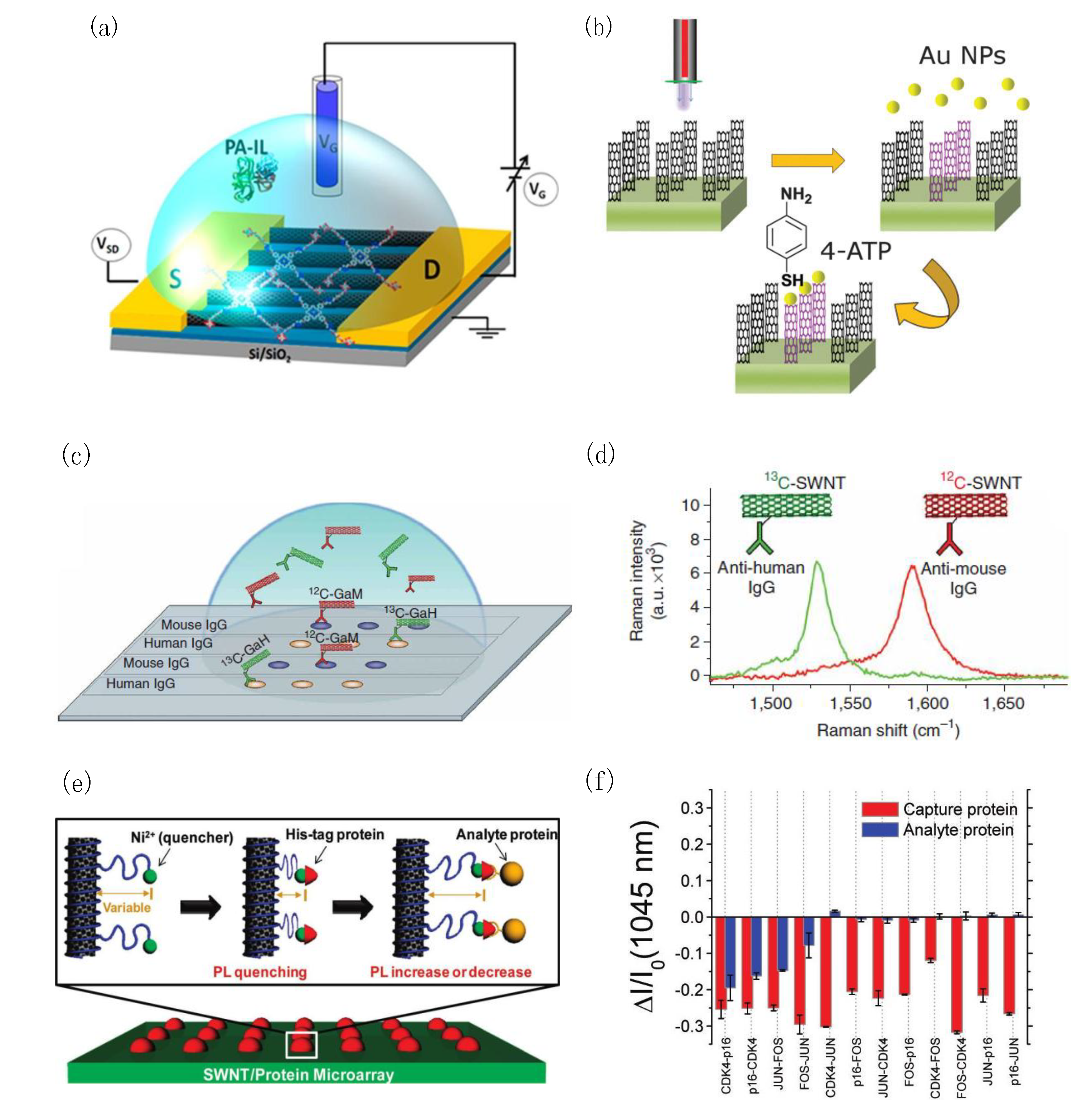
3.2. CNT-Based Optical Biosensors
3.3. Plasma-Processing: CNTs
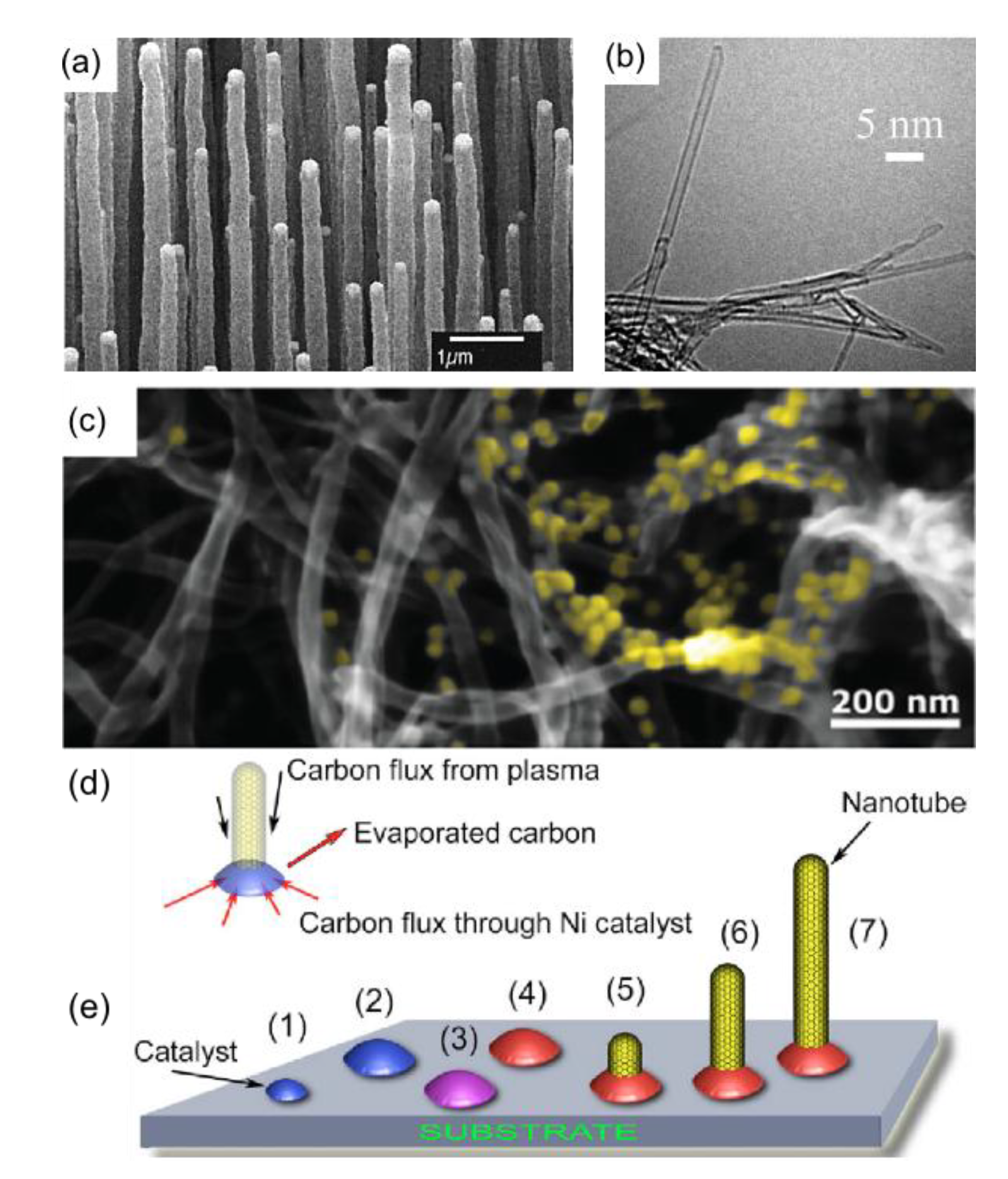
4. Carbon Nanowalls
4.1. CNW-Based Biosensors
4.2. Plasma-Processing: CNWs
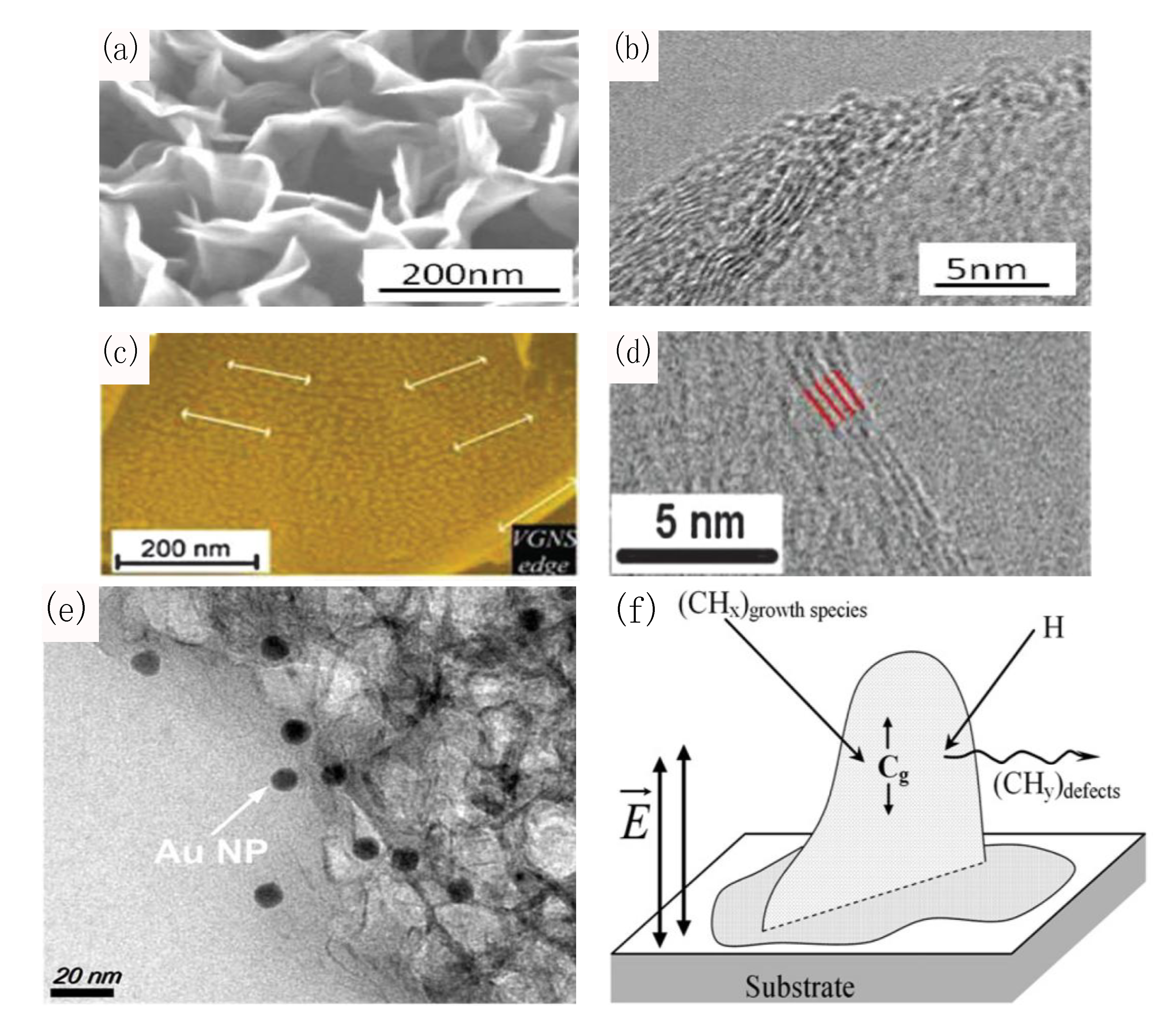
5. Conclusions and Outlook
| Sensors | Target analyte | Detection limit | Detection range | Reference |
|---|---|---|---|---|
| CNWs on graphite electrode | DNAs | 9.4 zM | 0.1 fM–10 mM | [199] |
| GO with sulfonated polyalinine | DNAs | 5.2 fM | 0.1 µM–10 fM | [225] |
| CNWs on Si | Metabolites | 0.17 µM | 1–100 µM | [226] |
| CNWs on Ti-coated Si | Metabolites | 0.3 µM | 0.01–50 mM | [227] |
| CNWs on Au | Immunoglobulins | 2 ng/mL | 2–20 ng/mL | [198] |
| CNTs | DNAs | 100 aM | 100 aM–1 pM | [228] |
| CNT-based FETs | DNAs | 0.1 mg/mL | – | [229] |
| Nitrogen-doped MWCNTs | Glucose | 10 µM | 0.02–1.02 mM | [230] |
| MWCNTs with GOx and Au NPs | Glucose | 20 µM | 0.05–22 mM | [231] |
| SWCNTs on SiO2 | Immunoglobulins | 1 pg/mL | 100 fg/mL–1000 pg/mL | [157] |
| SWCNTs on SiO2 | Immunoglobulins | 1 pg/mL | 1–1000 pg/mL | [145] |
| Functionalized SWCNTS | Immunoglobulins | 1 fM | 100 pM–1 fM | [164] |
| Graphene | DNAs | 0.12 pM | 1 pM–0.1 µM | [232] |
| Graphene on SiO2 | DNAs | 10 pM | 10 pM–100 nM | [93] |
| N-doped graphene oxide | Immunoglobulins | 0.012 U/mL | 0.1–20 U/mL | [75] |
| Graphene oxide with Au NPs | Immunoglobulins | 1 pM | 1 pM–1 µM | [70] |
| Graphene oxide with Au NPs | Immunoglobulins | 0.0375 µg/mL | 0.0375–40 µg/mL | [110] |
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.Q.; et al. Global prevalence of dementia: A delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Gray, S.; Kawas, C. Projections of alzheimer’s disease in the united states and the public health impact of delaying disease onset. Am. J. Public Health 1998, 88, 1337–1342. [Google Scholar] [CrossRef]
- Abbott, A. Dementia: A problem for our age. Nature 2011, 475, S2–S4. [Google Scholar] [CrossRef]
- Rapoport, S.I. In vivo pet imaging and postmortem studies suggest potentially reversible and irreversible stages of brain metabolic failure in alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 1999, 249, 46–55. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Klunk, W.E.; Abrahamson, E.E.; Mathis, C.A.; Price, J.C.; Tsopelas, N.D.; Lopresti, B.J.; Ziolko, S.; Bi, W.Z.; Paljug, W.R.; et al. Post-mortem correlates of in vivo pib-pet amyloid imaging in a typical case of alzheimer’s disease. Brain 2008, 131, 1630–1645. [Google Scholar] [CrossRef]
- Kung, M.P.; Hou, C.; Zhuang, Z.P.; Skovronsky, D.; Kung, H.F. Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with alzheimer’s disease. Brain Res. 2005, 1031, 302. [Google Scholar] [CrossRef]
- Darvesh, S.; Walsh, R.; Kumar, R.; Caines, A.; Roberts, S.; Magee, D.; Rockwood, K.; Martin, E. Inhibition of human cholinesterases by drugs used to treat alzheimer disease. Alzheimer Dis. Assoc. Dis. 2003, 17, 117–126. [Google Scholar] [CrossRef]
- Rösler, M.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stahelin, H.B.; Hartman, R.; Gharabawi, M. Efficacy and safety of rivastigmine in patients with alzheimer’s disease: International randomised controlled trial. BMJ 1999, 318, 633–638. [Google Scholar]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, D.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for alzheimer’s disease. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef]
- Factor, S.A.; Molho, E.S.; Feustel, P.J.; Brown, D.L.; Evans, S.M. Long-term comparative experience with tolcapone and entacapone in advanced parkinson’s disease. Clin. Neuropharmacol. 2001, 24, 295–299. [Google Scholar] [CrossRef]
- Holloway, R.G.; Shoulson, I.; Kieburtz, K.; McDermott, M.; Shinaman, A.; Kamp, C.; Fahn, S.; Lang, A.; Marek, K.; Seibyl, J.; et al. Pramipexole vs levodopa as initial treatment for parkinson disease—A 4-year randomized controlled trial. Arch. Neurol. (Chicago) 2004, 61, 1044–1053. [Google Scholar]
- Rascol, O.; Goetz, C.; Koller, W.; Poewe, W.; Sampaio, C. Treatment interventions for parkinson’s disease: An evidence based assessment. Lancet 2002, 359, 1589–1598. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Chung, K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for lewy-body formation in parkinson disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef]
- Kingwell, K. Alzheimer disease: Csf levels of mitochondrial DNA—A new biomarker for preclinical alzheimer disease? Nat. Rev. Neurol. 2013, 9, 420. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; Macarthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Ammar, M.; Smadja, C.; Giang Thi Phuong, L.; Azzouz, M.; Vigneron, J.; Etcheberry, A.; Taverna, M.; Dufour-Gergam, E. A new controlled concept of immune-sensing platform for specific detection of alzheimer’s biomarkers. Biosens. Bioelectron. 2013, 40, 329–335. [Google Scholar] [CrossRef]
- Chikae, M.; Fukuda, T.; Kerman, K.; Idegami, K.; Miura, Y.; Tamiya, E. Amyloid-beta detection with saccharide immobilized gold nanoparticle on carbon electrode. Bioelectrochemistry 2008, 74, 118–123. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Wu, X.; Ye, Z.; Li, G. Peptide-based electrochemical biosensor for amyloid beta 1–42 soluble oligomer assay. Talanta 2012, 93, 358–363. [Google Scholar] [CrossRef]
- Prabhulkar, S.; Piatyszek, R.; Cirrito, J.R.; Wu, Z.Z.; Li, C.Z. Microbiosensor for alzheimer’s disease diagnostics: Detection of amyloid beta biomarkers. J. Neurochem. 2012, 122, 374–381. [Google Scholar] [CrossRef]
- Rushworth, J.V.; Ahmed, A.; Griffiths, H.H.; Pollock, N.M.; Hooper, N.M.; Millner, P.A. A label-free electrical impedimetric biosensor for the specific detection of alzheimer’s amyloid-beta oligomers. Biosens. Bioelectron. 2013, 56C, 83–90. [Google Scholar]
- Sierks, M.R.; Chatterjee, G.; McGraw, C.; Kasturirangan, S.; Schulz, P.; Prasad, S. Csf levels of oligomeric alpha-synuclein and beta-amyloid as biomarkers for neurodegenerative disease. Integr. Biol. (UK) 2011, 3, 1188–1196. [Google Scholar] [CrossRef]
