Spark Plasma Sintering As a Solid-State Recycling Technique: The Case of Aluminum Alloy Scrap Consolidation
Abstract
:1. Introduction
1.1. Energy and Material Efficiency Challenges in Aluminum (Al) Recycling
1.2. Solid-State Recycling Techniques
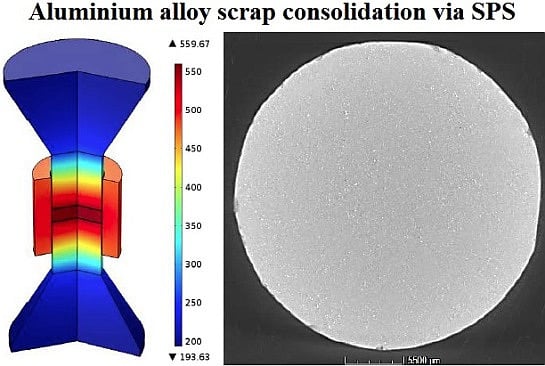
1.3. SPS Description and Advantages
1.4. Research Motivation and Objectives
2. Materials

| Sample | Measured density (g/cm3) | Oxide content (%) |
|---|---|---|
| S1–ΑΑ6061 | 2.807 | 8.48 |
| S2–ΑΑ6061 | 2.806 | 8.49 |
| Al6061-T0 reference | 2.686 | - |
| S3–ΑΑ6082 | 2.83 | 10.42 |
| S4–ΑΑ6082 | 2.832 | 10.52 |
| AA6082 T-651 reference | 2.701 | - |
3. Experimental Section
3.1. Process and Tool Set-Up

3.2. Sintering Cycle and Precipitation Hardening

3.3. Material Characterization Measurements
3.3.1. Microstructural Characterization
3.3.2. Hardness and Density Measurements

3.3.3. X-ray Industrial Computed Tomography (CT)
3.3.4. Impulse Excitation Technique (IET)
3.3.5. Compression Testing
4. Results and Discussion
4.1. Microstructural Investigations
4.1.1. Factors that Contribute to Oxide Layer Breakage and to Scrap Consolidation Process



4.1.2. Material Texture

4.1.3. Intermetallic and Grain Boundaries

| Peaks | Spot 1 | Spot 2 | Selected area |
|---|---|---|---|
| O K | 3.83/(15.47) | 9.91/(9.48) | - |
| Fe L | 1.51/(62.38) | - | - |
| Al K | 2.66/(9.9) | 86.47/(2.38) | 95.98/(1.9) |
| Bi M | 89.52/(1.46) | - | - |
| Cu K | 2.49/(14.42) | 3.63/(9.16) | 4.02/(6.8) |
4.2. Densification, Oxide Content and Impurities

X-ray Industrial CT Analysis


4.3. Mechanical Properties
4.3.1. Hardness
| Sample | Hardness after artificial aging (MPa) | Standard deviation σ |
|---|---|---|
| S1 top surface | 780 | 14.7 |
| S1 cross-section | 779 | 14.7 |
| S2 top surface | 777 | 17.6 |
| S2 cross-section | 802 | 4.9 |
| Al6061-T0 reference | 709 | 20.6 |
| S3 top surface | 758 | 27.5 |
| S3 cross-section | 803 | 36.3 |
| S4 top surface | 839 | 19.6 |
| S4 cross-section | 740 | 39.2 |
| AA6082 T-651 reference | 1106 | 23.5 |
4.3.2. Elastic Properties

| Sample | E-modulus (GPa) | G-modulus (GPa) | Poisson’s ratio | Bulk modulus ** (GPa) |
|---|---|---|---|---|
| S2 (AA6061 chips) | 77.97 ± 0.93 | 28.98 ± 0.34 | 0.345 | 83.84 ** |
| AA6061-T0 reference | 69.08 ± 2.48/68–78 * | 25–27 * | 0.325–0.335 * | 65–72 ** |
| S3 (AA6082 chips) | 77.34 ± 0.87 | 28.42 ± 0.32 | 0.36 | 92.07 ** |
| S4 (AA6082 chips) | 78.95 ± 5.28 | 29.13 ± 1.95 | 0.355 | 90.74 ** |
| AA6082-T651 reference | 69.69 ± 0.86/70–74 * | 25–27 * | 0.325–0.335 * | 65–72 ** |
4.3.3. Compression Testing


