New Gold Nanostructures for Sensor Applications: A Review
Abstract
:1. Uncommon Gold: Old Material with New Applications
2. Why Use Gold Nanostructures in Sensors?
2.1. Unique Properties of Gold Nanowires


| Properties of GNWs | Potential application | Reference |
|---|---|---|
| Large surface area | Nanoelectrode Sensor, catalytic, | [8] |
| Electron transport with organic OA layer | High resistivity, 106 Ω, temperature dependent non-linear behavior, lower catalytic ability | [15,17] |
| Electron transport without OA layer | Low resistivity, 103 Ω, high electron transport, electrochemical, nanoelectrode sensor, high catalytic ability | [15,17] |
| Mechanical properties | High engineering strength, interconnects | [14,20] |
| LSPR | SEF, SERS | [21,22,23,24] |
| Chirality of gold cluster | Asymmetric drugs, sensors and catalysts | [27] |
| Easy self-assemble | Active substrates for SERS | [4] |
2.2. Unique Properties of Gold Nanoparticles with Surface Modification
| Properties of GNPs | Potential applications | Reference |
|---|---|---|
| SPR | Colorimetric, SERS based sensing | [6,30,31,32,33] |
| Photoluminescence Fluorescence enhancement Fluorescence quenching | Fluorescence sensing | [30,31,32,33,35] |
| Raman scattering enhancement | SERS by electromagnetic and chemical mechanism | [37] |
| Strong light scattering | Dynamic light scattering (DLS) assay | [38] |
| Easy surface modification | Molecular recognition in different sensing systems | [1,45,46,47,48,49,50] |
2.3. Principles of LSPR
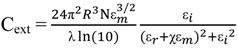
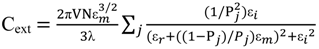
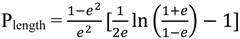
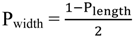
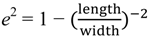
3. Synthesis of Gold Nanostructures
3.1. Synthesis of Gold Nanowires
3.1.1. Template Directed Methods
3.1.2. Non-Template Directed Methods
| Synthesis of GNWs | Advantages | Disadvantages | Diameter (nm) | Length (μm) | Reference |
|---|---|---|---|---|---|
| Soft Template OA | easy to make | longer time-several days, organic layer | 1–2 | 3–5 | [60] |
| OA and ascorbic acid | easy to make | longer time several days organic layer | 1–2 | 3–5 | [61] |
| OA and TIPs | fast-several hours | hard control, organic layer | 1–2 | 3–5 | [4] |
| Soft template CnAA | tune gap distance | CnAA dispersion, organic layer | 1–2 | 3–5 | [62] |
| Amphiphilic molecules CTAB | growth in water | organic layer | 1–2 | 2–3 | [63] |
| Hard template AAO | control diameter | etch the template | 2–100 | 1–10 | [64] |
| Hard template GO | well dispersed not bundles | hard to separate | 1–2 | 3–5 | [65] |
| Electron beam lithography | control morphology | expensive | 10 | 1–10 | [56,57,58] |
| LPNE | define thickness | polycrystalline | 10 | 1–10 | [58] |
| Nanoskiving | fast and low lost | need special instrument | 10 | 1–10 | [17] |
3.2. Synthesis of Gold Nanoparticles
| Size (nm) | Synthetic method | Capping agent | Reference |
|---|---|---|---|
| 1–2 | Reduction of HAuCl4 by NaBH4 in toluene | Phosphine | [76] |
| 1–5.2 | Reduction of HAuCl4 by NaBH4 in toluene | Alkanethiol | [74,75] |
| 3–5 | Reduction of HAuCl4 by NaBH4 in toluene | TOAB | [77] |
| 10–147 | Reduction of HAuCl4 by citrate in water | Citrate | [71,72] |
| 50–200 | Reduction of HAuCl4 by HQ in water | Citrate | [73] |
4. Recent Development in the Use of Gold Nanoparticles Based Materials in Sensors
4.1. Gold Nanoparticles Based Colorimetric, Fluorescence and SERS Sensing
| Sensing approach | Analyte | LOD | Reference |
|---|---|---|---|
| Colorimetric | Metal ion (Pb2+) | 0.4 nM | [80] |
| Anion (S2−) | 5 μM | [87] | |
| Oligonucleotide (ssDNA) | 0.1 aM | [92] | |
| Biomolecule (GSH) | 8 nM | [97] | |
| Fluorescence | Metal ion (Hg2+) | 16 nM | [84] |
| Anion (CN−) | 0.3 μM | [89] | |
