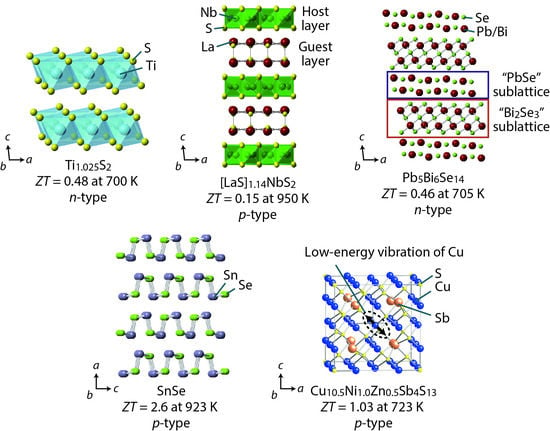
Hierarchical Architecturing for Layered Thermoelectric Sulfides and Chalcogenides
Abstract
:1. Introduction
2. CS2 Sulfurization and Pressurized Sintering


3. TiS2-Based Layered Sulfides

| Sample | Direction | S (μV·K−1) | ρ (μΩ·m) | μ (cm2·V−1·s−1) | S2/ρ (μW·K−2·m−1) | κlat (W·K−1·m−1) | ZT |
|---|---|---|---|---|---|---|---|
| Single crystal | In-plane | −251 | 17 | 15 | 3710 | 6.35 | 0.16 |
| Single crystal | Out-of-plane | - | 13,000 | 0.017 | - | 4.21 | - |
| Polycrystalline | In-plane | −80 | 6.2 | 2.3 | 1030 | 1.5 | 0.12 |
| Polycrystalline | Out-of-plane | −84 | 11 | 1.2 | 630 | 1.3 | 0.10 |


4. Misfit Layered Sulfides [MS]1+m[TS2]n
| Sample | Direction | T (K) | ρ (μΩ·m) | S (μV·K−1) | κtotal (W·K−1·m−1) | κlat (W·K−1·m−1) | S2/ρ (μW·K−2·m−1) | ZT | Reference |
|---|---|---|---|---|---|---|---|---|---|
| (Yb2S2)0.62NbS2 | In-plane | 300 | 19.0 | 60 | 0.80 | 0.41 | 200 | 0.1 | [81] |
| (La2S2)0.62NbS2 | In-plane | 300 | 11.5 | 22 | - | - | 50 | - | [81] |
| (LaS)1.14NbS2 a | In-plane | 300 | 7.6 | 37 | 2.50 | 1.50 | 177 | 0.02 | [32] |
| 950 | 22.0 | 83 | 2.00 | 0.93 | 316 | 0.15 | |||
| Out-of-plane | 300 | 13.3 | 25 | 2.04 | 1.48 | 49 | 0.01 | ||
| 950 | 32.1 | 72 | 1.62 | 0.88 | 162 | 0.09 | |||
| (LaS)1.14NbS2 b | In-plane | 300 | 5.2 | 35 | 4.88 | 3.45 | 233 | 0.02 | [32] |
| 950 | 16.9 | 83 | 3.25 | 1.86 | 405 | 0.15 | |||
| Out-of-plane | 300 | 9.3 | 25 | 1.56 | 0.75 | 70 | 0.01 | ||
| 950 | 28.5 | 56 | 1.34 | 0.52 | 111 | 0.09 | |||
| (LaS)1.2CrS2 a | In-plane | 950 | 207 | −172 | 1.16 | 1.04 | 143 | 0.12 | [32] |
| Out-of-plane | 950 | 223 | −174 | 1.02 | 0.91 | 137 | 0.13 | ||
| (LaS)1.2CrS2 b | In-plane | 950 | 171 | −172 | 1.25 | 1.11 | 174 | 0.13 | [32] |
| Out-of-plane | 950 | 278 | −154 | 0.92 | 0.84 | 84 | 0.08 |

5. Homologous Chalcogenides




| Sample | S (μV·K−1) | ρ (μΩ·m) | n (cm−3) | μ (cm2·V−1·s−1) | S2/ρ (μW·K−2·m−1) | κlat (W·K−1·m−1) | ZT |
|---|---|---|---|---|---|---|---|
| Pb5Bi6Se14 | −28 | 22 | 0.86 × 10−20 | 33 | 36 | 0.72 | 0.01 |
| Pb5Bi12Se23 | −27 | 27 | 1.15 × 10−20 | 20 | 27 | 0.59 | 0.01 |
| Pb5Bi18Se32 | −52 | 39 | 1.19 × 10−20 | 14 | 69 | 0.49 | 0.03 |
6. Accordion-Like Layered SnQ (Q = S, Se)

7. Thermoelectric Minerals
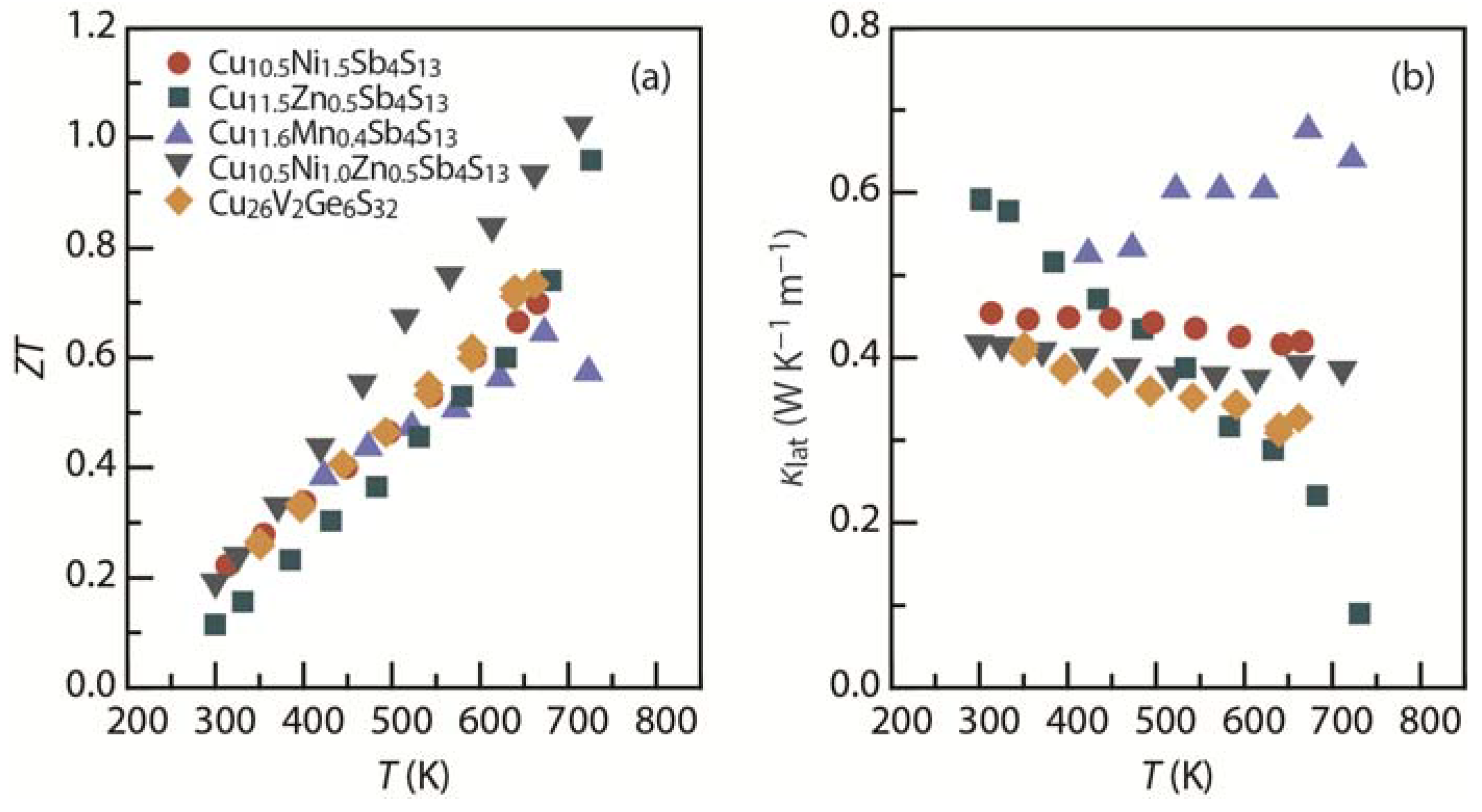
8. Conclusions and Insights
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kajikawa, T. Thermoelectric power generation system recovering industrial waste heat. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 50:1–50:28. [Google Scholar]
- Matsubara, K.; Matsuura, M. A Thermoelectric application to vehicles. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 52:1–52:11. [Google Scholar]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Crane, D.T.; LaGrandeur, J.W. Progress report on BSST-led US department of energy automotive waste heat recovery program. J. Electron. Mater. 2010, 39, 2142–2148. [Google Scholar] [CrossRef]
- Yang, J.; Stabler, F.R. Automotive applications of thermoelectric materials. In Thermoelectrics and Its Energy Harvesting: Modules, Systems, and Applications in Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 25:1–25:15. [Google Scholar]
- Slack, G.A. New materials and performance limits for thermoelectric cooling. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 1997, 56, R12685–R12687. [Google Scholar] [CrossRef]
- Masset, A.C.; Michel, C.; Maignan, A.; Hervieu, M.; Toulemonde, O.; Studer, F.; Raveau, B.; Hejtmanek, J. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys. Rev. B 2000, 62, 166–175. [Google Scholar] [CrossRef]
- Funahashi, R.; Matsubara, I.; Ikuta, H.; Takeuchi, T.; Mizutani, U.; Sodeoka, S. An oxide single crystal with high thermoelectric performance in air. Jpn. J. Appl. Phys. 2000, 39, L1127–L1129. [Google Scholar] [CrossRef]