- Chou, I.H.; Benford, M.; Beier, H.T.; Cote, G.L.; Wang, M.; Jing, N.; Kameoka, J.; Good, T.A. Nanofluidic biosensing for beta-amyloid detection using surface enhanced raman spectroscopy. Nano Lett. 2008, 8, 1729–1735. [Google Scholar] [CrossRef]
- Gheorghiu, M.; David, S.; Polonschii, C.; Olaru, A.; Gaspar, S.; Bajenaru, O.; Popescu, B.O.; Gheorghiu, E. Label free sensing platform for amyloid fibrils effect on living cells. Biosens. Bioelectron. 2014, 52, 89–97. [Google Scholar] [CrossRef]
- Stravalaci, M.; Bastone, A.; Beeg, M.; Cagnotto, A.; Colombo, L.; di Fede, G.; Tagliavini, F.; Cantu, L.; del Favero, E.; Mazzanti, M.; et al. Specific recognition of biologically active amyloid-beta oligomers by a new surface plasmon resonance-based immunoassay and an in vivo assay in caenorhabditis elegans. J. Biol. Chem. 2012, 287, 27796–27805. [Google Scholar] [CrossRef]
- Ostrikov, K.; Neyts, E.C.; Meyyappan, M. Plasma nanoscience: From nano-solids in plasmas to nano-plasmas in solids. Adv. Phys. 2013, 62, 113–224. [Google Scholar] [CrossRef]
- Azarenkov, N.A.; Denisenko, I.B.; Ostrikov, K.N. A model of a large-area planar plasma producer based on surface wave propagation in a plasma-metal structure with a dielectric sheath. J. Phys. D Appl. Phys. 1995, 28, 2465–2469. [Google Scholar] [CrossRef]
- Mariotti, D.; Ostrikov, K. Tailoring microplasma nanofabrication: From nanostructures to nanoarchitectures. J. Phys. D Appl. Phys. 2009, 42. [Google Scholar] [CrossRef]
- Ostrikov, K.N.; Yu, M.Y.; Sugai, H. Standing surface waves in a dust-contaminated large-area planar plasma source. J. Appl. Phys. 1999, 86, 2425–2430. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene-based materials: Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nano 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Tang, Y.B.; Yin, L.C.; Yang, Y.; Bo, X.H.; Cao, Y.L.; Wang, H.E.; Zhang, W.J.; Bello, I.; Lee, S.T.; Cheng, H.M.; et al. Tunable band gaps and p-type transport properties of boron-doped graphenes by controllable ion doping using reactive microwave plasma. ACS Nano 2012, 6, 1970–1978. [Google Scholar] [CrossRef]
- Park, C.S.; Zhao, Y.; Lee, J.H.; Whang, D.; Shon, Y.; Song, Y.H.; Lee, C.J. Tunable bandgap of a single layer graphene doped by the manganese oxide using the electrochemical doping. Appl. Phys. Lett. 2013, 102, 032106. [Google Scholar] [CrossRef]
- Matis, B.R.; Burgess, J.S.; Bulat, F.A.; Friedman, A.L.; Houston, B.H.; Baldwin, J.W. Surface doping and band gap tunability in hydrogenated graphene. ACS Nano 2012, 6, 17–22. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; Song, Z.M.; Li, X.B.; Wu, X.S.; Brown, N.; Naud, C.; Mayou, D.; Li, T.B.; Hass, J.; Marchenkov, A.N.; et al. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef]
- Li, G.; Andrei, E.Y. Observation of landau levels of dirac fermions in graphite. Nat. Phys. 2007, 3, 623–627. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Gweon, G.H.; Graf, J.; Fedorov, A.V.; Spataru, C.D.; Diehl, R.D.; Kopelevich, Y.; Lee, D.H.; Louie, S.G.; Lanzara, A. First direct observation of dirac fermions in graphite. Nat. Phys. 2006, 2, 595–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum hall effect and berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Mohanty, N.; Berry, V. Graphene-based single-bacterium resolution biodevice and DNA transistor: Interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 2008, 8, 4469–4476. [Google Scholar] [CrossRef]
- Baraket, M.; Stine, R.; Lee, W.K.; Robinson, J.T.; Tamanaha, C.R.; Sheehan, P.E.; Walton, S.G. Aminated graphene for DNA attachment produced via plasma functionalization. Appl. Phys. Lett. 2012, 100, 233123. [Google Scholar]
- Guo, S.R.; Lin, J.; Penchev, M.; Yengel, E.; Ghazinejad, M.; Ozkan, C.S.; Ozkan, M. Label free DNA detection using large area graphene based field effect transistor biosensors. J. Nanosci. Nanotechnol. 2011, 11, 5258–5263. [Google Scholar] [CrossRef]
- Stine, R.; Robinson, J.T.; Sheehan, P.E.; Tamanaha, C.R. Real-time DNA detection using reduced graphene oxide field effect transistors. Adv. Mater. 2010, 22, 5297–5300. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Gilje, S.; Han, S.; Wang, M.; Wang, K.L.; Kaner, R.B. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Yin, Z.Y.; He, Q.Y.; Huang, X.; Zhang, J.; Wu, S.X.; Chen, P.; Lu, G.; Chen, P.; Zhang, Q.C.; Yan, Q.Y.; et al. Real-time DNA detection using pt nanoparticle-decorated reduced graphene oxide field-effect transistors. Nanoscale 2012, 4, 293–297. [Google Scholar] [CrossRef]
- Dong, X.C.; Shi, Y.M.; Huang, W.; Chen, P.; Li, L.J. Electrical detection of DNA hybridization with single-base specificity using transistors based on CVD-grown graphene sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef]
- Chen, T.Y.; Phan, T.K.L.; Hsu, C.L.; Lee, Y.H.; Wang, J.T.W.; Wei, K.H.; Lin, C.T.; Li, L.J. Label-free detection of DNA hybridization using transistors based on cvd grown graphene. Biosens. Bioelectron. 2013, 41, 103–109. [Google Scholar] [CrossRef]
- Cai, B.; Wang, S.; Huang, L.; Ning, Y.; Zhang, Z.; Zhang, G.-J. Ultrasensitive label-free detection of pna-DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 2014, 8, 2632–2638. [Google Scholar] [CrossRef]
- Kim, D.J.; Sohn, I.Y.; Jung, J.H.; Yoon, O.J.; Lee, N.E.; Park, J.S. Reduced graphene oxide field-effect transistor for label-free femtomolar protein detection. Biosens. Bioelectron. 2013, 41, 621–626. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.H.; Yu, K.H.; Bo, Z.; Chen, J.H. Specific protein detection using thermally reduced graphene oxide sheet decorated with gold nanoparticle-antibody conjugates. Adv. Mater. 2010, 22, 3521–3526. [Google Scholar] [CrossRef]
- Mao, S.; Yu, K.H.; Lu, G.H.; Chen, J.H. Highly sensitive protein sensor based on thermally-reduced graphene oxide field-effect transistor. Nano Res. 2011, 4, 921–930. [Google Scholar] [CrossRef]