| Oligonucleotide (mRNA) | 0.3 nM & 0.5 nM | [96] | |
| Biomolecule (PfHsp70) | 2.4 μg·mL−1 | [98] | |
| SERS | Metal ion (Cd2+) | 1 μM | [85] |
| Anion (CN−) | 4 nM | [91] |
4.2. Gold Nanowires Based Sensing Applications
4.2.1. Gold Nanowires Based Electrochemical Sensing Applications
| GNWs based materials | Advantages | Properties and application | Reference | |||
|---|---|---|---|---|---|---|
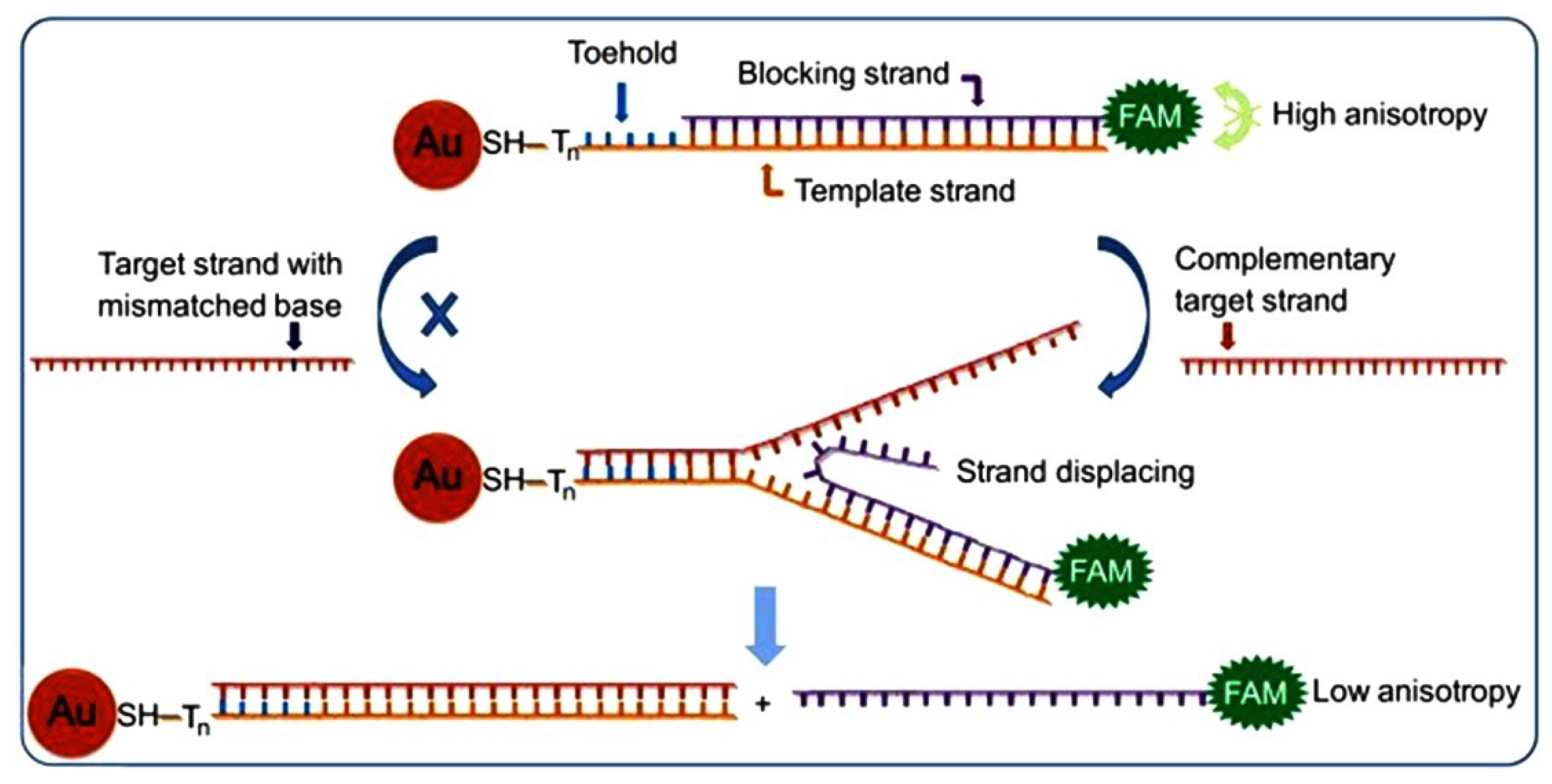
| GNWs NSEs | Microcapillary based electrochemical method and lithographic-free electrodeposition method | ET kinetics of GNWs K0 = 0.10 cm·s−1 | [100] | |||
| GNWs with tunable electron transport | Tunable electron transport | Crossover from a non-Fermin Liquid TLL ground state to a disordered state with VRH layer | [101] | |||
| Giant superlattice nanomembrane | Mechanical strong, optical transparent | Thickness 2.5 nm, resistance is 1142 kΩ | [102] | |||
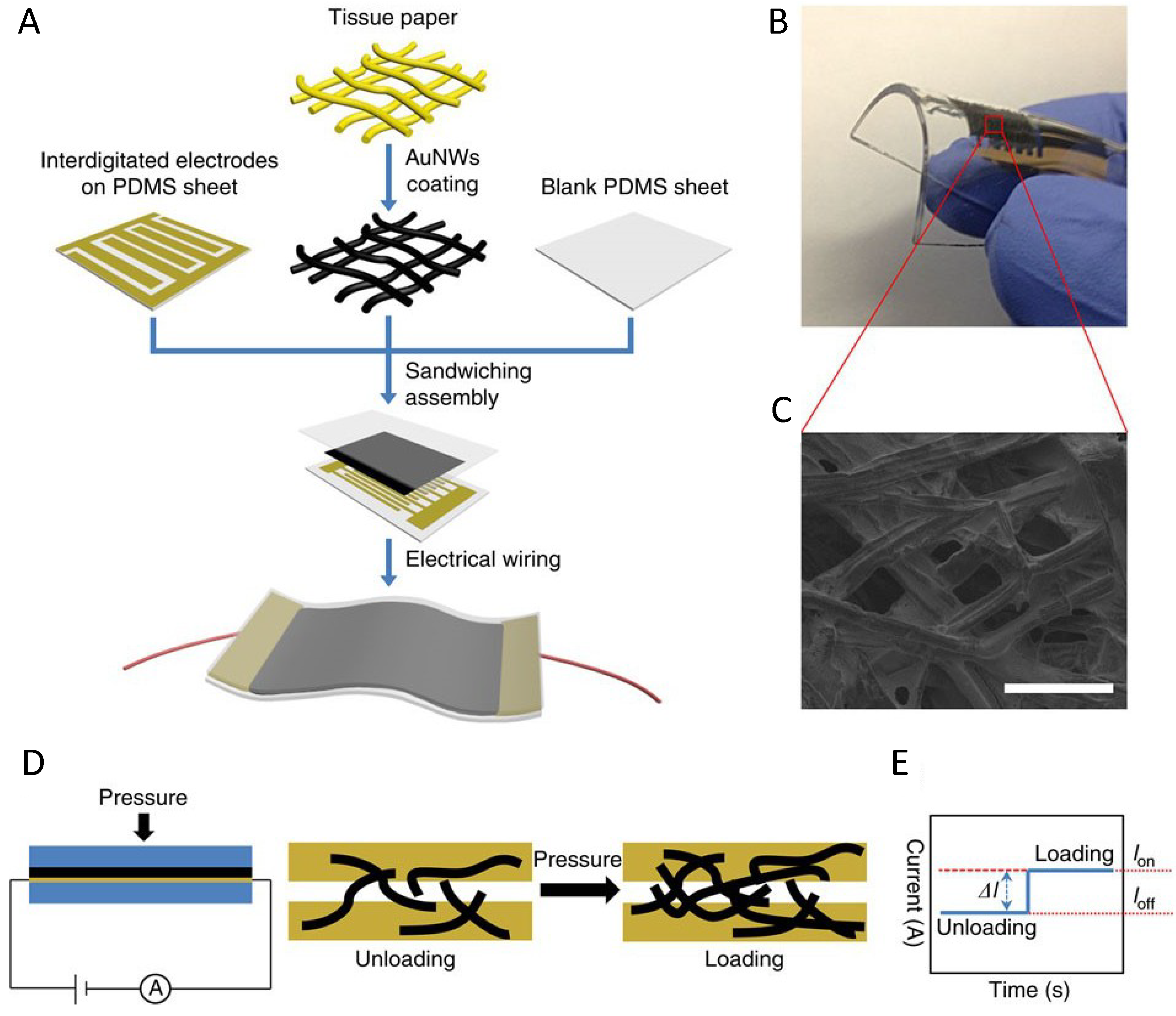
| Pressure sensor with GNWs | Low energy consumption High sensitivity >1.14 kPa−1 Fast response time <17 ms High stability >50,000 | Pressure force reduce the wire to wire spacing | [103] | |||
| Removal the layer of GNPs | Rapid removal using NaBH4 solution in water | Hydride has a higher binding affinity to gold than organothiols | [19] | |||
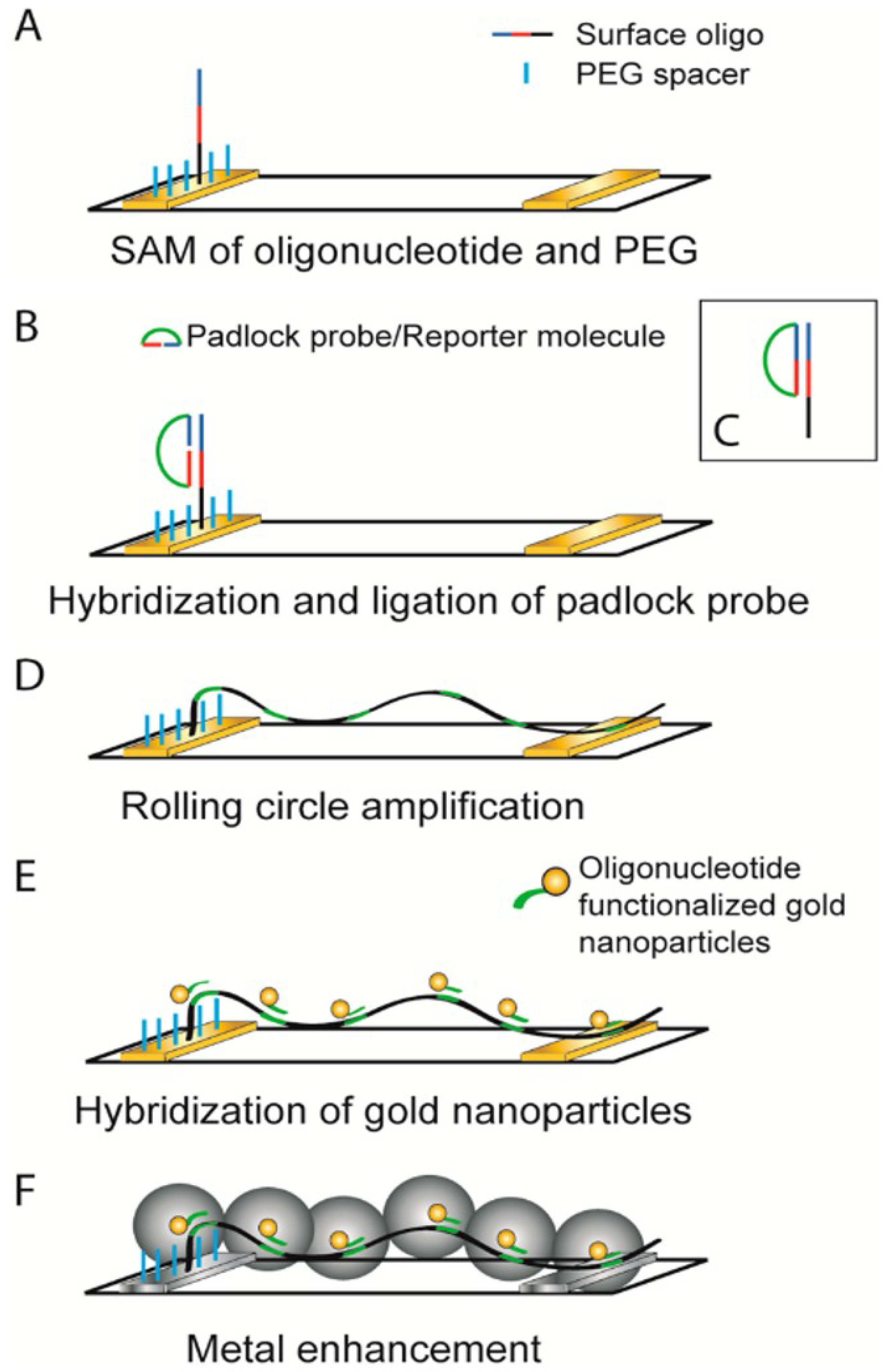
| DNA template GNWs sensor | Circle amplification of single strand DNA | DNA detection of limit LOD is 6.6 × 10−15 M | [104] | |||
| Lattice orientation protection in gold nanowires by a zipper | Ag blocks preserving the lattice of gold rings | Zipper mechanism shows ligand loss, lattice alignment and coalescence. | [105] | |||
4.2.2. Gold Nanowires Based Optical and SERS Sensing Applications
4.2.2.1. Gold Nanowires Based Optical Sensing Applications
| Materials | Methods and Properties | Results (Factor) | Reference |
|---|---|---|---|
| GNWs arrays | Optical Diffraction methods | Used for surface molecule adsorption process | [24] |
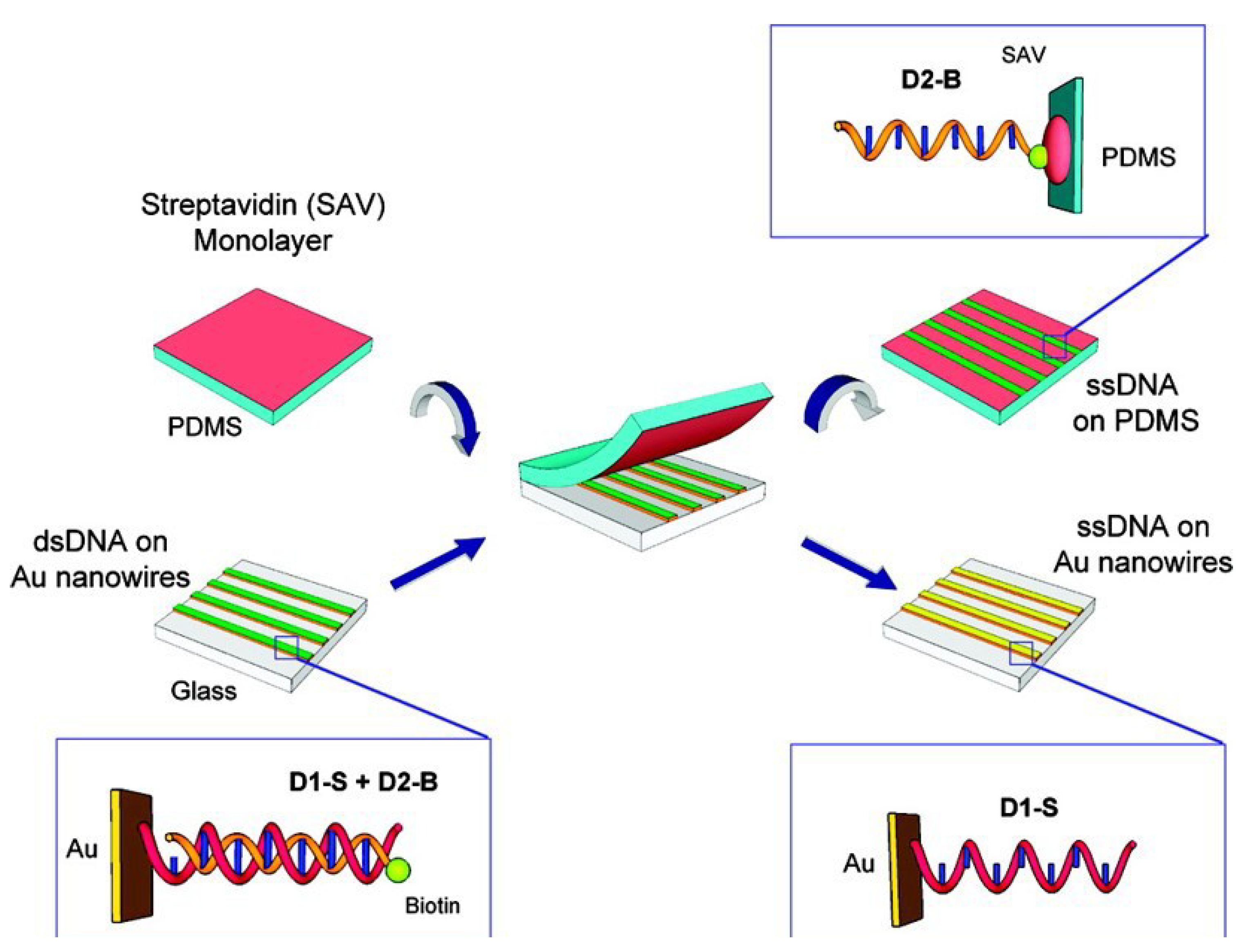
| GNWs arrays with DNA | ssDNA hybridizing, optical diffraction measurements | Detect sequence of unlabeled ssDNA | [109] |
| GNWs with different cross section | Scattering loss and joule heating with cross section | Scattering loss dependent on plasmon mode rather than cross section | [110] |
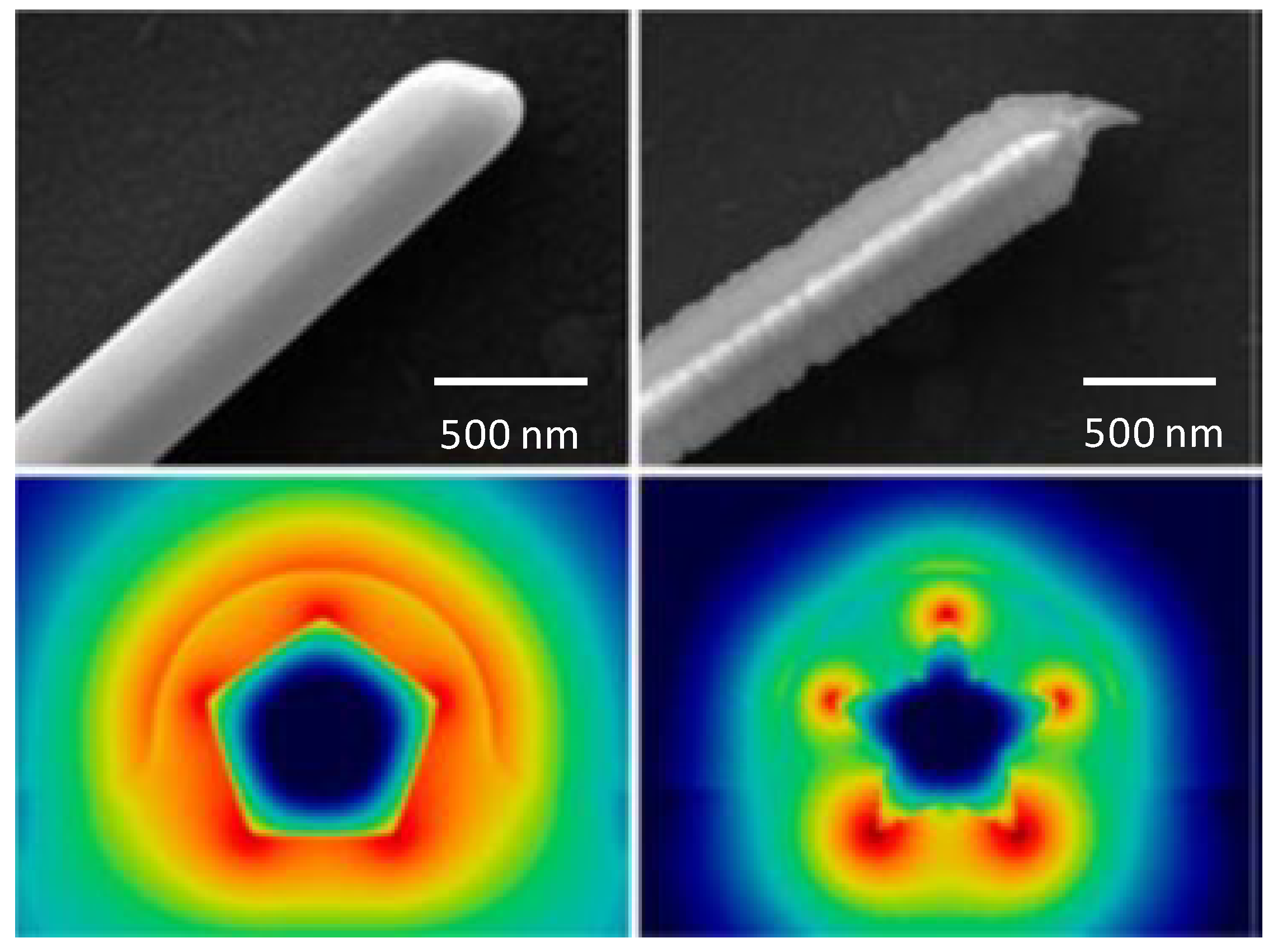
| Gold nanoantenna dimmers | Infrared spectroscopy | Bonding and antibonding combination show | [111] |
| Bowtie gold nanoantenna | Surface enhance fluorescence | Factor of 1340 | [112] |
4.2.2.2. Gold Nanowires Based SERS Sensing
| SERS substrate materials | Detection of molecules | Enhanced factors | Reference |
|---|---|---|---|
| Gold sphere with 5 nm gap single molecular detection | Directional radiated fields | EFs average value up to 1010 | [43] |
| DNA origami NPs two with 3.3 nm gap | Far field scattering | A small number of dye molecules | [114] |
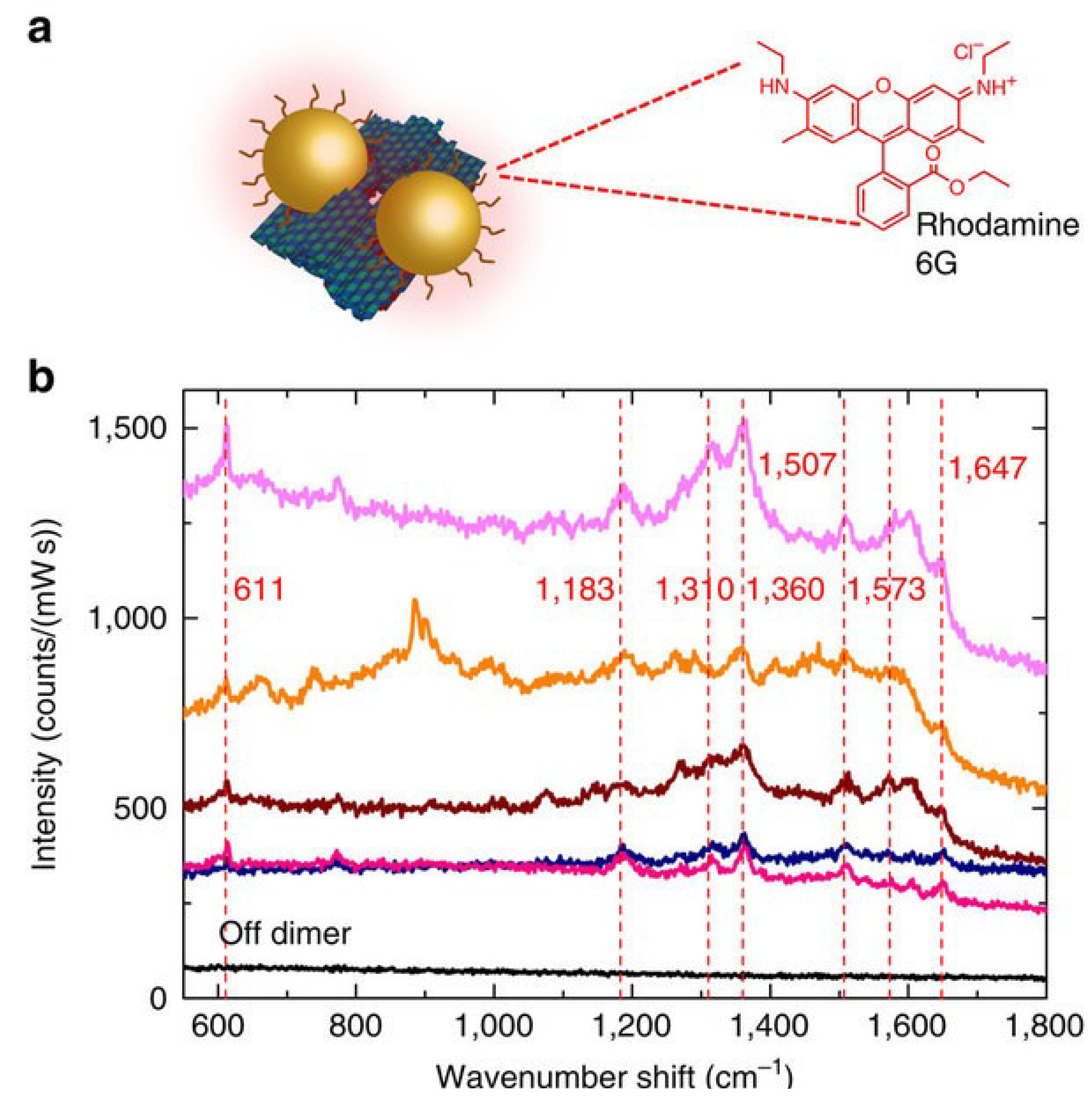
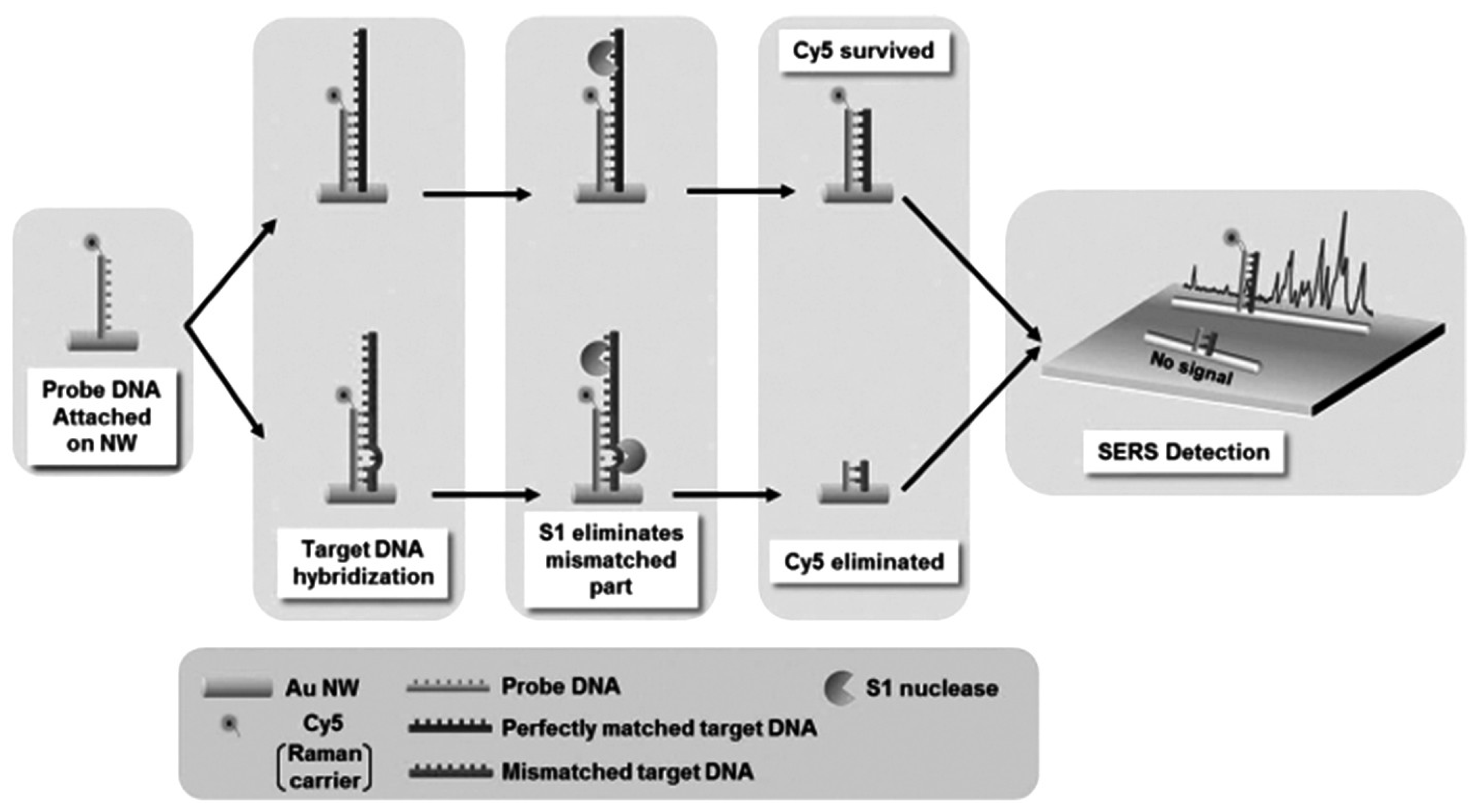
| GNWs based DNA | Raman carrier Cy5 | Strong SERS signal | [115] |
| DNA template GNPs | SERS | EFs up to 106 | [116] |
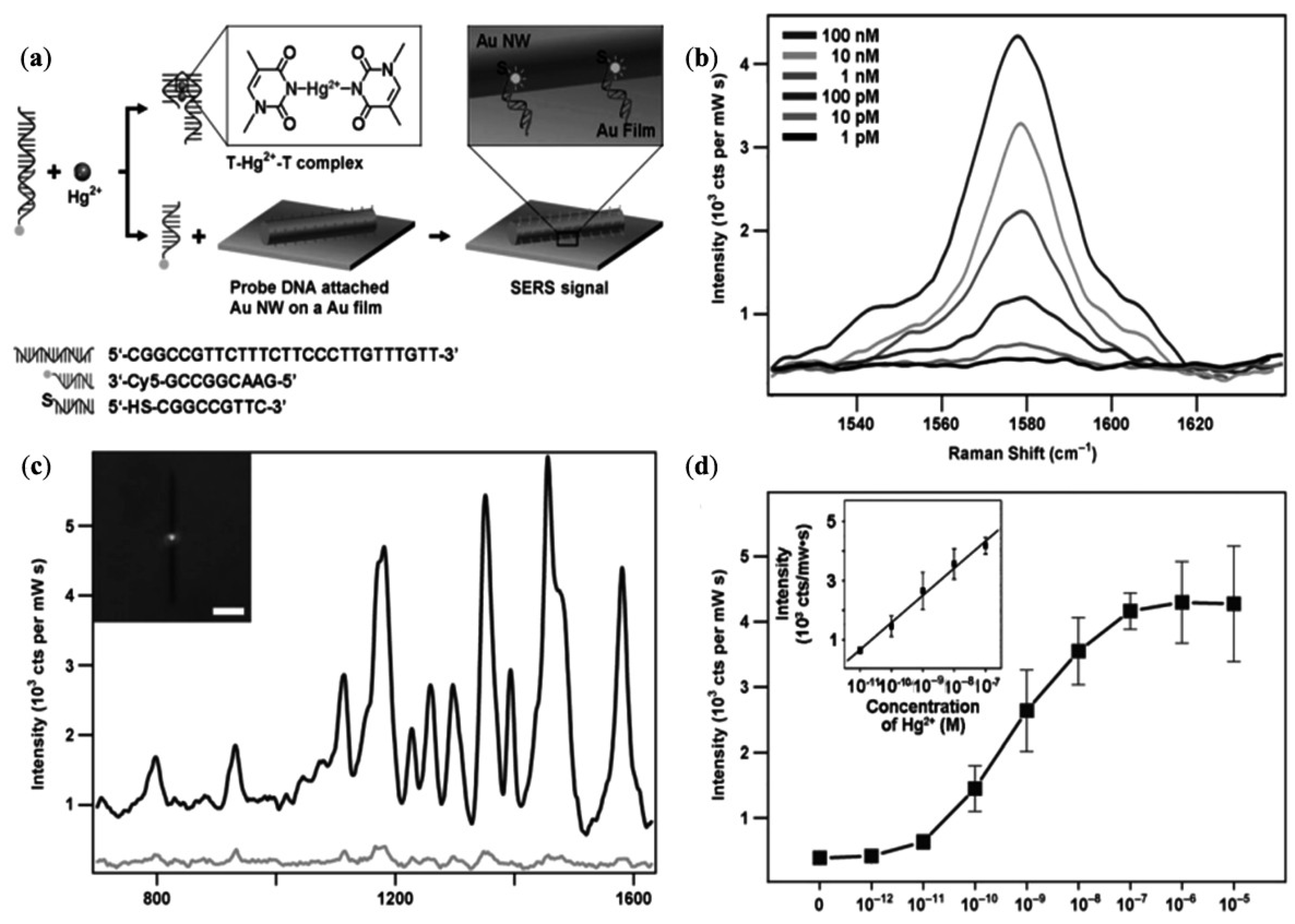
| Single nanowire based sensors of Hg2+ | Hg2+ | Detection limit up to 1 × 10−10 M | [117] |
| Ag-Au bimetal nanocages | – | SERS enhanced intensity | [118] |
| Au-Cu alloy nanotube | 4-Mpy as a single molecule | SERS enhanced intensity | [119] |
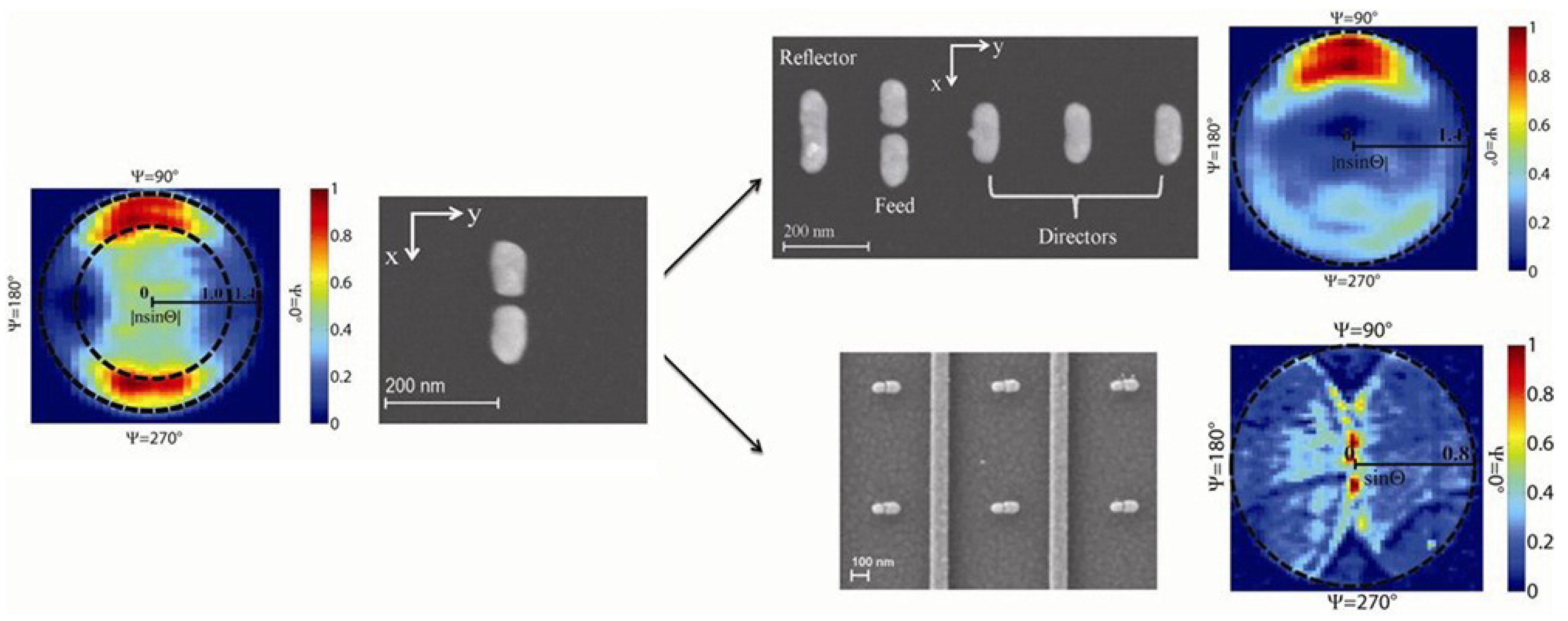
| Dimers antennas | Energy momentum spectroscope/radiated power | Perpendicular to the dipole orientation | [120] |

5. Conclusions and Future Perspectives
Acknowledgments
Nomenclature
| NPs | Nanoparticles |
| NWs | Nanowires |
| GNPs | Gold nanoparticles |
| GNWs | Gold nanowires |
| SERS | Surface Enhanced Raman Spectroscopy |