- Koumoto, K.; Terasaki, I.; Kajitani, T.; Ohtaki, M.; Funahashi, R. Oxide thermoelectrics. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 35:1–35:15. [Google Scholar]
- Koumoto, K.; Terasaki, I.; Funahashi, R. Complex oxide materials for potential thermoelectric applications. MRS Bull. 2006, 31, 206–210. [Google Scholar] [CrossRef]
- Koumoto, K.; Wang, Y.F.; Zhang, R.Z.; Kosuga, A.; Funahashi, R. Oxide thermoelectric materials: A nanostructuring approach. Annu. Rev. Mater. Res. 2010, 40, 363–394. [Google Scholar] [CrossRef]
- Koumoto, K.; Funahashi, R.; Guilmeau, E.; Miyazaki, Y.; Weidenkaff, A.; Wang, Y.F.; Wan, C.L. Thermoelectric ceramics for energy harvesting. J. Am. Ceram. Soc. 2013, 96, 1–23. [Google Scholar] [CrossRef]
- Hébert, S.; Kobayashi, W.; Muguerra, H.; Bréard, Y.; Raghavendra, N.; Gascoin, F.; Guilmeau, E.; Maignan, A. From oxides to selenides and sulfides: The richness of the CdI2 type crystallographic structure for thermoelectric properties. Phys. Status Solidi A 2013, 210, 69–81. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.Q.; Zhang, Q.C.; Wang, G.Y.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Biswas, K.; Lo, S.H.; He, J.Q.; Chung, D.Y.; Dravid, V.P.; Kanatzidis, M.G. Enhancement of thermoelectric figure of merit by the insertion of MgTe nanostructures in p-type PbTe doped with Na2Te. Adv. Energy Mater. 2012, 2, 1117–1123. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.Q.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- He, J.Q.; Kanatzidis, M.G.; Dravid, V.P. High performance bulk thermoelectrics via a panoscopic approach. Mater. Today 2013, 5, 166–176. [Google Scholar] [CrossRef]
- Zhao, L.D.; Wu, H.J.; Hao, S.Q.; Wu, C.I.; Zhou, X.Y.; Biswas, K.; He, J.Q.; Hogan, T.P.; Uher, C.; Wolverton, C.; et al. All-scale hierarchical thermoelectrics: MgTe in PbTe facilitates valence band convergence and suppresses bipolar thermal transport for high performance. Energy Environ. Sci. 2013, 6, 3346–3355. [Google Scholar] [CrossRef]
- Zhao, L.D.; Dravid, V.P.; Kanatzidis, M.G. The panoscopic approach to high performance thermoelectrics. Energy Environ. Sci. 2014, 7, 251–268. [Google Scholar] [CrossRef]
- Wu, H.J.; Zhao, L.D.; Zheng, F.S.; Wu, D.; Pei, Y.L.; Tong, X.; Kanatzidis, M.G.; He, J.Q. Broad temperature plateau for thermoelectric figure of merit ZT > 2 in phase-separated PbTe0.7S0.3. Nat. Commun. 2014, 5, 4515:1–4515:9. [Google Scholar]
- Sales, B.C.; Mandrus, D.; Williams, R.K. Filled skutterudite antimonides: A new class of thermoelectric materials. Science 1996, 272, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Nolas, G.S.; Cohn, J.L.; Slack, G.A.; Schujman, S.B. Semiconducting Ge clathrates: Promising candidates for thermoelectric applications. Appl. Phys. Lett. 1998, 73, 178–180. [Google Scholar] [CrossRef]
- Nolas, G.S.; Morelli, D.T.; Tritt, T.M. Skutterudites: A phonon-glass-electron crystal approach to advanced thermoelectric energy conversion applications. Annu. Rev. Mater. Sci. 1999, 29, 89–116. [Google Scholar] [CrossRef]
- Guo, J.Q.; Geng, H.Y.; Ochi, T.; Suzuki, S.; Kikuchi, M.; Yamaguchi, Y.; Ito, S. Development of skutterudite thermoelectric materials and modules. J. Electron. Mater. 2012, 41, 1036–1042. [Google Scholar] [CrossRef]
- Takabatake, T. Nano-cage structured materials: Clathrates. In Thermoelectric Nanomaterials: Materials Design and Applications; Koumoto, K., Mori, T., Eds.; Springer: Berlin, Germany, 2013; pp. 33–49. [Google Scholar]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Peranio, N.; Bauer, E.; Zehetbauer, M.; Eibl, O. n-type skutterudites (R,Ba,Yb)yCo4Sb12 (R = Sr, La, Mm, DD, SrMm, SrDD) approaching ZT ≈ 2.0. Acta Mater. 2014, 63, 30–43. [Google Scholar] [CrossRef]