- Kurkina, T.; Sundaram, S.; Sundaram, R.S.; Re, F.; Masserini, M.; Kern, K.; Balasubramanian, K. Self-assembled electrical biodetector based on reduced graphene oxide. ACS Nano 2012, 6, 5514–5520. [Google Scholar] [CrossRef]
- Sharon, E.; Liu, X.Q.; Freeman, R.; Yehezkeli, O.; Willner, I. Label-free analysis of thrombin or Hg2+ ions by nucleic acid-functionalized graphene oxide matrices assembled on field-effect transistors. Electroanalysis 2013, 25, 851–856. [Google Scholar] [CrossRef]
- Saltzgaber, G.; Wojcik, P.; Sharf, T.; Leyden, M.R.; Wardini, J.L.; Heist, C.A.; Adenuga, A.A.; Remcho, V.T.; Minot, E.D. Scalable graphene field-effect sensors for specific protein detection. Nanotechnology 2013, 24, 355502. [Google Scholar] [CrossRef]
- Kim, D.J.; Park, H.C.; Sohn, I.Y.; Jung, J.H.; Yoon, O.J.; Park, J.S.; Yoon, M.Y.; Lee, N.E. Electrical graphene aptasensor for ultra-sensitive detection of anthrax toxin with amplified signal transduction. Small 2013, 9, 3352–3360. [Google Scholar]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef]
- Myung, S.; Solanki, A.; Kim, C.; Park, J.; Kim, K.S.; Lee, K.B. Graphene-encapsulated nanoparticle-based biosensor for the selective detection of cancer biomarkers. Adv. Mater. 2011, 23, 2221–2225. [Google Scholar] [CrossRef]
- Johnsson, B.; Lofas, S.; Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface-plasmon resonance sensors. Anal. Biochem. 1991, 198, 268–277. [Google Scholar] [CrossRef]
- Turkova, J. Oriented immobilization of biologically active proteins as a tool for revealing protein interactions and function. J. Chromatogr. B 1999, 722, 11–31. [Google Scholar] [CrossRef]
- Cazzaniga, E.; Bulbarelli, A.; Lonati, E.; Orlando, A.; Re, F.; Gregori, M.; Masserini, M. Abeta peptide toxicity is reduced after treatments decreasing phosphatidylethanolamine content in differentiated neuroblastoma cells. Neurochem. Res. 2011, 36, 863–869. [Google Scholar] [CrossRef]
- Mao, K.X.; Wu, D.; Li, Y.; Ma, H.M.; Ni, Z.Z.; Yu, H.Q.; Luo, C.N.; Wei, Q.; Du, B. Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal. Biochem. 2012, 422, 22–27. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Li, S.; Turner, A.P. Electrochemical immunosensor with n-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15-3. Biosens. Bioelectron. 2013, 43, 25–29. [Google Scholar] [CrossRef]
- Li, B.Y.; Shi, X.H.; Gu, W.; Zhao, K.; Chen, N.N.; Xian, Y.Z. Graphene based electrochemical biosensor for label-free measurement of the activity and inhibition of protein tyrosine kinase. Analyst 2013, 138, 7212–7217. [Google Scholar] [CrossRef]
- Liu, J.; Lu, C.Y.; Zhou, H.; Xu, J.J.; Wang, Z.H.; Chen, H.Y. A dual-functional electrochemical biosensor for the detection of prostate specific antigen and telomerase activity. Chem. Commun. 2013, 49, 6602–6604. [Google Scholar] [CrossRef]
- Huang, K.J.; Niu, D.J.; Sun, J.Y.; Han, C.H.; Wu, Z.W.; Li, Y.L.; Xiong, X.Q. Novel electrochemical sensor based on functionalized graphene for simultaneous determination of adenine and guanine in DNA. Colloid Surface B 2011, 82, 543–549. [Google Scholar] [CrossRef]
- Goh, M.S.; Pumera, M. Oxidation of DNA bases influenced by the presence of other bases. Electroanalysis 2012, 24, 1147–1152. [Google Scholar] [CrossRef]
- Du, M.; Yang, T.; Jiao, K. Immobilization-free direct electrochemical detection for DNA specific sequences based on electrochemically converted gold nanoparticles/graphene composite film. J. Mater. Chem. 2010, 20, 9253–9260. [Google Scholar] [CrossRef]
- Li, D.; Song, S.P.; Fan, C.H. Target-responsive structural switching for nucleic acid-based sensors. Accounts Chem. Res. 2010, 43, 631–641. [Google Scholar] [CrossRef]
- Bo, Y.; Wang, W.Q.; Qi, J.F.; Huang, S.S. A DNA biosensor based on graphene paste electrode modified with prussian blue and chitosan. Analyst 2011, 136, 1946–1951. [Google Scholar] [CrossRef]
- Guo, S.J.; Du, Y.; Yang, X.; Dong, S.J.; Wang, E.K. Solid-state label-free integrated aptasensor based on graphene-mesoporous silica-gold nanoparticle hybrids and silver microspheres. Anal. Chem. 2011, 83, 8035–8040. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Tang, L.H.; Li, J.H. Electrochemical DNA sensor by the assembly of graphene and DNA-conjugated gold nanoparticles with silver enhancement strategy. Analyst 2011, 136, 4732–4737. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Qu, X.J.; Dong, J.; Ai, S.Y.; Han, R.X. Electrochemical detection of DNA damage induced by acrylamide and its metabolite at the graphene-ionic liquid-nafion modified pyrolytic graphite electrode. J. Hazard. Mater. 2011, 190, 480–485. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G.F.; Zhu, L.; Li, G.X. Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem. Commun. 2011, 13, 31–33. [Google Scholar] [CrossRef]
- Qi, X.W.; Gao, H.W.; Zhang, Y.Y.; Wang, X.Z.; Chen, Y.; Sun, W. Electrochemical DNA biosensor with chitosan-Co3O4 nanorod-graphene composite for the sensitive detection of staphylococcus aureus nuc gene sequence. Bioelectrochemistry 2012, 88, 42–47. [Google Scholar] [CrossRef]
- Gupta, V.K.; Yola, M.L.; Qureshi, M.S.; Solak, A.O.; Atar, N.; Ustundag, Z. A novel impedimetric biosensor based on graphene oxide/gold nanoplatform for detection of DNA arrays. Sens. Actuators B Chem. 2013, 188, 1201–1211. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Jiang, W. Decorating graphene sheets with gold nanoparticles for the detection of sequence-specific DNA. Electrochim. Acta 2012, 71, 239–245. [Google Scholar] [CrossRef]
- Bonanni, A.; Pumera, M. Graphene platform for hairpin-DNA-based impedimetric genosensing. ACS Nano 2011, 5, 2356–2361. [Google Scholar] [CrossRef]
- Loo, A.H.; Bonanni, A.; Pumera, M. Thrombin aptasensing with inherently electroactive graphene oxide nanoplatelets as labels. Nanoscale 2013, 5, 4758–4762. [Google Scholar] [CrossRef]
- Zhang, C.L.; Xu, J.; Zhang, S.M.; Ji, X.H.; He, Z.K. One-pot synthesized DNA-cdte quantum dots applied in a biosensor for the detection of sequence-specific oligonucleotides. Chem. Eur. J. 2012, 18, 8296–8300. [Google Scholar] [CrossRef]