| Sample | Strain hardening exponent (n) | Compressive strength | ||
|---|---|---|---|---|
| 8% strain | 9% strain | 10% strain | ||
| S1-Al6061 (top) | 0.46 ± 0.07 | 320 ± 5.9 | 337 ± 6.1 | 350 ± 5.9 |
| S2-Al6061 (cross-section) | 0.40 ± 0.03 | 336 ± 9.3 | 348 ± 9.5 | 360 ± 9.2 |
| Al6061-T0 reference | 0.49 | 240 | 256 | 270 |
| S3-Al6082 (top) | 0.55 ± 0.05 | 329 ± 8 | 351 ± 7 | 371 ± 6 |
| S4-Al6082 (cross-section) | 0.46 ± 0.08 | 304 ± 16 | 329 ± 1 | 342 ± 1 |
| Al6082-T651 reference | 0.24 | 368 | 376 | 386 |
5. Conclusions and Discussion
6. Future Research
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duflou, J.R.; Sutherland, J.W.; Dornfeld, D.; Herrmann, C.; Jeswiet, J.; Kara, S.; Hauschild, M.; Kellens, K. Towards energy and resource efficient manufacturing: A processes and systems approach. CIRP Ann. Manuf. Technol. 2012, 61, 587–609. [Google Scholar] [CrossRef]
- Gutowski, T.; Allwood, J.M.; Sahni, S.; Hermann, C. A global assessment of manufacturing: Economic development, energy use, carbon emmissions, and the potential for energy efficiency and materials recycling. Ann. Rev. Environ. Resour. 2013, 38, 81–106. [Google Scholar] [CrossRef]
- Allwood, J.M.; Cullen, J.M.; Milford, R.L. Options for achieving a 50% cut in industrial carbon emissions by 2050. Environ. Sci. Technol. 2010, 44, 1888–1894. [Google Scholar] [CrossRef]
- Liu, G.; Bangs, C.E.; Müller, D.B. Stock dynamics and emission pathways of the global aluminium cycle. Nat. Clim. Chang. 2013, 3, 338–342. [Google Scholar]
- Environmental Profile Report for the European Aluminium Industry—Life Cycle Inventory Data for Aluminium Production and Transformation Processes in Europe; European Aluminium Association (EAA): Brussels, Belgium, 2008. Available online: http://www.alueurope.eu/wp-content/uploads/2011/08/EAA_Environmental_profile_report-May081.pdf (accessed on 5 August 2014).
- Schwarz, H.G. Aluminum production and energy. Encycl. Energy 2004, 1, 81–95. [Google Scholar] [CrossRef]
- Milford, R.L.; Allwood, J.M.; Cullen, J.M. Assessing the potential of yield improvements, through process scrap reduction, for energy and CO2 abatement in the steel and aluminium sectors. Resour. Conserv. Recycl. 2011, 55, 1185–1195. [Google Scholar] [CrossRef]
- Reuter, M.A.; Boin, U.M.J.; van Schaik, A.; Verhoef, E.; Heiskanen, K.; Yang, Y.; Georgalli, G. The Metrics of Material and Metal Ecology: Harmonizing the Resource, Technology and Environmental Cycles; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Xiao, Y.; Reuter, M.A. Recycling of distributed aluminium turning scrap. Miner. Eng. 2002, 15, 963–970. [Google Scholar] [CrossRef]
- Geertruyden, W.H.; Prescott, C.A.; Misiolek, W.Z.; Peterson, R. Evaluation of light gauge Al scrap remelting after consolidation via the extrusion process. Light Met. Age 2005, 63, 14–18. [Google Scholar]
- Boin, U.M.J.; Bertram, M. Melting standardized aluminium scrap: A mass balance model for Europe. J. Met. Mater. Soc. 2005, 57, 26–33. [Google Scholar] [CrossRef]
- Tekkaya, A.E.; Schikorra, M.; Becker, D.; Biermann, D.; Hammer, N.; Pantke, K. Hot profile extrusion of AA-6060 aluminum chips. J. Mater. Process. Technol. 2009, 209, 3343–3350. [Google Scholar] [CrossRef]
- Güley, V.; Ben Khalifa, N.; Tekkaya, A.E. Direct recycling of 1050 aluminium scrap material mixed with 6060 aluminium alloy chips by hot extrusion. Int. J. Mater. Form. 2010, 3, 853–856. [Google Scholar]