- Salvador, J.R.; Cho, J.Y.; Ye, Z.; Moczygemba, J.E.; Thompson, A.J.; Sharp, J.W.; Koenig, J.D.; Maloney, R.; Thompson, T.; Sakamoto, J.; et al. Conversion efficiency of skutterudite-based thermoelectric modules. Phys. Chem. Chem. Phys. 2014, 16, 12510–12520. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Satoh, S.; Kuzuya, T.; Hirai, S.; Kunii, M.; Yamamoto, A. Thermoelectric properties of Ti1+xS2 prepared by CS2 sulfurization. Acta Mater. 2012, 60, 7232–7240. [Google Scholar] [CrossRef]
- Beaumale, M.; Barbier, T.; Bréard, Y.; Guelou, G.; Powell, A.V.; Vaqueiro, P.; Guilmeau, E. Electron doping and phonon scattering in Ti1+xS2 thermoelectric compounds. Acta Mater. 2014, 78, 86–92. [Google Scholar] [CrossRef]
- Meerschaut, A.; Rabu, P.; Rouxel, J. Preparation and characterization of new mixed sandwiched layered compounds Ln32Nb28S88 (Ln = La, Ce). J. Solid State Chem. 1989, 78, 35–45. [Google Scholar] [CrossRef]
- Jood, P.; Ohta, M.; Nishiate, H.; Yamamoto, A.; Lebedev, O.I.; Berthebaud, D.; Suekuni, K.; Kunii, M. Microstructural control and thermoelectric properties of misfit layered sulfides (LaS)1+mTS2 (T = Cr, Nb): The natural superlattice systems. Chem. Mater. 2014, 26, 2684–2692. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wilkinson, A.P.; Lee, P.L.; Shastri, S.D.; Shu, D.; Chung, D.Y.; Kanatzidis, M.G. Determining metal ion distributions using resonant scattering at very high-energy K-edges: Bi/Pb in Pb5Bi6Se14. J. Appl. Crystallogr. 2005, 38, 433–441. [Google Scholar] [CrossRef]
- Ohta, M.; Chung, D.Y.; Kunii, M.; Kanatzidis, M.G. Low lattice thermal conductivity in Pb5Bi6Se14, Pb3Bi2S6, and PbBi2S4: Promising thermoelectric materials in the cannizzarite, lillianite, and galenobismuthite homologous series. J. Mater. Chem. A 2014, 2, 20048–20058. [Google Scholar] [CrossRef]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.S.; Sun, H.; Tan, G.J.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Suekuni, K.; Tsuruta, K.; Kunii, M.; Nishiate, H.; Nishibori, E.; Maki, S.; Ohta, M.; Yamamoto, A.; Koyano, M. High-performance thermoelectric mineral Cu12−xNixSb4S13 tetrahedrite. J. Appl. Phys. 2013, 113, 043712:1–043712:5. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Ozolins, V. Increasing the thermoelectric figure of merit of tetrahedrites by Co-doping with nickel and zinc. Chem. Mater. 2015, 27, 408–413. [Google Scholar] [CrossRef]
- Rimmington, H.P.B.; Balchin, A.A. The growth by iodine vapour transport techniques and the crystal structures of layer compounds in the series TiSxSe2−x, TiSxTe2−x, TiSexTe2−x. J. Cryst. Growth. 1974, 21, 171–181. [Google Scholar] [CrossRef]
- Han, S.H.; Cook, B.A. An experimental search for high ZT semiconductors: A survey of the preparation and properties of several alloy systems. AIP Conf. Proc. 1995, 316, 66–70. [Google Scholar]
- Imai, H.; Shimakawa, Y.; Kubo, Y. Large thermoelectric power factor in TiS2 crystal with nearly stoichiometric composition. Phys. Rev. B 2001, 64, 241104(R):1–241104(R):4. [Google Scholar] [CrossRef]
- Abbott, E.E.; Kolis, J.W.; Lowhorn, N.D.; Sams, W.; Rao, A.; Tritt, T.M. Thermoelectric properties of doped titanium disulfides. Appl. Phys. Lett. 2006, 88, 262106:1–262106:3. [Google Scholar]
- Wan, C.L.; Wang, Y.F.; Wang, N.; Koumoto, K. Low-thermal-conductivity (MS)1+x(TiS2)2 (M = Pb, Bi, Sn) misfit layer compounds for bulk thermoelectric materials. Materials 2010, 3, 2606–2617. [Google Scholar] [CrossRef]
- Wan, C.L.; Wang, Y.F.; Wang, N.; Norimatsu, W.; Kusunoki, M.; Koumoto, K. Intercalation: Building a natural superlattice for better thermoelectric performance in layered chalcogenides. J. Electron. Mater. 2011, 40, 1271–1280. [Google Scholar] [CrossRef]
- Guilmeau, E.; Bréard, Y.; Maignan, A. Transport and thermoelectric properties in Copper intercalated TiS2 chalcogenide. Appl. Phys. Lett. 2011, 99, 052107:1–052107:3. [Google Scholar] [CrossRef]