| LSPR | Localized surface plasmon resonances |
| HPLC | High performance liquid chromatograph |
| SPR | Surface plasmon resonance |
| PET | Photoinduced electron transfer |
| FRET | Fluorescence resonance energy transfer |
| NSET | Nanosurface energy transfer |
| DLS | Dynamic light scattering |
| AAO | Anodic aluminium oxide |
| Hcp | Hexagonal Close Packed |
| Fcc | Face centred cubic |
| TOAB | Tetraoctylammonium bromide |
Author Contributions
Conflicts of Interest
References
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2003, 104, 293–346. [Google Scholar] [CrossRef]
- Faraday, M. The bakerian lecture: Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Feng, H.; Yang, Y.; You, Y.; Li, G.; Guo, J.; Yu, T.; Shen, Z.; Wu, T.; Xing, B. Simple and rapid synthesis of ultrathin gold nanowires, their self-assembly and application in surface-enhanced Raman scattering. Chem. Commun. 2009, 1984–1986. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Yang, W.; Qu, L.; Zheng, R.; Liu, Z.; Ratinac, K.R.; Shen, L.; Yu, D.; Yang, L.; Barrow, C.J.; Ringer, S.P.; et al. Self-assembly of gold nanowires along carbon nanotubes for ultrahigh-aspect-ratio hybrids. Chem. Mater. 2011, 23, 2760–2765. [Google Scholar] [CrossRef]
- Dawson, K.; O’Riordan, A. Towards nanowire (bio)sensors. J. Phys. Conf. Ser. 2011, 307, 012004. [Google Scholar] [CrossRef]
- Dawson, K.; Wahl, A.; Murphy, R.; O’Riordan, A. Electroanalysis at single gold nanowire electrodes. J. Phys. Chem. C 2012, 116, 14665–14673. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D. Nano Structures via Chemistry. Nanosci. Nanotechnol. Lett. 2011, 3, 337–364. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Repko, A.; Cademartiri, L. Recent advances in the synthesis of colloidal nanowires. Can. J. Chem. 2012, 90, 1032–1047. [Google Scholar] [CrossRef]
- Zhu, C.; Peng, H.-C.; Zeng, J.; Liu, J.; Gu, Z.; Xia, Y. Facile synthesis of gold wavy nanowires and investigation of their growth mechanism. J. Am. Chem. Soc. 2012, 134, 20234–20237. [Google Scholar] [CrossRef]
- Rubio-Bollinger, G.; Bahn, S.R.; Agrait, N.; Jacobsen, K.W.; Vieira, S. Mechanical properties and formation mechanisms of a wire of single gold atoms. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef]
- Pud, S.; Kisner, A.; Heggen, M.; Belaineh, D.; Temirov, R.; Simon, U.; Offenhaeusser, A.; Mourzina, Y.; Vitusevich, S. Features of transport in ultrathin gold nanowire structures. Small 2013, 9, 846–852. [Google Scholar] [CrossRef]
- Cederquist, K.B.; Kelley, S.O. Nanostructured biomolecular detectors: Pushing performance at the nanoscale. Curr. Opin. Chem. Biol. 2012, 16, 415–421. [Google Scholar] [CrossRef]
- Dawson, K.; Strutwolf, J.; Rodgers, K.P.; Herzog, G.; Arrigan, D.W.M.; Quinn, A.J.; O’Riordan, A. Single nanoskived nanowires for electrochemical applications. Anal. Chem. 2011, 83, 5535–5540. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzan, L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Ansar, S.M.; Ameer, F.S.; Hu, W.; Zou, S.; Pittman, C.U., Jr.; Zhang, D. Removal of molecular adsorbates on gold nanoparticles using sodium borohydride in water. Nano Lett. 2013, 13, 1226–1229. [Google Scholar] [CrossRef]
- Lu, Y.; Song, J.; Huang, J.Y.; Lou, J. Fracture of Sub-20 nm ultrathin gold nanowires. Adv. Funct. Mater. 2011, 21, 3982–3989. [Google Scholar] [CrossRef]
- Desai, A.V.; Haque, M.A. Mechanical properties of ZnO nanowires. Sens. Actuators A 2007, 134, 169–176. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Li, C.Z.; He, H.X.; Bogozi, A.; Bunch, J.S.; Tao, N.J. Molecular detection based on conductance quantization of nanowires. Appl. Phys. Lett. 2000, 76, 1333–1335. [Google Scholar] [CrossRef]
