Structural and Optical Properties of La1−xSrxTiO3+δ
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
3.1. Phase and Microstructure Characterization

| x | Phase | Lattice Parameters | Reported Values |
|---|---|---|---|
| 0 | La2Ti2O7 | a = 7.817 Å, b = 13.051 Å, c = 5.546 Å, α = 90°, β = 98.64°, γ = 90° | a = 7.800 Å, b = 13.01 Å, c = 5.55 Å, α = 90°, β = 98.6°, γ = 90° [11] |
| 0.1 | SrLa8Ti9O31 | a = 7.779 Å, b = 56.853 Å, c = 5.536 Å, α = 90°, β = 90°, γ = 90° | a = 7.81 Å, b = 57.01 Å, c = 5.533 Å, α = 90°, β = 90°, γ = 90° [17] |
| 0.25 | SrLa4Ti5O17 | a = 3.918 Å, b = 5.531 Å, c = 31.514 Å, α = 90°, β = 97.12°, γ = 90° | a = 3.982 Å, b = 5.532 Å, c = 31.466 Å, α = 90°, β = 97.14°, γ = 90° [18] |
| 0.75 | La0.25Sr0.75TiO3 | a = b = c = 3.911 Å, α = β = γ = 90° | a = b = c = 3.905 Å, α = β = γ = 90° [19] |

| x | Phase | Crystal Structure | Crystal System |
|---|---|---|---|
| 0 | La2Ti2O7 | Layered structure m = 4 | Monoclinic |
| 0.05 | La2Ti2O7 | Layered structure m = 4 | Monoclinic |
| SrLa8Ti9O31 | Layered structure m = 4.5 | Orthorhombic | |
| 0.1 | SrLa8Ti9O31 | Layered structure m = 4.5 | Orthorhombic |
| 0.2 | SrLa4Ti5O17 | Layered structure m = 5 | Monoclinic |
| 0.25 | SrLa4Ti5O17 | Layered structure m = 5 | Monoclinic |
| 0.5 | SrLa4Ti5O17 | Layered structure m = 5 | Monoclinic |
| La1−ySryTiO3 (0.5 < y < 1) | – | Cubic | |
| 0.75 | La0.25Sr0.75TiO3 | – | Cubic |

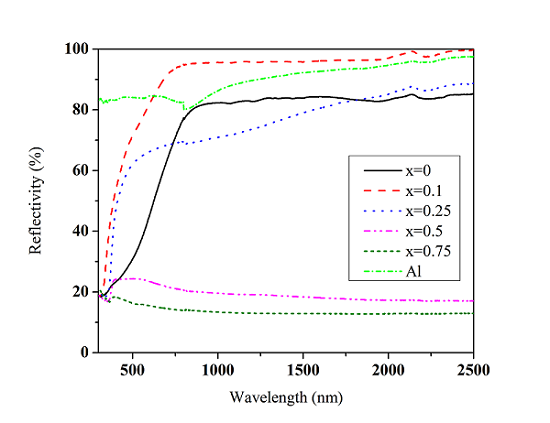
3.2. Reflectivity and Its Effect Factors
3.2.1. Reflectivity