- Gascoin, F.; Raghavendra, N.; Guilmeau, E.; Bréard, Y. CdI2 structure type as potential thermoelectric materials: Synthesis and high temperature thermoelectric properties of the solid solution TiSxSe2−x. J. Alloy. Compd. 2012, 521, 121–125. [Google Scholar] [CrossRef]
- Beaumale, M.; Barbier, T.; Bréard, Y.; Hébert, S.; Kinemuchi, Y.; Guilmeau, E. Thermoelectric properties in the series Ti1−xTaxS2. J. Appl. Phys. 2014, 115, 043704:1–043704:7. [Google Scholar] [CrossRef]
- Beaumale, M.; Barbier, T.; Bréard, Y.; Raveau, B.; Kinemuchi, Y.; Funahashi, R.; Guilmeau, E. Mass fluctuation effect in Ti1−xNbxS2 bulk compounds. J. Electron. Mater. 2014, 43, 1590–1596. [Google Scholar] [CrossRef]
- Henderson, J.R.; Muramoto, M.; Loh, E.; Gruber, J.B. Electronic structure of rare-earth sesquisulfide crystals. J. Chem. Phys. 1967, 47, 3347–3356. [Google Scholar] [CrossRef]
- Toide, T.; Utsunomiya, T.; Sato, M.; Hoshino, Y.; Hatano, T.; Akimoto, Y. Preparation of lanthanum sulfides using carbon disulfide as sulfurization agent and the change of these sulfides on heating in air. Bull. Tokyo Inst. Technol. 1973, 117, 41–48. [Google Scholar]
- Guittard, M.; Flahaut, J. Preparation of rare earth sulfides and selenides. In Synthesis of Lanthanide and Actinide Compounds (Topics in f-Element Chemistry, Volume 2); Meyer, G., Morss, L.R., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1991; pp. 321–352. [Google Scholar]
- Hirai, S.; Shimakage, K.; Saitou, Y.; Nishimura, T.; Uemura, Y.; Mitomo, M.; Brewer, L. Synthesis and sintering of cerium(III) sulfide powders. J. Am. Ceram. Soc. 1998, 81, 145–151. [Google Scholar] [CrossRef]
- Hirai, S.; Suzuki, K.; Shimakage, K.; Nishimura, T.; Uemura, Y.; Mitomo, M. Preparations of γ-Pr2S3 and γ-Nd2S3 powders by sulfurization of Pr6O11 and Nd2O3 powders using CS2 gas, and their sintering. J. Jpn. Inst. Metals 2003, 67, 15–21. [Google Scholar]
- Miyazaki, Y.; Ogawa, H.; Kajitani, T. Preparation and thermoelectric properties of misfit layered sulfide [Yb1.90S2]0.62NbS2. Jpn. J. Appl. Phys. 2004, 43, L1202–L1204. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O. Thermochemical Properties of Inorganic Substances; Springer-Verlag: Berlin, Germany, 1973. [Google Scholar]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances: Supplement; Springer-Verlag: Berlin, Germany, 1977. [Google Scholar]
- Aoki, T.; Wan, C.L.; Ishiguro, H.; Morimitsu, H.; Koumoto, K. Evaluation of layered TiS2-based thermoelectric elements fabricated by a centrifugal heating technique. J. Ceram. Soc. Jpn. 2011, 119, 382–385. [Google Scholar] [CrossRef]
- Li, D.; Qin, X.Y.; Zhang, J.; Wang, L.; Li, H.J. Enhanced thermoelectric properties of bismuth intercalated compounds BixTiS2. Solid State Commun. 2005, 135, 237–240. [Google Scholar] [CrossRef]
- Li, D.; Qin, X.Y.; Zhang, J.; Li, H.J. Enhanced thermoelectric properties of neodymium intercalated compounds NdxTiS2. Phys. Lett. A 2006, 348, 379–385. [Google Scholar] [CrossRef]
- Li, D.; Qin, X.Y.; Zhang, J. Improved thermoelectric properties of gadolinium intercalated compounds GdxTiS2 at the temperatures from 5 to 310 K. J. Mater. Res. 2006, 21, 480–483. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.Y.; Li, D.; Dong, H.Z. The electrical and thermal conductivity and thermopower of nickel doped compounds (NixTi1−x)1+yS2 at low temperatures. J. Phys. D Appl. Phys. 2006, 39, 1230–1236. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zhang, J.; Li, D.; Dong, H.Z.; Wang, L. The effect of Mg substitution for Ti on transport and thermoelectric properties of TiS2. J. Appl. Phys. 2007, 102, 073703:1–073703:7. [Google Scholar]
- Zhang, J.; Qin, X.Y.; Li, D.; Xin, H.X.; Pan, L.; Zhang, K.X. The transport and thermoelectric properties of Cd doped compounds (CdxTi1−x)1+yS2. J. Alloy. Compd. 2009, 479, 816–820. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.Y.; Xin, H.X.; Li, D.; Song, C.J. Thermoelectric properties of Co-doped TiS2. J. Electron. Mater. 2011, 40, 980–986. [Google Scholar] [CrossRef]