- Han, Y.; Corn, R.M. Characterization and application of surface plasmon-enhanced optical diffraction from electrodeposited gold nanowire arrays. J. Phys. Chem. Lett. 2011, 2, 1601–1606. [Google Scholar] [CrossRef]
- Dale, E.B.; Ganta, D.; Yu, D.-J.; Flanders, B.N.; Wicksted, J.P.; Rosenberger, A.T. Spatially localized enhancement of evanescent coupling to Whispering-Gallery modes at 1550 nm due to surface plasmon resonances of AU nanowires. IEEE J. Sel. Top. 2011, 17, 979–984. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Knoppe, S.; Bürgi, T. Chirality in thiolate-protected gold clusters. Acc. Chem. Res. 2014, 47, 1318–1326. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.X.; Sun, H.; Zhang, W.Q.; Chen, G.; Wang, Y.W.; Shen, X.S.; Han, Y.; Lu, X.M.; Chen, H.Y. Chiral transformation: From single nanowire to double helix. J. Am. Chem. Soc. 2011, 133, 20060–20063. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Trügler, A.; Tinguely, J.-C.; Krenn, J.R.; Hohenau, A.; Hohenester, U. Influence of surface roughness on the optical properties of plasmonic nanoparticles. Phys. Rev. B 2011, 83, 081412. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef]
- Philip, R.; Chantharasupawong, P.; Qian, H.; Jin, R.; Thomas, J. Evolution of nonlinear optical properties: From gold atomic clusters to plasmonic nanocrystals. Nano Lett. 2012, 12, 4661–4667. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet-visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Dulkeith, E.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; von Plessen, G.; Gittins, D.I.; Mayya, K.S.; Caruso, F. Plasmon emission in photoexcited gold nanoparticles. Phys. Rev. B 2004, 70, 205424. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, C.; Dickson, R.M. Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys. Rev. Lett. 2004, 93, 077402. [Google Scholar] [CrossRef]
- Kang, K.A.; Wang, J.; Jasinski, J.B.; Achilefu, S. Fluorescence manipulation by gold nanoparticles: from complete quenching to extensive enhancement. J. Nanobiotechnol. 2011, 9. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence quenching of dye molecules near gold nanoparticles: Radiative and nonradiative effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef]
- Barazzouk, S.; Kamat, P.V.; Hotchandani, S. Photoinduced electron transfer between chlorophyll a and gold nanoparticles. J. Phys. Chem. B 2004, 109, 716–723. [Google Scholar] [CrossRef]
- Acuna, G.P.; Bucher, M.; Stein, I.H.; Steinhauer, C.; Kuzyk, A.; Holzmeister, P.; Schreiber, R.; Moroz, A.; Stefani, F.D.; Liedl, T.; et al. Distance dependence of single-fluorophore quenching by gold nanoparticles studied on DNA origami. ACS Nano 2012, 6, 3189–3195. [Google Scholar] [CrossRef]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced raman scattering from individual Au nanoparticles and nanoparticle dimer substrates. Nano Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef]
- Crozier, K.B.; Zhu, W.; Wang, D.; Lin, S.; Best, M.D.; Camden, J.P. Plasmonics for surface enhanced raman scattering: Nanoantennas for single molecules. 2013 20. [CrossRef]
- Di Felice, R.; Selloni, A. Adsorption modes of cysteine on Au(111): Thiolate, amino-thiolate, disulfide. J. Chem. Phys. 2004, 120, 4906–4914. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef]
- Fendler, J.H. Self-assembled nanostructured materials. Chem. Mater. 1996, 8, 1616–1624. [Google Scholar] [CrossRef]
- Lal, M.; Plummer, M.; Smith, W. Solvent density effects on the solvation behavior and configurational structure of bare and passivated 38-atom gold nanoparticle in supercritical ethane. J. Phys. Chem. B 2006, 110, 20879–20888. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef]