- Haase, M.; Ben Khalifa, N.; Tekkaya, E.A.; Misiolek, W.Z. Improving mechanical properties of chip-based aluminum extrudates by integrated extrusion and equal channel angular pressing (iECAP). Mater. Sci. Eng. A 2012, 539, 194–204. [Google Scholar]
- Widerøe, F.; Welo, T.; Vestøl, H. A new testing machine to determine the behaviour of aluminium granulate under combined pressure and shear. Int. J. Mater. Form. 2010, 3, 861–864. [Google Scholar] [CrossRef]
- Güley, V.; Güzel, A.; Jäger, A.; Khalifa, N.B.; Tekkaya, A.E.; Misiolek, W.Z. Effect of die design on the welding quality during solid state recycling of AA6060 chips by hot extrusion. Mater. Sci. Eng. A 2013, 574, 163–175. [Google Scholar]
- Sherafat, Z.; Paydar, M.H.; Ebrahimi, R. Fabrication of Al7075/Al, two phase material, by recycling Al7075 alloy chips using powder metallurgy route. J. Alloy. Compd. 2009, 487, 395–399. [Google Scholar] [CrossRef]
- Wu, S.; Ji, Z.; Zhang, T. Microstructure and mechanical properties of AZ31B magnesium alloy recycled by solid-state process from different size chips. J. Mater. Process. Technol. 2009, 209, 5319–5324. [Google Scholar]
- Luo, P.; Xie, H.; Paladugu, M.; Palanisamy, S.; Dargusch, M.S.; Xia, K. Recycling of titanium machining chips by severe plastic deformation consolidation. J. Mater. Sci. 2010, 45, 4606–4612. [Google Scholar] [CrossRef]
- Stern, M. Method for Treating Aluminum or Aluminum Alloy Scrap. U.S. Patent 2,391,752 A, 25 December 1945. [Google Scholar]
- Paraskevas, D.; Kellens, K.; Renaldi, R.; Dewulf, W.; Duflou, J.R. Resource Efficiency in Manufacturing: Identifying Low Impact Paths. In Proceedings of the 10th Global Conference on Sustainable Manufacturing (GCSM 2012), Istanbul, Turkey, 30 October–2 November 2012; pp. 271–276.
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Groza, J.R. Field Assisted Sintering. In ASM Handbook, Powder Metallurgy Technologies and Applications; ASM International: Materials Park, OH, USA, 1998; Volume 7, pp. 583–589. [Google Scholar]
- Newman, D.C. Novel uses of electric fields and electric currents in powder metal (P/M) processing. Mater. Sci. Eng. A 2000, 287, 198–204. [Google Scholar] [CrossRef]
- Vanmeensel, K.; Sastry, K.Y.; Hennicke, J.; Anné, G.; Jiang, D.; Laptev, A.; Vleugels, J.; van der Biest, O. Microstructure and mechanical properties of spark plasma sintered ZrO2-Al2O3-TiC0.5N0.5 nanocomposites. Solid State Phenom. 2005, 106, 153–160. [Google Scholar] [CrossRef]
- Nygren, M.; Shen, Z. On the preparation of bio-, nano- and structural ceramics and composites by spark plasma sintering. Solid State Sci. 2003, 5, 125–131. [Google Scholar] [CrossRef]
- Olevsky, E.A.; Aleksamdrova, E.V.; Ilyina, A.M.; Dudina, D.V.; Novoselov, A.N.; Pelve, K.Y.; Grigoryev, E.G. Outside mainstream electronic databases: Review of studies conducted in the USSR and post-Soviet countries on electric current-assisted consolidation of powder materials. Materials 2013, 6, 4375–4440. [Google Scholar] [CrossRef]
- Groza, J.R.; Zavaliangos, A. Sintering activation by external electrical field. Mater. Sci. Eng. A 2000, 287, 171–177. [Google Scholar] [CrossRef]
- Vanmeensel, K.; Laptev, A.; Hennicke, J.; Vleugels, J.; van der Biest, O. Modelling of the temperature distribution during field assisted sintering. Acta Mater. 2005, 53, 4379–4388. [Google Scholar] [CrossRef]