- Wan, C.L.; Wang, Y.F.; Norimatsu, W.; Kusunoki, M.; Koumoto, K. Nanoscale stacking faults induced low thermal conductivity in thermoelectric layered metal sulfides. Appl. Phys. Lett. 2012, 100, 101913:1–101913:4. [Google Scholar]
- Putri, Y.E.; Wan, C.L.; Wang, Y.F.; Norimatsu, W.; Kusunoki, M.; Koumoto, K. Effects of alkaline earth doping on the thermoelectric properties of misfit layer sulfides. Scr. Mater. 2012, 66, 895–898. [Google Scholar] [CrossRef]
- Putri, Y.E.; Wan, C.L.; Dang, F.; Mori, T.; Ozawa, Y.; Norimatsu, W.; Kusunoki, M.; Koumoto, K. Effects of transition metal substitution on the thermoelectric properties of metallic (BiS)1.2(TiS2)2 misfit layer sulfide. J. Electron. Mater. 2014, 43, 1870–1874. [Google Scholar] [CrossRef]
- Takeuchi, S.; Katsuta, H. Characteristics of nonstoichiometry and lattice defects of the TiS2 phase. J. Jpn. Inst. Metals 1970, 34, 758–763. [Google Scholar]
- Thompson, A.H.; Gamble, F.R.; Symon, C.R. The verification of the existence of TiS2. Mater. Res. Bull. 1975, 10, 915–919. [Google Scholar] [CrossRef]
- Murray, J.L. The S-Ti (Sulfur-Titanium) system. J. Phase Equilib. 1986, 7, 156–163. [Google Scholar]
- Kobayashi, H.; Sakashita, K.; Sato, M.; Nozue, T.; Suzuki, T.; Kamimura, T. Electronic specific heat of Ti1+xS2 (0 < x < 0.1). Physica B 1997, 237–238, 169–171. [Google Scholar] [CrossRef]
- Cutler, M.; Leavy, J.F.; Fitzpatrick, R.L. Electronic transport in semimetallic cerium sulfide. Phys. Rev. 1964, 133, A1143–A1152. [Google Scholar] [CrossRef]
- Snyder, G.J.; Tobere, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- May, A.F.; Fleurial, J.-P.; Snyder, G.J. Thermoelectric performance of lanthanum telluride produced via mechanical alloying. Phys. Rev. B 2008, 78, 125205:1–125205:12. [Google Scholar] [CrossRef]
- Logothetis, E.M.; Kaiser, W.J.; Kukkonen, C.A.; Faile, S.P.; Colella, R.; Gambold, J. Transport properties and the semiconducting nature of TiS2. Physica B+C 1980, 99, 193–198. [Google Scholar] [CrossRef]
- Kukkonen, C.A.; Kaiser, W.J.; Logothetis, E.M.; Blumenstock, B.J.; Schroeder, P.A.; Faile, S.P.; Colella, R.; Gambold, J. Transport and optical properties of Ti1+xS2. Phys. Rev. B 1981, 24, 1691–1709. [Google Scholar] [CrossRef]
- Koyano, M.; Negishi, H.; Ueda, Y.; Sasaki, M.; Inoue, M. Electrical resistivity and thermoelectric power of intercalation compounds MxTiS2 (M = Mn, Fe, Co, and Ni). Phys. Status Solidi B 1986, 138, 357–363. [Google Scholar] [CrossRef]
- Amara, A.; Frongillo, Y.; Aubin, M.J.; Jandl, S.; Lopez-Castillo, J.M.; Jay-Gerin, J.-P. Thermoelectric power of TiS2. Phys. Rev. B 1987, 36, 6415–6419. [Google Scholar] [CrossRef]
- Wiegers, G.A. Misfit layer compounds: Structures and physical properties. Prog. Solid State Chem. 1996, 24, 1–139. [Google Scholar] [CrossRef]
- Van Smaalen, S. Superspace-group approach to the modulated structure of the inorganic misfit layer compound (LaS)1.14NbS2. J. Phys. Condens. Matter 1991, 3, 1247–1263. [Google Scholar] [CrossRef]
- Wiegers, G.A.; Meetsma, A.; Haange, R.J.; van Smaalen, S.; de Boer, J.L.; Meerschaut, A.; Rabu, P.; Rouxel, J. The incommensurate misfit layer structure of (PbS)1.14NbS2, “PbNbS3” and (LaS)1.14NbS2, “LaNbS3”: An X-ray diffraction study. Acta Crystallogr. B 1990, 46, 324–332. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Ogawa, H.; Nakajo, T.; Kikuchii, Y.; Hayashi, K. Crystal structure and thermoelectric properties of misfit-layered sulfides [Ln2S2]pNbS2 (Ln = Lanthanides). J. Electron. Mater. 2013, 42, 1335–1339. [Google Scholar] [CrossRef]