- He, S.J.; Liu, K.K.; Su, S.; Yan, J.; Mao, X.H.; Wang, D.F.; He, Y.; Li, L.J.; Song, S.P.; Fan, C.H. Graphene-based high-efficiency surface-enhanced raman scattering-active platform for sensitive and multiplex DNA detection. Anal. Chem. 2012, 84, 4622–4627. [Google Scholar]
- Bonanni, A.; Chua, C.K.; Pumera, M. Rational design of carboxyl groups perpendicularly attached to a graphene sheet: A platform for enhanced biosensing applications. Chemistry 2014, 20, 217–222. [Google Scholar] [CrossRef]
- Han, X.W.; Fang, X.; Shi, A.Q.; Wang, J.; Zhang, Y.Z. An electrochemical DNA biosensor based on gold nanorods decorated graphene oxide sheets for sensing platform. Anal. Biochem. 2013, 443, 117–123. [Google Scholar] [CrossRef]
- Wu, Y.M.; Xu, W.J.; Bai, L.J.; Yuan, Y.L.; Yi, H.Y.; Chai, Y.Q.; Yuan, R. Ultrasensitive thrombin detection based on direct electrochemistry of highly loaded hemoglobin spheres-encapsulated platinum nanoparticles as labels and electrocatalysts. Biosens. Bioelectron. 2013, 50, 50–56. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, M.L.; Zhang, Y.Y.; Li, H.T.; Lin, Y.H.; Yao, S.Z. Apoferritin protein nanoparticles dually labeled with aptamer and horseradish peroxidase as a sensing probe for thrombin detection. Anal. Chim. Acta 2013, 759, 53–60. [Google Scholar] [CrossRef]
- Grigorenko, A.N.; Polini, M.; Novoselov, K.S. Graphene plasmonics. Nat. Photon. 2012, 6, 749–758. [Google Scholar] [CrossRef]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A platform for surface-enhanced raman spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef]
- Swathi, R.S.; Sebastian, K.L. Distance dependence of fluorescence resonance energy transfer. J. Chem. Sci. 2009, 121, 777–787. [Google Scholar] [CrossRef]
- Swathi, R.S.; Sebastian, K.L. Long range resonance energy transfer from a dye molecule to graphene has (distance)−4 dependence. J. Chem. Phys. 2009, 130. [Google Scholar] [CrossRef]
- Swathi, R.S.; Sebastian, K.L. Resonance energy transfer from a dye molecule to graphene. J. Chem. Phys. 2008, 129. [Google Scholar] [CrossRef]
- Li, X.; Ma, K.; Zhu, S.J.; Yao, S.Y.; Liu, Z.Y.; Xu, B.; Yang, B.; Tian, W.J. Fluorescent aptasensor based on aggregation-induced emission probe and graphene oxide. Anal. Chem. 2014, 86, 298–303. [Google Scholar] [CrossRef]
- Dong, H.; Gao, W.; Yan, F.; Ji, H.; Ju, H. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem. 2010, 82, 5511–5517. [Google Scholar] [CrossRef]
- Chang, H.; Tang, L.; Wang, Y.; Jiang, J.; Li, J. Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal. Chem. 2010, 82, 2341–2346. [Google Scholar] [CrossRef]
- Jung, J.H.; Cheon, D.S.; Liu, F.; Lee, K.B.; Seo, T.S. A graphene oxide based immuno-biosensor for pathogen detection. Angew. Chem. Int. Edit. 2010, 49, 5708–5711. [Google Scholar] [CrossRef]
- Liu, F.; Choi, J.Y.; Seo, T.S. Graphene oxide arrays for detecting specific DNA hybridization by fluorescence resonance energy transfer. Biosens. Bioelectron. 2010, 25, 2361–2365. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, C.; Han, L.; Jin, L.; Zhou, M.; Dong, S. Label-free, regenerative and sensitive surface plasmon resonance and electrochemical aptasensors based on graphene. Chem. Commun. 2011, 47, 7794–7796. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Wu, Q.; Zhang, H.; Bai, Y.; Song, D. A protein a modified Au-graphene oxide composite as an enhanced sensing platform for SPR-based immunoassay. Analyst 2013, 138, 7175–7181. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Xu, B.; Zhang, H.; Gao, Y.; Song, D. A novel surface plasmon resonance biosensor based on graphene oxide decorated with gold nanorod-antibody conjugates for determination of transferrin. Biosens. Bioelectron. 2013, 45, 230–236. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef]
- Kim, Y.K.; Han, S.W.; Min, D.H. Graphene oxide sheath on ag nanoparticle/graphene hybrid films as an antioxidative coating and enhancer of surface-enhanced raman scattering. ACS Appl. Mater. Interfaces 2012, 4, 6545–6551. [Google Scholar] [CrossRef]
- Lu, G.; Li, H.; Liusman, C.; Yin, Z.Y.; Wu, S.X.; Zhang, H. Surface enhanced raman scattering of Ag or Au nanoparticle-decorated reduced graphene oxide for detection of aromatic molecules. Chem. Sci. 2011, 2, 1817–1821. [Google Scholar] [CrossRef]
- Bakhori, N.; Yusof, N.; Abdullah, A.; Hussein, M. Development of a fluorescence resonance energy transfer (fret)-based DNA biosensor for detection of synthetic oligonucleotide of ganoderma boninense. Biosensors 2013, 3, 419–428. [Google Scholar] [CrossRef]
- Huang, P.-J.J.; Liu, J. Molecular beacon lighting up on graphene oxide. Anal. Chem. 2012, 84, 4192–4198. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Q.; Tong, Y.; Wei, W.; Liu, S. Detection of DNA damage by using hairpin molecular beacon probes and graphene oxide. Talanta 2012, 99, 625–630. [Google Scholar] [CrossRef]
- Al-Ogaidi, I.; Gou, H.L.; Aguilar, Z.P.; Guo, S.W.; Melconian, A.K.; Al-Kazaz, A.K.A.; Meng, F.K.; Wu, N.Q. Detection of the ovarian cancer biomarker CA-125 using chemiluminescence resonance energy transfer to graphene quantum dots. Chem. Commun. 2014, 50, 1344–1346. [Google Scholar] [CrossRef]
- Xu, W.; Ling, X.; Xiao, J.; Dresselhaus, M.S.; Kong, J.; Xu, H.; Liu, Z.; Zhang, J. Surface Enhanced Raman Spectroscopy on a Flat Graphene Surface. In Proceedings of the National Academy of Sciences, Melbourne, Australia, 12 June 2012; pp. 9281–9286.
- Liu, X.J.; Cao, L.Y.; Song, W.; Ai, K.L.; Lu, L.H. Functionalizing metal nanostructured film with graphene oxide for ultrasensitive detection of aromatic molecules by surface-enhanced raman spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 2944–2952. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; van Duyne, R.R. Surface-enhanced raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Chan, S.H.; Chen, S.H.; Lin, W.T.; Li, M.C.; Lin, Y.C.; Kuo, C.C. Low-temperature synthesis of graphene on cu using plasma-assisted thermal chemical vapor deposition. Nanoscale Res. Lett. 2013, 8, 285. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, R.; Zhang, L.C.; Shi, Z.W.; Yang, W.; Wang, D.M.; Xie, G.B.; Shi, D.X.; Zhang, G.Y. Restoration of graphene from graphene oxide by defect repair. Carbon 2012, 50, 2581–2587. [Google Scholar] [CrossRef]