3.2.2. Effect of Phase Structure on the Reflectivity of La1−xSrxTiO3+δ




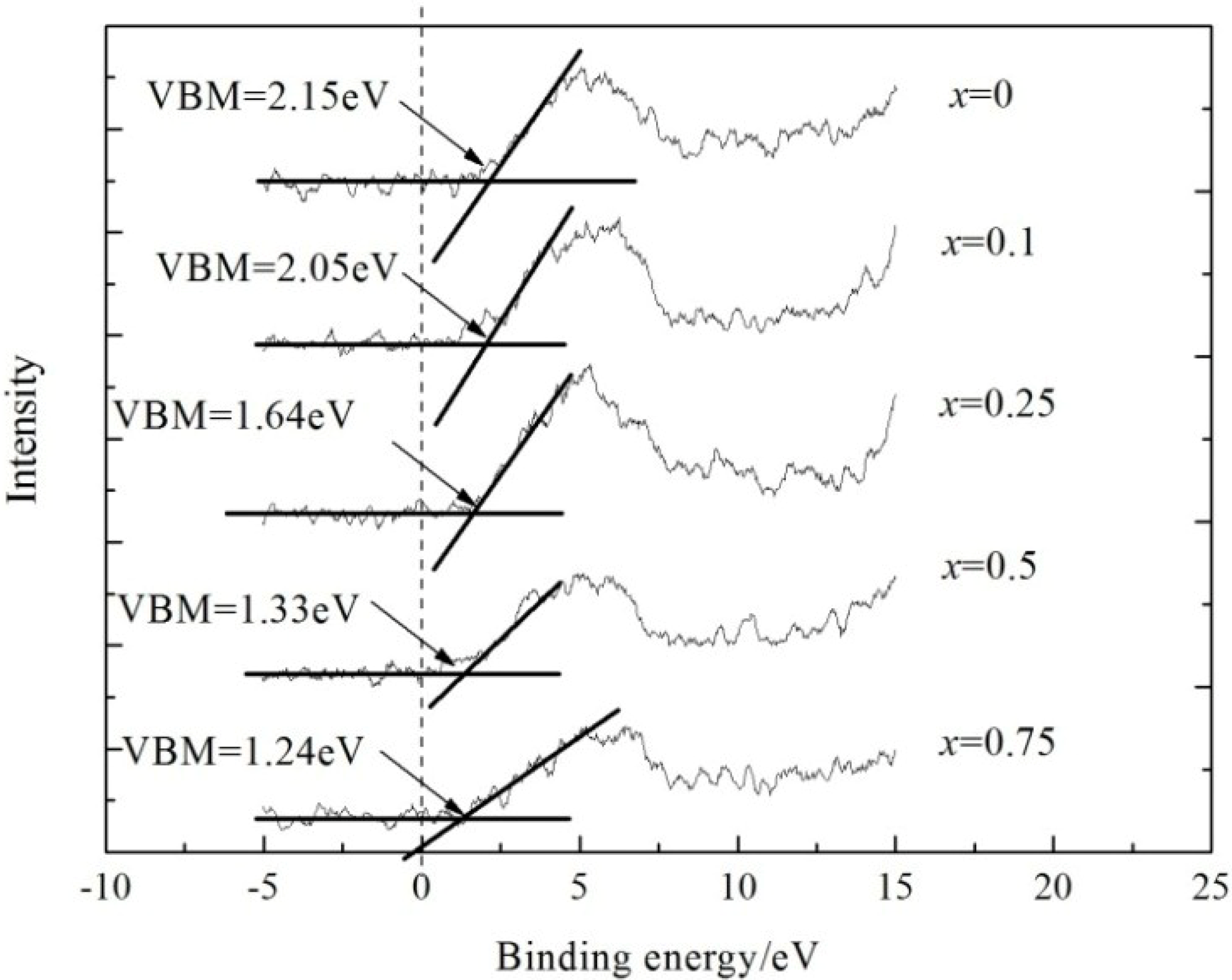
3.2.3. Effect of Energy Band Structure on the Reflectivity of La1−xSrxTiO3+δ
3.2.4. Effect of Density on the Reflectivity of La1−xSrxTiO3+δ