- Terashima, T.; Kojima, N. Electrical transport properties of incommensurate layer compounds (RES)xNbS2 (RE = rare-earth metals; x = 1.2, 0.6). J. Phys. Soc. Jpn. 1994, 63, 658–673. [Google Scholar] [CrossRef]
- Suzuki, K.; Enoki, T.; Imaeda, K. Synthesis, characterization and physical properties of incommensurate layered compounds/(RES)xTaS2 (RE = rare earth metal). Solid State Commun. 1991, 78, 73–77. [Google Scholar] [CrossRef]
- Suzuki, K.; Enoki, T.; Bandow, S. Electronic properties and valence state of Sm in (SmS)1.19TaS2. Phys. Rev. B 1993, 48, 11077–11085. [Google Scholar] [CrossRef]
- Cho, N.; Kikkawa, S.; Kanamaru, F.; Takeda, Y.; Yamamoto, O.; Kido, H.; Hoshikawa, T. Crystal structural, electric and magnetic studies on the misfit layer compounds “LnMS3” (Ln = rare-earth metal; M = Ti, V, Cr). Solid State Ion. 1993, 63–65, 696–701. [Google Scholar] [CrossRef]
- Kato, K.; Kawada, I.; Takahashi, T. Die Kristallstruktur von LaCrS3. Acta Crystallogr. B 1977, 33, 3437–3443. [Google Scholar] [CrossRef]
- Wiegers, G.A.; Meerschaut, A. Structures of misfit layer compounds (MS)nTS2 (M = Sn, Pb, Bi, rare earth metals; T = Nb, Ta, Ti, V, Cr; 1.08 < n < 1.23). J. Alloy. Compd. 1992, 178, 351–368. [Google Scholar] [CrossRef]
- Fang, C.M.; van Smaalen, S.; Wiegers, G.A.; Haas, C.; de Groot, R.A. Electronic structure of the misfit layer compound (LaS)1.14NbS2: Band-structure calculations and photoelectron spectra. J. Phys. Condens. Matter 1996, 8, 5367–5382. [Google Scholar] [CrossRef]
- Fang, C.M.; Ettema, A.R.H.; Haas, C.; Wiegers, G.A.; van Leuken, H.; de Groot, R.A. Electronic structure of the misfit-layer compound (SnS)1.17NbS2 deduced from band-structure calculations and photoelectron spectra. Phys. Rev. B 1995, 52, 2336–2347. [Google Scholar] [CrossRef]
- Fang, C.M.; de Groot, R.A.; Wiegers, G.A.; Haas, C. Electronic structure of the misfit layer compound (SnS)1.20TiS2: Band structure calculations and photoelectron spectra. J. Phys. Condens. Matter 1996, 8, 1663–1676. [Google Scholar] [CrossRef]
- Ohta, M.; Hirai, S.; Ma, Z.; Nishimura, T.; Uemura, Y.; Shimakage, K. Phase transformation and microstructures of Ln2S3 (Ln = La, Sm) with different impurities content of oxygen and carbon. J. Alloy. Compd. 2006, 408–412, 551–555. [Google Scholar] [CrossRef]
- Makovicky, E. The building principles and classification of bismuth-lead sulphosalts and related compounds. Fortschr. Mineral. 1981, 59, 137–190. [Google Scholar]
- Mrotzek, A.; Kanatzidis, M.G. “Design” in solid-state chemistry based on phase homologies. The concept of structural evolution and the new megaseries Am[M1+lSe2+l]2m[M2l+nSe2+3l+n]. Acc. Chem. Res. 2003, 36, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kanatzidis, M.G. New bulk materials for thermoelectric applications: Synthetic strategies based on phase homologies. In Chemistry, Physics, and Materials Science of Thermoelectric Materials; Kanatzidis, M.G., Mahanti, S.D., Hogan, T.P., Eds.; Kluwer Acadmeic/Plenum Publishers: New York, NY, USA, 2003; pp. 35–54. [Google Scholar]
- Kanatzidis, M.G. Structural evolution and phase homologies for “design” and prediction of solid-state compounds. Acc. Chem. Res. 2005, 38, 361–370. [Google Scholar] [CrossRef]
- Makovicky, E. Crystal structures of sulfides and other chalcogenides. Rev. Mineral. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Segawa, K.; Taskin, A.A.; Ando, Y. Pb5Bi24Se41: A new member of the homologous series forming topological insulator heterostructures. J. Solid State Chem. 2015, 221, 196–201. [Google Scholar] [CrossRef]
- Chung, D.-Y.; Choi, K.-S.; Iordanidis, L.; Schindler, J.L.; Brazis, P.W.; Kannewurf, C.R.; Chen, B.; Hu, S.; Uher, C.; Kanatzidis, M.G. High thermopower and low thermal conductivity in semiconducting ternary K-Bi-Se compounds. Synthesis and properties of β-K2Bi8Se13 and K2.5Bi8.5Se14 and their Sb analogues. Chem. Mater. 1997, 9, 3060–3071. [Google Scholar] [CrossRef]