- Giuntini, D.; Olensky, E.A.; Garcia-Cardona, C.; Maximenko, A.L.; Yurlova, M.S.; Haines, C.D.; Martin, D.G.; Karpoor, D. Localized overheating phenomena and optimization of spark-plasma sintering tooling design. Materials 2013, 6, 2612–2632. [Google Scholar] [CrossRef]
- Sastry, K.Y.; Froyen, L.; Vleugels, J.; van der Biest, O.; Schattevoy, R.; Hennicke, J. Mechanical milling and field assisted sintering consolidation of nanocrystalline Al-Si-Fe-X powder. Rev. Adv. Mater. Sci. 2004, 8, 27–32. [Google Scholar]
- Kwon, H.; Park, D.H.; Park, Y.; Silvain, J.F.; Kawasaki, A.; Park, Y. Spark plasma sintering behavior of pure aluminum depending on various sintering temperatures. Met. Mater. Int. 2010, 16, 71–75. [Google Scholar] [CrossRef]
- Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio for Advanced Ceramics by Impulse Excitation of Vibration; ASTM Standard C1259-08; ASTM International: West Conshohocken, PA, USA, 2008. [CrossRef]
- Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature; ASTM Standard E9-89a; ASTM International: West Conshohocken, PA, USA, 2008.
- Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio by Impulse Excitation of Vibration; ASTM Standard E1876-09; ASTM International: West Conshohocken, PA, USA, 2009. [CrossRef]
- Xie, G.; Ohashi, O.; Yamaguchi, N.; Song, M.; Mitsuishi, K.; Furuya, K.; Noda, T. Reduction of surface oxide films in Al–Mg alloy powders by pulse electric current sintering. J. Mater. Res. 2004, 19, 815–819. [Google Scholar] [CrossRef]
- Xie, G.; Ohashi, O.; Yamaguchi, N. Sintering behaviour of aluminium powder by spark plasma sintering. Trans. Mater. Res. Soc. Jpn. 2002, 27, 743–746. [Google Scholar]
- Le, G.M.; Godfrey, A.; Hansen, N. Structure and strength of aluminum with sub-micrometer/micrometer grain size prepared by spark plasma sintering. Mater. Des. 2013, 49, 360–367. [Google Scholar] [CrossRef]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Metall. 2002, 45, 322–328. [Google Scholar] [CrossRef]
- Shen, Z.J.; Johnson, M.; Zhao, Z.; Nygren, M. Spark plasma sintering of alumina. J. Am. Ceram. Soc. 2002, 85, 1921–1927. [Google Scholar] [CrossRef]
- Nieh, T.J.; Luo, P.; Nellis, W.; Lesuser, D.; Benson, D. Dynamic compaction of aluminium nanocrystals. Acta Mater. 1996, 44, 3781–3788. [Google Scholar] [CrossRef]
- Risbud, S.H.; Groza, J.R.; Kim, M.J. Clean grain boundaries in AlN ceramics densified without additives by a plasma-activated sintering process. Philos. Mag. 1994, 69, 525–533. [Google Scholar] [CrossRef]
- Granta’s CES EduPack: Teaching Resources for Materials and Process Education. Available online: http://www.grantadesign.com/education/ (accessed on 5 August 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Paraskevas, D.; Vanmeensel, K.; Vleugels, J.; Dewulf, W.; Deng, Y.; Duflou, J.R. Spark Plasma Sintering As a Solid-State Recycling Technique: The Case of Aluminum Alloy Scrap Consolidation. Materials 2014, 7, 5664-5687. https://doi.org/10.3390/ma7085664
Paraskevas D, Vanmeensel K, Vleugels J, Dewulf W, Deng Y, Duflou JR. Spark Plasma Sintering As a Solid-State Recycling Technique: The Case of Aluminum Alloy Scrap Consolidation. Materials. 2014; 7(8):5664-5687. https://doi.org/10.3390/ma7085664
Chicago/Turabian StyleParaskevas, Dimos, Kim Vanmeensel, Jef Vleugels, Wim Dewulf, Yelin Deng, and Joost R. Duflou. 2014. "Spark Plasma Sintering As a Solid-State Recycling Technique: The Case of Aluminum Alloy Scrap Consolidation" Materials 7, no. 8: 5664-5687. https://doi.org/10.3390/ma7085664