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dagotto, E. Complexity in strongly correlated electronic systems. Science 2005, 309, 257–262. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Lei, H.; Zhu, X.; Li, G.; Wang, B.; Song, W.; Yang, Z.; Dai, J.; Sun, Y.; et al. Chemical solution deposition of transparent and metallic La0.5Sr0.5TiO3+x/2 films using topotactic reduction. J. Am. Ceram. Soc. 2009, 92, 800–804. [Google Scholar] [CrossRef]
- Wu, W.B.; Lu, F.; Wong, K.H.; Pang, G.; Choy, C.L.; Zhang, Y.H. Epitaxial and highly electrical conductive La0.5Sr0.5TiO3 films grown by pulsed laser deposition in vacuum. J. Appl. Phys. 2000, 88, 700–704. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.L.; Su, W.B.; Wang, H.C.; Zheng, P.; Li, J.C.; Zhang, J.L.; Mei, L.M. Enhancement of thermoelectric efficiency in oxygen-deficient Sr1−xLaxTiO3−δ ceramics. Appl. Phys. Lett. 2009, 9. [Google Scholar] [CrossRef]
- Ohta, S.; Nomura, T.; Ohta, H.; Koumoto, K. High-temperature carrier transport and thermoelectric properties of heavily La- or Nb-doped SrTiO3 single crystals. J. Appl. Phys. 2005, 97. [Google Scholar] [CrossRef]
- Tokura, Y.; Taguchi, Y.; Okada, Y.; Fujishima, Y.; Arima, T.; Kumagai, K.; Iye, Y. Filling dependence of electronic properties on the verge of metal–Mott-insulator transition in Sr1−xLaxTiO3. Phys. Rev. Lett. 1993, 70, 2126–2129. [Google Scholar]
- Kumagai, K.; Suzuki, T.; Taguchi, Y.; Okada, Y.; Fujishima, Y.; Tokura, Y. Metal-insulator transition in La1−xSrxTiO3 and Y1−xCaxTiO3 investigated by specific-heat measurements. Phys. Rev. B 1993, 48, 7636–7642. [Google Scholar]
- Onoda, M.; Kohno, M. Antiferromagnetic correlation effects in the metal-insulator crossover of the perovskite-type La1−xSrxTiO3 system. J. Phys. Condens. Matter 1998, 10, 1003–1011. [Google Scholar]
- Yun, J.N.; Zhang, Z.Y.; Yan, J.F.; Zhao, W. First-principles study of structural stability and electronic structure of La-doped Sr1.9375La0.0625TiO3.96875. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Arao, M.; Koyama, Y.; Inoue, Y. Incomplete structural phase transition and lattice destruction in Sr1−xLaxTiO3+δ. J. Appl. Phys. 1999, 86, 2759–2763. [Google Scholar]
- Howard, S.A.; Yau, J.K.; Anderson, H.U. Structural characteristics of Sr1−xLaxTi3+δ as a function of oxygen partial pressure at 1400 °C. J. Appl. Phys. 1989, 65, 1492–1498. [Google Scholar]
- Hays, C.C.; Zhou, J.-S.; Markert, J.T.; Goodenough, J.B. Electronic transition in La1−xSrxTiO3. Phys. Rev. B 1999, 60, 10367–10373. [Google Scholar]
- Yun, J.N.; Zhang, Z.Y.; Yan, J.F.; Zhao, W. Electronic structure and optical properties of la-doped SrTiO3 and Sr2TiO4 by density function theory. Chin. Phys. Lett. 2009, 26. [Google Scholar] [CrossRef]
- Suzuki, T.; Jasinski, P.; Petrovsky, V.; Anderson, H.U. The optical properties and band gap energy of nanocrystalline La0.4Sr0.6TiO3 thin films. J. Am. Ceram. Soc. 2005, 88, 1186–1189. [Google Scholar] [CrossRef]
- Cho, J.H.; Cho, H.J. Optical transparency of metallic La0.5Sr0.5TiO3+δ thin films. Appl. Phys. Lett. 2001, 79, 1426–1428. [Google Scholar] [CrossRef]
- Gao, L.H.; Ma, Z.; Fan, Q.B. First-principle studies of the electronic structure and reflectivity of LaTiO3 and Sr doped LaTiO3 (La1−xSrxTiO3). J. Electroceram. 2011, 27, 114–119. [Google Scholar] [CrossRef]
- Bowden, M.E.; Jefferson, D.A.; Brown, I.W.M. Determination of layer structure in Sr1−xLaxTiO3+0.5x (0 < x < 1) compounds by high-resolution electron microscopy. J. Solid State Chem. 1995, 117, 88–96. [Google Scholar] [CrossRef]
- Williams, T.; Schmalle, H.; Reller, A.; Lichtenberg, F.; Widmer, D.; Bednorz, G. On the crystal structures of La2Ti2O7 and La5Ti5O17: High-resolution electron microscopy. J. Solid State Chem. 1991, 93, 534–548. [Google Scholar] [CrossRef]
- Wang, Z.; Mori, M.; Itoh, T. Thermal expansion properties of Sr1−xLaxTiO3 (0 ≤ x ≤ 0.3) perovskites in oxidizing and reducing atmospheres. J. Electrochem. Soc. 2010, 157, B1783–B1789. [Google Scholar] [CrossRef]
- Zhang, F.X.; Lian, J.; Becker, U.; Ewing, R.C.; Wang, L.M.; Hud, J.; Saxena, S.K. Structural change of layered perovskite La2Ti2O7 at high pressures. J. Solid State Chem. 2007, 180, 571–576. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, J.S.; Li, W.; Oh, S.H. Electronic band structure and photocatalytic activity of Ln2Ti2O7 (Ln = La, Pr, Nd). J. Phys. Chem. B 2003, 107, 4963–4970. [Google Scholar] [CrossRef]
- Cao, Y.; He, T.; Chen, Y.; Cao, Y. Fabrication of rutile TiO2-Sn/Anatase TiO2-N heterostructure and its application in visible-light photocatalysis. J. Phys. Chem. C 2010, 114, 3627–3633. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, L.; Ma, Z.; Wang, S.; Wang, F.; Yang, C. Structural and Optical Properties of La1−xSrxTiO3+δ. Materials 2014, 7, 4982-4993. https://doi.org/10.3390/ma7074982
Gao L, Ma Z, Wang S, Wang F, Yang C. Structural and Optical Properties of La1−xSrxTiO3+δ. Materials. 2014; 7(7):4982-4993. https://doi.org/10.3390/ma7074982
Chicago/Turabian StyleGao, Lihong, Zhuang Ma, Song Wang, Fuchi Wang, and Cai Yang. 2014. "Structural and Optical Properties of La1−xSrxTiO3+δ" Materials 7, no. 7: 4982-4993. https://doi.org/10.3390/ma7074982