- Lee, S.W.; Mattevi, C.; Chhowalla, M.; Sankaran, R.M. Plasma-assisted reduction of graphene oxide at low temperature and atmospheric pressure for flexible conductor applications. J. Phys. Chem. Lett. 2012, 3, 772–777. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Juang, Z.-Y.; Wu, C.-Y.; Lo, C.-W.; Chen, W.-Y.; Huang, C.-F.; Hwang, J.-C.; Chen, F.-R.; Leou, K.-C.; Tsai, C.-H. Synthesis of graphene on silicon carbide substrates at low temperature. Carbon 2009, 47, 2026–2031. [Google Scholar] [CrossRef]
- Oostinga, J.B.; Heersche, H.B.; Liu, X.; Morpurgo, A.F.; Vandersypen, L.M.K. Gate-induced insulating state in bilayer graphene devices. Nat. Mater. 2008, 7, 151–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, T.-T.; Girit, C.; Hao, Z.; Martin, M.C.; Zettl, A.; Crommie, M.F.; Shen, Y.R.; Wang, F. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 2009, 459, 820–823. [Google Scholar] [CrossRef]
- Chen, W.F.; Yan, L.F. Preparation of graphene by a low-temperature thermal reduction at atmosphere pressure. Nanoscale 2010, 2, 559–563. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Wang, D.B.; Kim, S.; Kim, S.Y.; Hu, Y.K.; Yakes, M.K.; Laracuente, A.R.; Dai, Z.T.; Marder, S.R.; Berger, C.; et al. Nanoscale tunable reduction of graphene oxide for graphene electronics. Science 2010, 328, 1373–1376. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Guo, G.L.; Ji, L.C.; Wang, T.; Xie, Y.Q.; Liu, F.; Liu, A.Y. Pulsed laser assisted reduction of graphene oxide as a flexible transparent conducting material. J. Nanosci. Nanotechnol. 2012, 12, 6480–6483. [Google Scholar]
- Huang, L.; Liu, Y.; Ji, L.C.; Xie, Y.Q.; Wang, T.; Shi, W.Z. Pulsed laser assisted reduction of graphene oxide. Carbon 2011, 49, 2431–2436. [Google Scholar] [CrossRef]
- Kumar, N.A.; Nolan, H.; McEvoy, N.; Rezvani, E.; Doyle, R.L.; Lyons, M.E.G.; Duesberg, G.S. Plasma-assisted simultaneous reduction and nitrogen doping of graphene oxide nanosheets. J. Mater. Chem. A 2013, 1, 4431–4435. [Google Scholar]
- Yu, Y.; Li, Y.Z.; Pan, Y.X.; Liu, C.J. Fabrication of palladium/graphene oxide composite by plasma reduction at room temperature. Nanoscale Res. Lett. 2012, 7, 234. [Google Scholar] [CrossRef]
- Levchenko, I.; Kumar, S.; Yajadda, M.M.A.; Han, Z.J.; Furman, S.; Ostrikov, K. Self-organization in arrays of surface-grown nanoparticles: Characterization, control, driving forces. J. Phys. D Appl. Phys. 2011, 44, 174020. [Google Scholar] [CrossRef]
- Bockrath, M.; Cobden, D.H.; McEuen, P.L.; Chopra, N.G.; Zettl, A.; Thess, A.; Smalley, R.E. Single-electron transport in ropes of carbon nanotubes. Science 1997, 275, 1922–1925. [Google Scholar] [CrossRef]
- McEuen, P.L.; Park, J.Y. Electron transport in single-walled carbon nanotubes. MRS Bull. 2004, 29, 272–275. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.L.; Lieber, C.M. Single-walled carbon nanotubes - from fundamental studies to new device concepts. Ann. N. Y. Acad. Sci. 2002, 960, 203–215. [Google Scholar] [CrossRef]
- Charlier, J.-C.; de Vita, A.; Blase, X.; Car, R. Microscopic growth mechanisms for carbon nanotubes. Science 1997, 275, 647–649. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Tomanek, D. Electronic and structural properties of multiwall carbon nanotubes. Phys. Rev. B 1998, 58, 16001–16004. [Google Scholar] [CrossRef]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Electronic-structure of chiral graphene tubules. Appl. Phys. Lett. 1992, 60, 2204–2206. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, G.; Chang, Y.W.; Kim, H.J.; Jose, J.; Kim, E.; Pyun, J.C.; Yoo, K.H. A carbon nanotube metal semiconductor field effect transistor-based biosensor for detection of amyloid-beta in human serum. Biosens. Bioelectron. 2013, 50, 345–350. [Google Scholar] [CrossRef]
- Li, B.R.; Chen, C.W.; Yang, W.L.; Lin, T.Y.; Pan, C.Y.; Chen, Y.T. Biomolecular recognition with a sensitivity-enhanced nanowire transistor biosensor. Biosens. Bioelectron. 2013, 45, 252–259. [Google Scholar] [CrossRef]
- Chang, J.B.; Mao, S.; Zhang, Y.; Cui, S.M.; Steeber, D.A.; Chen, J.H. Single-walled carbon nanotube field-effect transistors with graphene oxide passivation for fast, sensitive, and selective protein detection. Biosens. Bioelectron. 2013, 42, 186–192. [Google Scholar] [CrossRef]
- Lerner, M.B.; D’Souza, J.; Pazina, T.; Dailey, J.; Goldsmith, B.R.; Robinson, M.K.; Johnson, A.T.C. Hybrids of a genetically engineered antibody and a carbon nanotube transistor for detection of prostate cancer biomarkers. ACS Nano 2012, 6, 5143–5149. [Google Scholar] [CrossRef]
- Croce, R.A.; Vaddiraju, S.; Chan, P.Y.; Seyta, R.; Jain, F.C. Label-free protein detection based on vertically aligned carbon nanotube gated field-effect transistors. Sens. Actuators B Chem. 2011, 160, 154–160. [Google Scholar] [CrossRef]
- Wang, J.P.; Yau, S.T. Field-effect amperometric immuno-detection of protein biomarker. Biosens. Bioelectron. 2011, 29, 210–214. [Google Scholar] [CrossRef]
- Kim, J.P.; Hong, S.; Sim, S.J. Apta-biosensors for nonlabeled real time detection of human ige based on carbon nanotube field effect transistors. J. Nanosci. Nanotechnol. 2011, 11, 4182–4187. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.H.; Yu, K.H.; Chen, J.H. Specific biosensing using carbon nanotubes functionalized with gold nanoparticle-antibody conjugates. Carbon 2010, 48, 479–486. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.G.; Byon, H.R.; Shin, H.J.; Ban, C.; Choi, H.C. Recognition of single mismatched DNA using muts-immobilized carbon nanotube field effect transistor devices. J. Phys. Chem. B 2009, 113, 12164–12168. [Google Scholar] [CrossRef]