- Nayar, D.; Yadav, H.O.S.; Jabes, B.S.; Chakravarty, C. Relating structure, entropy, and energy of solvation of nanoscale solutes: Application to gold nanoparticle dispersions. J. Phys. Chem. B 2012, 116, 13124–13132. [Google Scholar] [CrossRef]
- Aslan, K.; Pérez-Luna, V.H. Surface modification of colloidal gold by chemisorption of alkanethiols in the presence of a nonionic surfactant. Langmuir 2002, 18, 6059–6065. [Google Scholar] [CrossRef]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Estevez, M.; Otte, M.A.; Sepulveda, B.; Lechuga, L.M. Trends and challenges of refractometric nanoplasmonic biosensors: A review. Anal. Chim. Acta 2014, 806, 55–73. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Guo, X. Surface plasmon resonance based biosensor technique: A review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Sensing using localised surface plasmon resonance sensors. Chem. Commun. 2012, 48, 8999–9010. [Google Scholar] [CrossRef]
- Zayats, A.V.; Smolyaninov, I.I.; Maradudin, A.A. Nano-optics of surface plasmon polaritons. Phys. Rep. 2005, 408, 131–314. [Google Scholar] [CrossRef]
- Menke, E.; Thompson, M.; Xiang, C.; Yang, L.; Penner, R. Lithographically patterned nanowire electrodeposition. Nat. Mater. 2006, 5, 914–919. [Google Scholar] [CrossRef]
- Shi, S.; Lu, N.; Lu, Y.; Wang, Y.; Qi, D.; Xu, H.; Chi, L. Fabrication of periodic metal nanowires with microscale mold by nanoimprint lithography. ACS Appl. Mater. 2011, 3, 4174–4179. [Google Scholar] [CrossRef]
- Lu, X.; Yavuz, M.S.; Tuan, H.-Y.; Korgel, B.A.; Xia, Y. Ultrathin gold nanowires can be obtained by reducing polymeric strands of Oleylamine−AuCl complexes formed via aurophilic interaction. J. Am. Chem. Soc. 2008, 130, 8900–8901. [Google Scholar] [CrossRef]
- Halder, A.; Ravishankar, N. Ultrafine single-crystalline gold nanowire arrays by oriented attachment. Adv. Mater. 2007, 19, 1854–1858. [Google Scholar] [CrossRef]
- Morita, C.; Tanuma, H.; Kawai, C.; Ito, Y.; Imura, Y.; Kawai, T. Room-temperature synthesis of two-dimensional ultrathin gold nanowire parallel array with tunable spacing. Langmuir 2013, 29, 1669–1675. [Google Scholar] [CrossRef]
- Imura, Y.; Tanuma, H.; Sugimoto, H.; Ito, R.; Hojo, S.; Endo, H.; Morita, C.; Kawai, T. Water-dispersible ultrathin Au nanowires prepared using a lamellar template of a long-chain amidoamine derivative. Chem. Commun. 2011, 47, 6380–6382. [Google Scholar]
- Kline, T.R.; Tian, M.; Wang, J.; Sen, A.; Chan, M.W.; Mallouk, T.E. Template-grown metal nanowires. Inorg. Chem. 2006, 45, 7555–7565. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Wu, S.; Huang, Y.; Boey, F.; Gan, C.L.; Zhang, H. Graphene oxide-templated synthesis of ultrathin or tadpole-shaped au nanowires with alternating hcp and fcc domains. Adv. Mater. 2012, 24. [Google Scholar] [CrossRef]
- Jiang, R.; Chen, H.; Shao, L.; Li, Q.; Wang, J. Unraveling the evolution and nature of the plasmons in (Au core)-(Ag shell) nanorods. Adv. Mater. 2012, 24, OP200–OP207. [Google Scholar]
- Li, H.; Li, Y.-J.; Sun, L.-L.; Zhao, X.-L. One-step, template-free electrochemical preparation of three-dimensional porous Au nanowire network and its enhanced activity toward methanol electrooxidation. Electrochim. Acta 2013, 108, 74–78. [Google Scholar] [CrossRef]
- Haldar, K.K.; Pradhan, N.; Patra, A. Formation of heteroepitaxy in different shapes of Au-CdSe metal-semiconductor hybrid nanostructures. Small 2013, 9, 3424–3432. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, G.; Chang, F.; Hu, B.; Zhong, C.-J. Gold-platinum alloy nanowires as highly sensitive materials for electrochemical detection of hydrogen peroxide. Anal. Chim. Acta 2012, 757, 56–62. [Google Scholar] [CrossRef]