- Chung, D.Y.; Hogan, T.; Brazis, P.; Rocci-Lane, M.; Kannewurf, C.; Bastea, M.; Uher, C.; Kanatzidis, M.G. CsBi4Te6: A high-performance thermoelectric material for low-temperature applications. Science 2000, 287, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, L.A.; Kuznetsov, V.L.; Rowe, D.M. Thermoelectric properties and crystal structure of ternary compounds in the Ge(Sn,Pb)Te–Bi2Te3 systems. J. Phys. Chem. Solids 2000, 61, 1269–1274. [Google Scholar] [CrossRef]
- Caillat, T.; Huang, C.K.; Fleurial, J.-P.; Snyder, G.J.; Borshchevsky, A. Synthesis and thermoelectric properties of some materials with the PbBi4Te7 crystal structure. In Proceedings of the 19th International Conference on Thermoelectrics, Cardiff, UK, 20–24 August 2000; Rowe, D.M., Ed.; Babrow Press: Wales, UK, 2000; pp. 151–154. [Google Scholar]
- Chung, D.Y.; Hogan, T.P.; Rocci-Lane, M.; Brazis, P.; Ireland, J.R.; Kannewurf, C.R.; Bastea, M.; Uher, C.; Kanatzidis, M.G. A new thermoelectric material: CsBi4Te6. J. Am. Chem. Soc. 2004, 126, 6414–6428. [Google Scholar] [CrossRef] [PubMed]
- Shelimova, L.E.; Karpinskii, O.G.; Konstantinov, P.P.; Avilov, E.S.; Kretova, M.A.; Lubman, G.U.; Nikhezina, I.Yu.; Zemskov, V.S. Composition and properties of compounds in the PbSe-Bi2Se3 system. Inorg. Mater. 2010, 46, 120–126. [Google Scholar] [CrossRef]
- Zemskov, V.S.; Shelimova, L.E.; Konstantinov, P.P.; Avilov, E.S.; Kretova, M.A.; Nikhezina, I.Y. Thermoelectric materials with low heat conductivity based on PbSe-Bi2Se3 compounds. Inorg. Mater. Appl. Res. 2011, 2, 405–413. [Google Scholar] [CrossRef]
- Zemskov, V.S.; Shelimova, L.E.; Konstantinov, P.P.; Avilov, E.S.; Kretova, M.A.; Nikhezina, I.Yu. Thermoelectric materials based on layered chalcogenides of bismuth and lead. Inorg. Mater. Appl. Res. 2012, 3, 61–68. [Google Scholar] [CrossRef]
- Kuropatwa, B.A.; Kleinke, H. Thermoelectric properties of stoichiometric compounds in the (SnTe)x(Bi2Te3)y system. Z. Anorg. Allg. Chem. 2012, 638, 2640–2647. [Google Scholar] [CrossRef]
- Kuropatwa, B.A.; Assoud, A.; Kleinke, H. Effects of cation site substitutions on the thermoelectric performance of layered SnBi2Te4 utilizing the triel elements Ga, In, and Tl. Z. Anorg. Allg. Chem. 2013, 639, 2411–2420. [Google Scholar] [CrossRef]
- Medlin, D.L.; Snyder, G.J. Atomic-scale interfacial structure in rock salt and tetradymite chalcogenide thermoelectric materials. JOM 2013, 65, 390–400. [Google Scholar] [CrossRef]
- Olvera, A.; Shi, G.G.; Djieutedjeu, H.; Page, A.; Uher, C.; Kioupakis, E.; Poudeu, P.F.P. Pb7Bi4Se13: A lillianite homologue with promising thermoelectric properties. Inorg. Chem. 2015, 54, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl phases for thermoelectric devices. Dalton Trans. 2007, 2099–2107. [Google Scholar] [CrossRef]
- Lefebvre, I.; Szymanski, M.A.; Olivier-Fourcade, J.; Jumas, J.C. Electronic structure of tin monochalcogenides from SnO to SnTe. Phys. Rev. B 1998, 58, 1896–1906. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Pannetier, J.; von Schnering, H.G. Neutron diffraction study of the structural phase transition in SnS and SnSe. J. Phys. Chem. Solids 1986, 47, 879–885. [Google Scholar] [CrossRef]
- Peters, M.J.; McNeil, L.E. High-pressure Mössbauer study of SnSe. Phys. Rev. B 1990, 41, 5893–5897. [Google Scholar] [CrossRef]
- Fadavieslam, M.R.; Shahtahmasebi, N.; Rezaee-Roknabadi, M.; Bagheri-Mohagheghi, M.M. A study of the photoconductivity and thermoelectric properties of SnxSy optical semiconductor thin films deposited by the spray pyrolysis technique. Phys. Scr. 2011, 84, 035705:1–035705:8. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.F. Thermoelectric properties of Sn-S bulk materials prepared by mechanical alloying and spark plasma sintering. J. Electron. Mater. 2014, 43, 2435–2439. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, L.D.; Li, J.F.; Wu, C.F.; Wei, T.R.; Xing, Z.B.; Kanatzidis, M.G. Thermoelectrics with earth abundant elements: Low thermal conductivity and high thermopower in doped SnS. J. Mater. Chem. A 2014, 2, 17302–17306. [Google Scholar] [CrossRef]