- Abe, M.; Murata, K.; Ataka, T.; Ifuku, Y.; Matsumoto, K. Selective protein sensing using a carbon nanotube field-effect transistor. J. Nanosci. Nanotechnol. 2009, 9, 1947–1950. [Google Scholar] [CrossRef]
- Cid, C.C.; Riu, J.; Maroto, A.; Rius, F.X. Detection of human immunoglobulin g at physiological conditions with chemically functionalizated carbon nanotube field effect transistors. Curr. Nanosci. 2008, 4, 314–317. [Google Scholar] [CrossRef]
- Abe, M.; Murata, K.; Kojima, A.; Ifuku, Y.; Shimizu, M.; Ataka, T.; Matsumoto, K. Quantitative detection of protein using a top-gate carbon nanotube field effect transistor. J. Phys. Chem. C 2007, 111, 8667–8670. [Google Scholar] [CrossRef]
- Kim, J.P.; Lee, B.Y.; Hong, S.; Sim, S.J. Ultrasensitive carbon nanotube-based biosensors using antibody-binding fragments. Anal. Biochem. 2008, 381, 193–198. [Google Scholar] [CrossRef]
- Maehashi, K.; Matsumoto, K.; Takamura, Y.; Tamiya, E. Aptamer-based label-free immunosensors using carbon nanotube field-effect transistors. Electroanalysis 2009, 21, 1285–1290. [Google Scholar] [CrossRef]
- Qureshi, A.; Roci, I.; Gurbuz, Y.; Niazi, J.H. An aptamer based competition assay for protein detection using cnt activated gold-interdigitated capacitor arrays. Biosens. Bioelectron. 2012, 34, 165–170. [Google Scholar] [CrossRef]
- So, H.-M.; Won, K.; Kim, Y.H.; Kim, B.-K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.-O. Single-walled carbon nanotube biosensors using aptamers as molecular recognition elements. J. Am. Chem. Soc. 2005, 127, 11906–11907. [Google Scholar]
- Lo, Y.-S.; Nam, D.H.; So, H.-M.; Chang, H.; Kim, J.-J.; Kim, Y.H.; Lee, J.-O. Oriented immobilization of antibody fragments on Ni-decorated single-walled carbon nanotube devices. ACS Nano 2009, 3, 3649–3655. [Google Scholar] [CrossRef]
- Vedala, H.; Chen, Y.A.; Cecioni, S.; Imberty, A.; Vidal, S.; Star, A. Nanoelectronic detection of lectin-carbohydrate interactions using carbon nanotubes. Nano Lett. 2011, 11, 170–175. [Google Scholar] [CrossRef]
- Yick, S.; Han, Z.J.; Ostrikov, K. Atmospheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine au nanodots for sers sensing. Chem. Commun. 2013, 49, 2861–2863. [Google Scholar] [CrossRef]
- Yang, R.H.; Jin, J.Y.; Chen, Y.; Shao, N.; Kang, H.Z.; Xiao, Z.; Tang, Z.W.; Wu, Y.R.; Zhu, Z.; Tan, W.H. Carbon nanotube-quenched fluorescent oligonucleotides: Probes that fluoresce upon hybridization. J. Am. Chem. Soc. 2008, 130, 8351–8358. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, J.-H.; Reuel, N.F.; Barone, P.W.; Boghossian, A.A.; Zhang, J.; Yoon, H.; Chang, A.C.; Hilmer, A.J.; Strano, M.S. Label-free, single protein detection on a near-infrared fluorescent single-walled carbon nanotube/protein microarray fabricated by cell-free synthesis. Nano Lett. 2011, 11, 2743–2752. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Souza, A.G.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotube samples with resonance raman scattering. New J. Phys. 2003, 5. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Lee, S.; Hahm, M.G.; Vajtai, R.; Hashim, D.P.; Thurakitseree, T.; Chipara, A.C.; Ajayan, P.M.; Hafner, J.H. Utilizing 3D SERS active volumes in aligned carbon nanotube scaffold substrates. Adv. Mater. 2012, 24, 5261–5266. [Google Scholar] [CrossRef]
- Goldberg-Oppenheimer, P.; Hutter, T.; Chen, B.A.; Robertson, J.; Hofmann, S.; Mahajan, S. Optimized vertical carbon nanotube forests for multiplex surface-enhanced raman scattering detection. J. Phys. Chem. Lett. 2012, 3, 3486–3492. [Google Scholar] [CrossRef]
- Scolari, M.; Mews, A.; Fu, N.; Myalitsin, A.; Assmus, T.; Balasubramanian, K.; Burghard, M.; Kern, K. Surface enhanced raman scattering of carbon nanotubes decorated by individual fluorescent gold particles. J. Phys. Chem. C 2008, 112, 391–396. [Google Scholar] [CrossRef]
- D’Acunto, M.; Colantonio, S.; Moroni, D.; Salvetti, O. Detection limit of biomarkers using the near-infrared band-gap fluorescence of single-walled carbon nanotubes. J. Mod. Opt. 2010, 57, 1695–1699. [Google Scholar] [CrossRef]
- Jeng, E.S.; Moll, A.E.; Roy, A.C.; Gastala, J.B.; Strano, M.S. Detection of DNA hybridization using the near-infrared band-gap fluorescence of single-walled carbon nanotubes. Nano Lett. 2006, 6, 371–375. [Google Scholar] [CrossRef]
- Levchenko, I.; Keidar, M.; Xu, S.Y.; Kersten, H.; Ostrikov, K. Low-temperature plasmas in carbon nanostructure synthesis. J. Vac. Sci. Technol. B 2013, 31, 050801. [Google Scholar]
- Levchenko, I.; Ostrikov, K.; Xu, S. Thermodynamical and plasma-driven kinetic growth of high-aspect-ratio nanostructures: Effect of hydrogen termination. J. Phys. D Appl. Phys. 2009, 42, 125207. [Google Scholar] [CrossRef]
- Denysenko, I.B.; Xu, S.; Long, J.D.; Rutkevych, P.P.; Azarenkov, N.A.; Ostrikov, K. Inductively coupled Ar/CH4/H2 plasmas for low-temperature deposition of ordered carbon nanostructures. J. Appl. Phys. 2004, 95, 2713–2724. [Google Scholar] [CrossRef]
- Shariat, M.; Hosseini, S.I.; Shokri, B.; Neyts, E.C. Plasma enhanced growth of single walled carbon nanotubes at low temperature: A reactive molecular dynamics simulation. Carbon 2013, 65, 269–276. [Google Scholar] [CrossRef]
- Meyyappan, M.; Delzeit, L.; Cassell, A.; Hash, D. Carbon nanotube growth by PECVD: A review. Plasma Sources Sci. Technol. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Hofmann, S.; Cantoro, M.; Kleinsorge, B.; Casiraghi, C.; Parvez, A.; Robertson, J.; Ducati, C. Effects of catalyst film thickness on plasma-enhanced carbon nanotube growth. J. Appl. Phys. 2005, 98, 034308. [Google Scholar] [CrossRef]
- Ostrikov, K.; Murphy, A.B. Plasma-aided nanofabrication: Where is the cutting edge? J. Phys. D Appl. Phys. 2007, 40, 2223–2241. [Google Scholar] [CrossRef]
- Han, Z.J.; Levchenko, I.; Yick, S.; Ostrikov, K. 3-orders-of-magnitude density control of single-walled carbon nanotube networks by maximizing catalyst activation and dosing carbon supply. Nanoscale 2011, 3, 4848–4853. [Google Scholar] [CrossRef]