- Tiwari, I.; Gupta, M. Neutral red interlinked gold nanoparticles/multiwalled carbon nanotubes hybrid nanomaterial and its application for the detection of NADH. Mater. Res. Bull. 2014, 49, 94–101. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Perrault, S.D.; Chan, W.C.W. Synthesis and surface modification of highly monodispersed, spherical gold nanoparticles of 50–200 nm. J. Am. Chem. Soc. 2009, 131, 17042–17043. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 1994, 801–802. [Google Scholar]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.-J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: Core and monolayer properties as a function of core size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Weare, W.W.; Reed, S.M.; Warner, M.G.; Hutchison, J.E. Improved synthesis of small (Dcore ≈ 1.5 nm) phosphine-stabilized gold nanoparticles. J. Am. Chem. Soc. 2000, 122, 12890–12891. [Google Scholar] [CrossRef]
- Fink, J.; Kiely, C.J.; Bethell, D.; Schiffrin, D.J. Self-Organization of nanosized gold particles. Chem. Mater. 1998, 10, 922–926. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.-Y.; Takeda, Y.; Kondow, T. Dissociation and aggregation of gold nanoparticles under laser irradiation. J. Phys. Chem. B 2001, 105, 9050–9056. [Google Scholar] [CrossRef]
- Sun, Y.; Jose, D.; Sorensen, C.; Klabunde, K. Alkyl and aromatic amines as digestive ripening/size focusing agents for gold nanoparticles. Nanomaterials 2013, 3, 370–392. [Google Scholar]
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl. Mater. 2011, 3, 668–673. [Google Scholar]
- Zhu, D.; Li, X.; Liu, X.; Wang, J.; Wang, Z. Designing bifunctionalized gold nanoparticle for colorimetric detection of Pb2+ under physiological condition. Biosens. Bioelectron. 2012, 31, 505–509. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, H.; Vongsvivut, J.; Li, R.; Glushenkov, A.M.; He, J.; Chen, Y.; Barrow, C.J.; Yang, W. Self-assembly of core-satellite gold nanoparticles for colorimetric detection of copper ions. Anal. Chim. Acta 2013, 803, 128–134. [Google Scholar] [CrossRef]
- Durgadas, C.V.; Sharma, C.P.; Sreenivasan, K. Fluorescent gold clusters as nanosensors for copper ions in live cells. Analyst 2011, 136, 933–940. [Google Scholar] [CrossRef]
- Tan, D.; He, Y.; Xing, X.; Zhao, Y.; Tang, H.; Pang, D. Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution. Talanta 2013, 113, 26–30. [Google Scholar] [CrossRef]
- Yin, J.; Wu, T.; Song, J.; Zhang, Q.; Liu, S.; Xu, R.; Duan, H. SERS-active nanoparticles for sensitive and selective detection of cadmium ion (Cd2+). Chem. Mater. 2011, 23, 4756–4764. [Google Scholar] [CrossRef]
- He, G.; Zhao, L.; Chen, K.; Liu, Y.; Zhu, H. Highly selective and sensitive gold nanoparticle-based colorimetric assay for PO43− in aqueous solution. Talanta 2013, 106, 73–78. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, X. Highly specific colorimetric recognition and sensing of sulfide with glutathione-modified gold nanoparticle probe based on an anion-for-molecule ligand exchange reaction. Analyst 2012, 137, 1556–1558. [Google Scholar] [CrossRef]
- Wei, S.-C.; Hsu, P.-H.; Lee, Y.-F.; Lin, Y.-W.; Huang, C.-C. Selective detection of iodide and cyanide anions using gold-nanoparticle-based fluorescent probes. ACS Appl. Mater. 2012, 4, 2652–2658. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, Y.; Qin, J.; Li, Z. A highly sensitive and selective fluorescent probe for cyanide based on the dissolution of gold nanoparticles and its application in real samples. Chem. Eur. J. 2011, 17, 9691–9696. [Google Scholar] [CrossRef]
- Lou, X.; Zeng, Q.; Zhang, Y.; Wan, Z.; Qin, J.; Li, Z. Functionalized polyacetylenes with strong luminescence: “Turn-On” fluorescent detection of cyanide based on the dissolution of gold nanoparticles and its application in real samples. J. Mater. Chem. 2012, 22, 5581–5586. [Google Scholar]