- Sassi, S.; Candolfi, C.; Vaney, J.-B.; Ohorodniichuk, V.; Masschelein, P.; Dauscher, A.; Lenoir, B. Assessment of the thermoelectric performance of polycrystalline p-type SnSe. Appl. Phys. Lett. 2014, 104, 212105:1–212105:4. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, H.; Chen, Y.Y.; Day, T.; Snyder, G.J. Thermoelectric properties of p-type polycrystalline SnSe doped with Ag. J. Mater. Chem. A 2014, 2, 11171–11176. [Google Scholar] [CrossRef]
- Carrete, J.; Mingo, N.; Curtarolo, S. Low thermal conductivity and triaxial phononic anisotropy of SnSe. Appl. Phys. Lett. 2014, 105, 101907:1–101907:4. [Google Scholar] [CrossRef]
- Wasscher, J.D.; Albers, W.; Haas, C. Simple evaluation of the maximum thermoelectric figure of merit, with application to mixed crystals SnS1-xSex. Solid-State Electron. 1963, 6, 261–264. [Google Scholar] [CrossRef]
- Chen, S.; Cai, K.; Zhao, W. The effect of Te doping on the electronic structure and thermoelectric properties of SnSe. Physica B 2012, 407, 4154–4159. [Google Scholar] [CrossRef]
- Parker, D.; Singh, D.J. First Principles investigations of the thermoelectric behavior of tin sulfide. J. Appl. Phys. 2010, 108, 083712:1–083712:3. [Google Scholar] [CrossRef]
- Bera, C.; Jacob, S.; Opahle, I.; Gunda, N.S.H.; Chmielowski, R.; Dennler, G.; Madsen, G.K.H. Integrated computational materials discovery of silver doped tin sulfide as a thermoelectric material. Phys. Chem. Chem. Phys. 2014, 16, 19894–19899. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Morelli, D.T.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.Y.; Uher, C. High performance thermoelectricity in earth-abundant compounds based on natural mineral tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Chetty, R.; Kumar, D.S.P.; Rogl, G.; Rogl, P.; Bauer, E.; Michor, H.; Suwas, S.; Puchegger, S.; Giester, G.; Mallik, R.C.; et al. Thermoelectric properties of a Mn substituted synthetic tetrahedrite. Phys. Chem. Chem. Phys. 2015, 17, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Suekuni, K.; Kim, F.S.; Nishiate, H.; Ohta, M.; Tanaka, H.I.; Takabatake, T. High-performance thermoelectric minerals: Colusites Cu26V2M6S32 (M = Ge, Sn). Appl. Phys. Lett. 2014, 105, 132107:1–132107:4. [Google Scholar] [CrossRef]
- Suekuni, K.; Tsuruta, K.; Ariga, T.; Koyano, M. Thermoelectric properties of mineral tetrahedrites Cu10Tr2Sb4S13 with low thermal conductivity. Appl. Phys. Express 2012, 5, 051201:1–051201:3. [Google Scholar] [CrossRef]
- Tsujii, N.; Mori, T. Stability and thermoelectric property of Cu9Fe9S16: Sulfide mineral as a promising thermoelectric material. MRS Proc. 2014, 1680. [Google Scholar] [CrossRef]
- Barbier, T.; Lemoine, P.; Gascoin, S.; Lebedev, O.I.; Kaltzoglou, A.; Vaqueiro, P.; Powell, A.V.; Smith, R.I.; Guilmeau, E. Structural stability of the synthetic thermoelectric ternary and nickel-substituted tetrahedrite phases. J. Alloy. Compd. 2015, 634, 253–262. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jood, P.; Ohta, M. Hierarchical Architecturing for Layered Thermoelectric Sulfides and Chalcogenides. Materials 2015, 8, 1124-1149. https://doi.org/10.3390/ma8031124
Jood P, Ohta M. Hierarchical Architecturing for Layered Thermoelectric Sulfides and Chalcogenides. Materials. 2015; 8(3):1124-1149. https://doi.org/10.3390/ma8031124
Chicago/Turabian StyleJood, Priyanka, and Michihiro Ohta. 2015. "Hierarchical Architecturing for Layered Thermoelectric Sulfides and Chalcogenides" Materials 8, no. 3: 1124-1149. https://doi.org/10.3390/ma8031124