- Han, Z.J.; Ostrikov, K. Uniform, dense arrays of vertically aligned, large-diameter single-walled carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 6018–6024. [Google Scholar] [CrossRef]
- Ostrikov, K.; Mehdipour, H. Thin single-walled carbon nanotubes with narrow chirality distribution: Constructive interplay of plasma and gibbs–thomson effects. ACS Nano 2011, 5, 8372–8382. [Google Scholar] [CrossRef]
- Kumar, S.; Mehdipour, H.; Ostrikov, K. Plasma-enabled graded nanotube biosensing arrays on a si nanodevice platform: Catalyst-free integration and in situ detection of nucleation events. Adv. Mater. 2013, 25, 69–74. [Google Scholar] [CrossRef]
- Kumar, S.; Levchenko, I.; Ostrikov, K.; McLaughlin, J.A. Plasma-enabled, catalyst-free growth of carbon nanotubes on mechanically-written Si features with arbitrary shape. Carbon 2012, 50, 325–329. [Google Scholar] [CrossRef]
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef]
- Qu, L.T.; Du, F.; Dai, L.M. Preferential syntheses of semiconducting vertically aligned single-walled carbon nanotubes for direct use in fets. Nano Lett. 2008, 8, 2682–2687. [Google Scholar] [CrossRef]
- Robertson, J.; Zhong, G.; Telg, H.; Thomsen, C.; Warner, J.H.; Briggs, G.A.D.; Dettlaff-Weglikowska, U.; Roth, S. Growth and characterization of high-density mats of single-walled carbon nanotubes for interconnects. Appl. Phys. Lett. 2008, 93, 163111. [Google Scholar] [CrossRef]
- Zheng, G.; Li, Q.; Jiang, K.; Zhang, X.; Chen, J.; Ren, Z.; Fan, S. Transition of single-walled carbon nanotubes from metallic to semiconducting in field-effect transistors by hydrogen plasma treatment. Nano Lett. 2007, 7, 1622–1625. [Google Scholar] [CrossRef]
- Maehashi, K.; Katsura, T.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-free protein biosensor based on aptamer-modified carbon nanotube field-effect transistors. Anal. Chem. 2007, 79, 782–787. [Google Scholar] [CrossRef]
- Khare, B.N.; Wilhite, P.; Quinn, R.C.; Chen, B.; Schingler, R.H.; Tran, B.; Imanaka, H.; So, C.R.; Bauschlicher, C.W., Jr.; Meyyappan, M. Functionalization of carbon nanotubes by ammonia glow-discharge: Experiments and modeling. J. Phys. Chem. B 2004, 108, 8166–8172. [Google Scholar] [CrossRef]
- Plank, N.O.V.; Forrest, G.A.; Cheung, R.; Alexander, A.J. Electronic properties of n-type carbon nanotubes prepared by CF4 plasma fluorination and amino functionalization. J. Phys. Chem. B 2005, 109, 22096–22101. [Google Scholar] [CrossRef]
- Malesevic, A.; Vitchev, R.; Schouteden, K.; Volodin, A.; Zhang, L.; van Tendeloo, G.; Vanhulsel, A.; van Haesendonck, C. Synthesis of few-layer graphene via microwave plasma-enhanced chemical vapour deposition. Nanotechnology 2008, 19, 305604. [Google Scholar] [CrossRef]
- Wang, Z.; Shoji, M.; Ogata, H. Carbon nanosheets by microwave plasma enhanced chemical vapor deposition in CH4–Ar system. Appl. Surface Sci. 2011, 257, 9082–9085. [Google Scholar] [CrossRef]
- Yu, K.H.; Bo, Z.; Lu, G.H.; Mao, S.; Cui, S.M.; Zhu, Y.W.; Chen, X.Q.; Ruoff, R.S.; Chen, J.H. Growth of carbon nanowalls at atmospheric pressure for one-step gas sensor fabrication. Nanoscale Res. Lett. 2011, 6, 202. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Outlaw, R.A.; Bagge-Hansen, M.; Chen, H.J.; Manos, D.M. Enhanced field emission of vertically oriented carbon nanosheets synthesized by C2H2/H2 plasma enhanced CVD. Carbon 2011, 49, 2526–2531. [Google Scholar] [CrossRef]
- Wu, Y.H. Effects of localized electric field on the growth of carbon nanowalls. Nano Lett. 2002, 2, 355–359. [Google Scholar] [CrossRef]
- Yu, K.H.; Wang, P.X.; Lu, G.H.; Chen, K.H.; Bo, Z.; Chen, J.H. Patterning vertically oriented graphene sheets for nanodevice applications. J. Phys. Chem. Lett. 2011, 2, 537–542. [Google Scholar] [CrossRef]
- Mao, S.; Yu, K.H.; Chang, J.B.; Steeber, D.A.; Ocola, L.E.; Chen, J.H. Direct growth of vertically-oriented graphene for field-effect transistor biosensor. Sci. Rep. (UK) 2013, 3, 1696. [Google Scholar]
- Akhavan, O.; Ghaderi, E.; Rahighi, R. Toward single-DNA electrochemical biosensing by graphene nanowalls. ACS Nano 2012, 6, 2904–2916. [Google Scholar] [CrossRef]
- Vansweevelt, R.; Malesevic, A.; van Gompel, M.; Vanhulsel, A.; Wenmackers, S.; D’Haen, J.; Vermeeren, V.; Ameloot, M.; Michiels, L.; van Haesendonck, C.; et al. Biological modification of carbon nanowalls with DNA strands and hybridization experiments with complementary and mismatched DNA. Chem. Phys. Lett. 2010, 485, 196–201. [Google Scholar] [CrossRef]
- Seo, D.H.; Rider, A.E.; Kumar, S.; Randeniya, L.K.; Ostrikov, K. Vertical graphene gas- and bio-sensors via catalyst-free, reactive plasma reforming of natural honey. Carbon 2013, 60, 221–228. [Google Scholar] [CrossRef]
- Seo, D.H.; Kumar, S.; Rider, A.E.; Han, Z.J.; Ostrikov, K. Deterministic control of structural and optical properties of plasma-grown vertical graphene nanosheet networks via nitrogen gas variation. Opt. Mater. Express 2012, 2, 700–707. [Google Scholar] [CrossRef]
- Rout, C.S.; Kumar, A.; Fisher, T.S. Carbon nanowalls amplify the surface-enhanced raman scattering from ag nanoparticles. Nanotechnology 2011, 22, 395704. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, P.; Chong, T.; Shen, Z. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhu, M.Y.; Outlaw, R.A.; Zhao, X.; Manos, D.M.; Holloway, B.C.; Mammana, V.P. Free-standing subnanometer graphite sheets. Appl. Phys. Lett. 2004, 85, 1265–1267. [Google Scholar] [CrossRef]
- Mori, T.; Hiramatsu, M.; Yamakawa, K.; Takeda, K.; Hori, M. Fabrication of carbon nanowalls using electron beam excited plasma-enhanced chemical vapor deposition. Diam. Relat. Mater. 2008, 17, 1513–1517. [Google Scholar] [CrossRef]
- Sato, G.; Morio, T.; Kato, T.; Hatakeyama, R. Fast growth of carbon nanowalls from pure methane using helicon plasma-enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2006, 45, 5210–5212. [Google Scholar] [CrossRef]