- Senapati, D.; Dasary, S.S.R.; Singh, A.K.; Senapati, T.; Yu, H.; Ray, P.C. A Label-free gold-nanoparticle-based SERS assay for direct cyanide detection at the parts-per-trillion Level. Chem. Eur. J. 2011, 17, 8445–8451. [Google Scholar] [CrossRef]
- Yin, H.; Huang, X.; Ma, W.; Xu, L.; Zhu, S.; Kuang, H.; Xu, C. Ligation chain reaction based gold nanoparticle assembly for ultrasensitive DNA detection. Biosens. Bioelectron. 2014, 52, 8–12. [Google Scholar] [CrossRef]
- Shen, W.; Deng, H.; Gao, Z. Gold nanoparticle-enabled real-time ligation chain reaction for ultrasensitive detection of DNA. J. Am. Chem. Soc. 2012, 134, 14678–14681. [Google Scholar] [CrossRef]
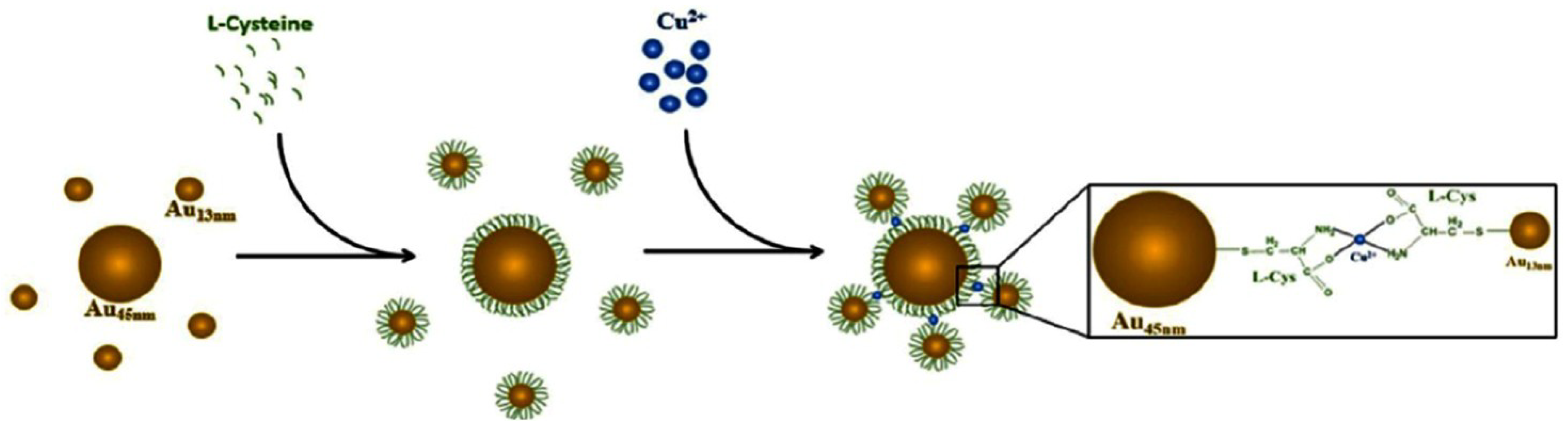
- Wang, X.; Zou, M.; Huang, H.; Ren, Y.; Li, L.; Yang, X.; Li, N. Gold nanoparticle enhanced fluorescence anisotropy for the assay of single nucleotide polymorphisms (SNPs) based on toehold-mediated strand-displacement reaction. Biosens. Bioelectron. 2013, 41, 569–575. [Google Scholar] [CrossRef]
- Xue, J.; Shan, L.; Chen, H.; Li, Y.; Zhu, H.; Deng, D.; Qian, Z.; Achilefu, S.; Gu, Y. Visual detection of STAT5B gene expression in living cell using the hairpin DNA modified gold nanoparticle beacon. Biosens. Bioelectron. 2013, 41, 71–77. [Google Scholar] [CrossRef]
- Qiao, G.; Gao, Y.; Li, N.; Yu, Z.; Zhuo, L.; Tang, B. Simultaneous detection of intracellular tumor mRNA with Bi-Color imaging based on a gold nanoparticle/molecular beacon. Chem. Eur. J. 2011, 17, 11210–11215. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Xu, H.; Zhang, H.; Zhong, X. Anti-Aggregation of gold nanoparticle-based colorimetric sensor for glutathione with excellent selectivity and sensitivity. Analyst 2011, 136, 196–200. [Google Scholar] [CrossRef]
- Guirgis, B.S.; Sá e Cunha, C.; Gomes, I.; Cavadas, M.; Silva, I.; Doria, G.; Blatch, G.; Baptista, P.; Pereira, E.; Azzazy, H.E.; Mota, M.; Prudêncio, M.; Franco, R. Gold nanoparticle-based fluorescence immunoassay for malaria antigen detection. Anal. Bioanal. Chem. 2012, 402, 1019–1027. [Google Scholar] [CrossRef]
- Crew, E.; Yan, H.; Lin, L.; Yin, J.; Skeete, Z.; Kotlyar, T.; Tchah, N.; Lee, J.; Bellavia, M.; Goodshaw, I.; et al. DNA assembly and enzymatic cutting in solutions: A gold nanoparticle based SERS detection strategy. Analyst 2013, 138, 4941–4949. [Google Scholar] [CrossRef]
- Dudin, P.V.; Snowden, M.E.; Macpherson, J.V.; Unwin, P.R. Electrochemistry at nanoscale electrodes: Individual single-walled carbon nanotubes (SWNTs) and SWNT-templated metal nanowires. ACS Nano 2011, 5, 10017–10025. [Google Scholar] [CrossRef]
- Chandni, U.; Kundu, P.; Kundu, S.; Ravishankar, N.; Ghosh, A. Tunability of electronic states in ultrathin gold nanowires. Adv. Mater. 2013, 25, 2486–2491. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, Z.; Gu, M.; Cheng, W. Mechanically strong, optically transparent, giant metal superlattice nanomembranes from ultrathin gold nanowires. Adv. Mater. 2013, 25, 80–85. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Russell, C.; Welch, K.; Jarvius, J.; Cai, Y.; Brucas, R.; Nikolajeff, F.; Svedlindh, P.; Nilsson, M. Gold nanowire based electrical DNA detection using rolling circle amplification. ACS Nano 2014, 8, 1147–1153. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Qi, X.; Liu, C.; He, J.; Zhang, H.; Chen, H. Preservation of lattice orientation in coalescing imperfectly aligned gold nanowires by a zipper mechanism. Angew. Chem. 2013, 125, 6135–6139. [Google Scholar]
- Neubrech, F.; Pucci, A.; Cornelius, T.W.; Karim, S.; García-Etxarri, A.; Aizpurua, J. Resonant plasmonic and vibrational coupling in a tailored nanoantenna for infrared detection. Phys. Rev. Lett. 2008, 101, 157403. [Google Scholar] [CrossRef]
- Neubrech, F.; Weber, D.; Lovrincic, R.; Pucci, A.; Lopes, M.; Toury, T.; de la Chapelle, M.L. Resonances of individual lithographic gold nanowires in the infrared. Appl. Phys. Lett. 2008, 93, 163105. [Google Scholar]
- Guo, X.; Ma, Y.; Wang, Y.; Tong, L. Nanowire plasmonic waveguides, circuits and devices. Laser Photonics Rev. 2013, 7, 855–881. [Google Scholar] [CrossRef]
- Chen, Y.L.; Kung, S.C.; Taggart, D.K.; Halpern, A.R.; Penner, R.M.; Corn, R.M. Fabricating nanoscale DNA patterns with gold nanowires. Anal. Chem. 2010, 82, 3365–3370. [Google Scholar] [CrossRef]
- Nauert, S.; Paul, A.; Zhen, Y.-R.; Solis, D.; Vigderman, L.; Chang, W.-S.; Zubarev, E.R.; Nordlander, P.; Link, S. Influence of cross sectional geometry on surface plasmon polariton propagation in gold nanowires. ACS Nano 2013, 8, 572–580. [Google Scholar]
- Neubrech, F.; Weber, D.; Katzmann, J.; Huck, C.; Toma, A.; di Fabrizio, E.; Pucci, A.; Härtling, T. Infrared optical properties of nanoantenna dimers with photochemically narrowed gaps in the 5 nm regime. ACS Nano 2012, 6, 7326–7332. [Google Scholar] [CrossRef]
- Kinkhabwala, A.; Zongfu, Y.; Shanhui, F.; Yuri, A.; Klaus, M.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Stokes, J.L.; Yu, Y.; Yuan, Z.H.; Pugh, J.R.; Lopez-Garcia, M.; Ahmad, N.; Cryan, M.J. Analysis and design of a cross dipole nanoantenna for fluorescence-sensing applications. J. Opt. Soc. Am. B 2014, 31, 302–310. [Google Scholar] [CrossRef]
- Thacker, V.V.; Herrmann, L.O.; Sigle, D.O.; Zhang, T.; Liedl, T.; Baumberg, J.J.; Keyser, U.F. DNA origami based assembly of gold nanoparticle dimers for surface-enhanced Raman scattering. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Yoo, S.M.; Kang, T.; Kim, B.; Lee, S.Y. Detection of single nucleotide polymorphisms by a gold nanowire-on-film SERS sensor coupled with S1 nuclease treatment. Chem. Eur. J. 2011, 17, 8657–8662. [Google Scholar] [CrossRef]
- Kundu, S.; Jayachandran, M. The self-assembling of DNA-templated Au nanoparticles into nanowires and their enhanced SERS and catalytic applications. Rsc. Adv. 2013, 3, 16486–16498. [Google Scholar] [CrossRef]
- Kang, T.; Yoo, S.M.; Yoon, I.; Lee, S.; Choo, J.; Lee, S.Y.; Kim, B. Au nanowire-on-film SERRS sensor for ultrasensitive Hg2+ detection. Chem. Eur. J. 2011, 17, 2211–2214. [Google Scholar]
- Rycenga, M.; Hou, K.K.; Cobley, C.M.; Schwartz, A.G.; Camargo, P.H.C.; Xia, Y. Probing the surface-enhanced Raman scattering properties of Au-Ag nanocages at two different excitation wavelengths. Phys. Chem. Chem. Phys. 2009, 11, 5903–5908. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Q.; Zong, C.; Liu, B.-J.; Ren, B.; Xie, Z.; Zheng, L. Cu-Au alloy nanotubes with five-fold twinned structure and their application in surface-enhanced Raman scattering. J. Mater. Chem. 2012, 22, 18192–18197. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, D.; Crozier, K.B. Direct observation of beamed Raman scattering. Nano Lett. 2012, 12, 6235–6243. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, D.; Li, R.; Wang, X.; Yang, W. A hybrid material by ultrathin gold nanowires and single Walled carbon nanotube for trace level detection. Biosens. Bioelectron. 2014. submitted. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New Gold Nanostructures for Sensor Applications: A Review. Materials 2014, 7, 5169-5201. https://doi.org/10.3390/ma7075169
Zhang Y, Chu W, Foroushani AD, Wang H, Li D, Liu J, Barrow CJ, Wang X, Yang W. New Gold Nanostructures for Sensor Applications: A Review. Materials. 2014; 7(7):5169-5201. https://doi.org/10.3390/ma7075169
Chicago/Turabian StyleZhang, Yuanchao, Wendy Chu, Alireza Dibaji Foroushani, Hongbin Wang, Da Li, Jingquan Liu, Colin J. Barrow, Xin Wang, and Wenrong Yang. 2014. "New Gold Nanostructures for Sensor Applications: A Review" Materials 7, no. 7: 5169-5201. https://doi.org/10.3390/ma7075169