- Kurita, S.; Yoshimura, A.; Kawamoto, H.; Uchida, T.; Kojima, K.; Tachibana, M.; Molina-Morales, P.; Nakai, H. Raman spectra of carbon nanowalls grown by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2005, 97, 104320. [Google Scholar] [CrossRef]
- Zhang, H.; Yoshimura, I.; Kusano, E.; Kogure, T.; Kinbara, A. Formation of carbon nano-flakes by RF magnetron sputtering method. SHINKU 2004, 47, 82–86. [Google Scholar] [CrossRef]
- Itoh, T. Synthesis of carbon nanowalls by hot-wire chemical vapor deposition. Thin Solid Films 2011, 519, 4589–4593. [Google Scholar] [CrossRef]
- Giorgi, L.; Makris, T.D.; Giorgi, R.; Lisi, N.; Salernitano, E. Electrochemical properties of carbon nanowalls synthesized by HF-CVD. Sens. Actuators B Chem. 2007, 126, 144–152. [Google Scholar] [CrossRef]
- Wang, B.B.; Ostrikov, K.; van der Laan, T.; Zheng, K.; Wang, J.J.; Yan, Y.P.; Quan, X.J. Carbon nanorods and graphene-like nanosheets by hot filament CVD: Growth mechanisms and electron field emission. J. Mater. Chem. C 2013, 1, 7703–7708. [Google Scholar] [CrossRef]
- Seo, D.H.; Han, Z.J.; Kumar, S.; Ostrikov, K. Structure-controlled, vertical graphene-based, binder-free electrodes from plasma-reformed butter enhance supercapacitor performance. Adv. Energy Mater. 2013, 3, 1316–1323. [Google Scholar] [CrossRef]
- Seo, D.H.; Rider, A.E.; Han, Z.J.; Kumar, S.; Ostrikov, K. Plasma break-down and re-build: Same functional vertical graphenes from diverse natural precursors. Adv. Mater. 2013, 25, 5638–5642. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Wang, J.J.; Holloway, B.C.; Outlaw, R.A.; Zhao, X.; Hou, K.; Shutthanandan, V.; Manos, D.M. A mechanism for carbon nanosheet formation. Carbon 2007, 45, 2229–2234. [Google Scholar] [CrossRef]
- Tai, L.M.; Bilousova, T.; Jungbauer, L.; Roeske, S.K.; Youmans, K.L.; Yu, C.; Poon, W.W.; Cornwell, L.B.; Miller, C.A.; Vinters, H.V.; et al. Levels of soluble apolipoprotein E/Amyloid-β (Aβ) complex are reduced and oligomeric Aβ increased with APOE4 and alzheimer disease in a transgenic mouse model and human samples. J. Biol. Chem. 2013, 288, 5914–5926. [Google Scholar] [CrossRef]
- Glabe, C.G. Structural classification of toxic amyloid oligomers. J. Biol. Chem. 2008, 283, 29639–29643. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Gubbens, J.; Ruijter, E.; de Fays, L.E.V.; Damen, J.M.A.; de Kruijff, B.; Slijper, M.; Rijkers, D.T.S.; Liskamp, R.M.J.; de Kroon, A.I.P.M. Photocrosslinking and click chemistry enable the specific detection of proteins interacting with phospholipids at the membrane interface. Chem. Biol. 2009, 16, 3–14. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.H.; Ryu, J.; Kim, D.J. Multivalent & multifunctional ligands to beta-amyloid. Curr. Pharm. Des. 2009, 15, 637–658. [Google Scholar] [CrossRef]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar]
- Yan, R.; Vassar, R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef]
- Vom Berg, J.; Prokop, S.; Miller, K.R.; Obst, J.; Kalin, R.E.; Lopategui-Cabezas, I.; Wegner, A.; Mair, F.; Schipke, C.G.; Peters, O.; et al. Inhibition of IL-12/IL-23 signaling reduces alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012, 18, 1812–1819. [Google Scholar] [CrossRef]
- Han, Z.J.; Levchenko, I.; Kumar, S.; Yajadda, M.M.A.; Yick, S.; Seo, D.H.; Martin, P.J.; Peel, S.; Kuncic, Z.; Ostrikov, K. Plasma nanofabrication and nanomaterials safety. J. Phys. D Appl. Phys. 2011, 44, 174019. [Google Scholar] [CrossRef]
- Yang, T.; Guan, Q.; Meng, L.; Yang, R.R.; Li, Q.H.; Jiao, K. A simple preparation method for large-area, wavy graphene oxide nanowalls and their application to freely switchable impedimetric DNA detection. RSC Adv. 2013, 3, 22430–22435. [Google Scholar] [CrossRef]
- Shang, N.G.; Papakonstantinou, P.; McMullan, M.; Chu, M.; Stamboulis, A.; Potenza, A.; Dhesi, S.S.; Marchetto, H. Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv. Funct. Mater. 2008, 18, 3506–3514. [Google Scholar] [CrossRef]
- Claussen, J.C.; Kumar, A.; Jaroch, D.B.; Khawaja, M.H.; Hibbard, A.B.; Porterfield, D.M.; Fisher, T.S. Nanostructuring platinum nanoparticles on multilayered graphene petal nanosheets for electrochemical biosensing. Adv. Funct. Mater. 2012, 22, 3399–3405. [Google Scholar] [CrossRef]
- Kurkina, T.; Vlandas, A.; Ahmad, A.; Kern, K.; Balasubramanian, K. Label-free detection of few copies of DNA with carbon nanotube impedance biosensors. Angew. Chem. Int. Ed. 2011, 50, 3710–3714. [Google Scholar] [CrossRef]
- Martínez, M.T.; Tseng, Y.-C.; Ormategui, N.; Loinaz, I.; Eritja, R.; Bokor, J. Label-free DNA biosensors based on functionalized carbon nanotube field effect transistors. Nano Lett. 2009, 9, 530–536. [Google Scholar] [CrossRef]
- Deng, S.; Jian, G.; Lei, J.; Hu, Z.; Ju, H. A glucose biosensor based on direct electrochemistry of glucose oxidase immobilized on nitrogen-doped carbon nanotubes. Biosens. Bioelectron. 2009, 25, 373–377. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Sethupathi, K.; Ramaprabhu, S. A glucose biosensor based on deposition of glucose oxidase onto crystalline gold nanoparticle modified carbon nanotube electrode. J. Phys. Chem. B 2009, 113, 3190–3194. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, L.; Wang, Z. DNA electrochemical biosensor based on thionine-graphene nanocomposite. Biosens. Bioelectron. 2012, 35, 507–511. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pineda, S.; Han, Z.J.; Ostrikov, K. Plasma-Enabled Carbon Nanostructures for Early Diagnosis of Neurodegenerative Diseases. Materials 2014, 7, 4896-4929. https://doi.org/10.3390/ma7074896
Pineda S, Han ZJ, Ostrikov K. Plasma-Enabled Carbon Nanostructures for Early Diagnosis of Neurodegenerative Diseases. Materials. 2014; 7(7):4896-4929. https://doi.org/10.3390/ma7074896
Chicago/Turabian StylePineda, Shafique, Zhao Jun Han, and Kostya Ostrikov. 2014. "Plasma-Enabled Carbon Nanostructures for Early Diagnosis of Neurodegenerative Diseases" Materials 7, no. 7: 4896-4929. https://doi.org/10.3390/ma7074896





