Towards Lead-Free Piezoceramics: Facing a Synthesis Challenge
Abstract
:1. Introduction
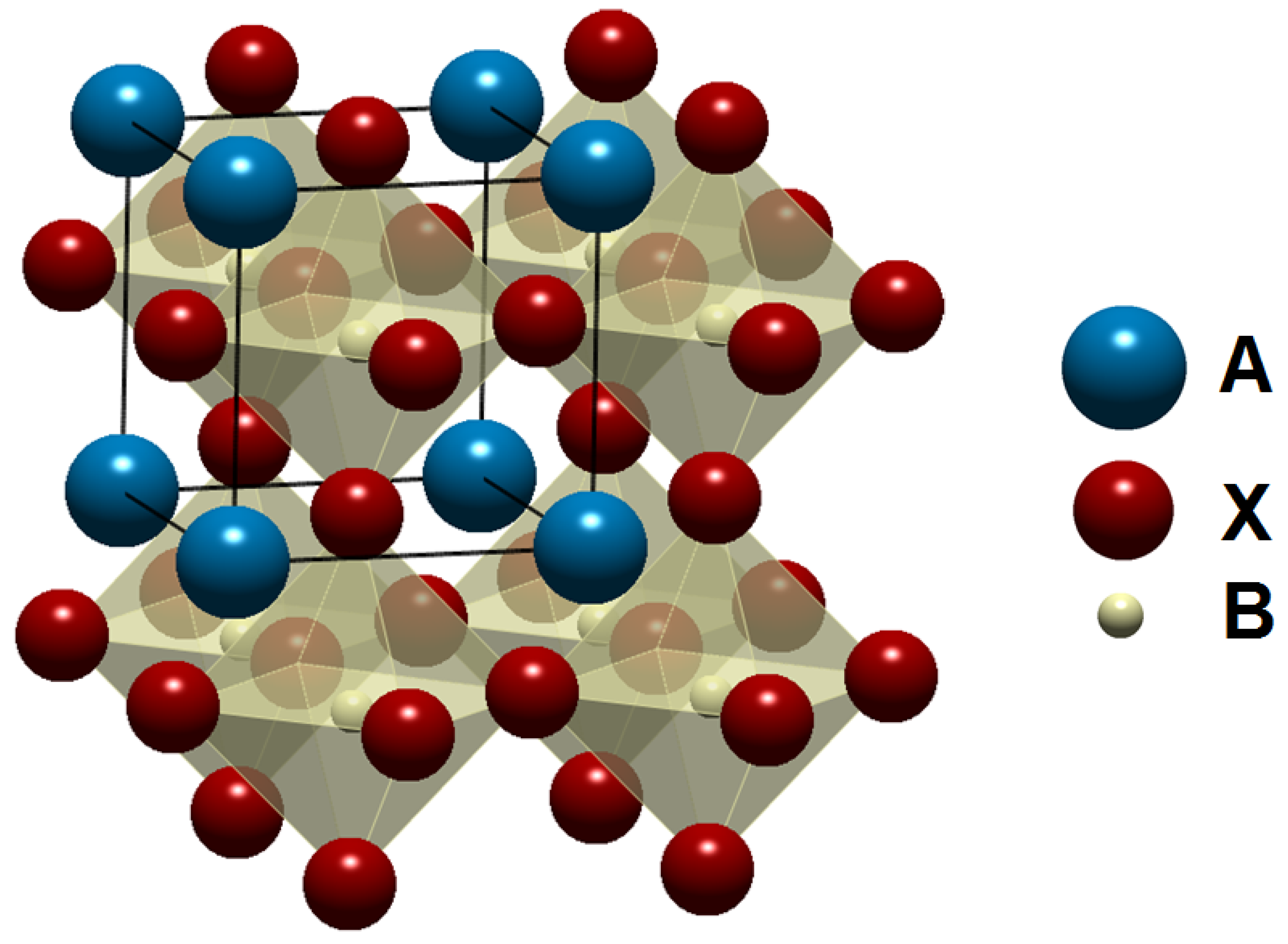
2. BaTiO3 (BT)



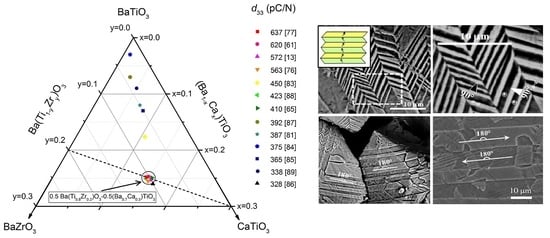
3. BaTiO3-BaZrO3-CaTiO3 (BCZT)

| Composition (%) | Synthesis Method | Synthesis (T (t)) (°C (h)) | Sintering (T (t)) (°C (h)) | Grain Size (μm) | εT33/tan δ (%) (at RT) | TC (°C) | d33/kp (pCN−1/%) | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Ba(Ti0.8Zr0.2)O3-x(Ba0.7Ca0.3)TiO3 | x = 0.00−1.00 | Solid State | 1350 (–) | 1450–1500 (–) | – | 2500–3060/– | 20–80 | 200–620/– | [61] |
| Ba(Zr0.15Ti0.85)O3-x(Ba0.8Ca0.2)TiO3 | x = 0.00−1.00 | Solid State | 1300 (2) | 1450 (3) | 10–30 | 1000–2250/– | 70–120 | 450–600/– | [83] |
| (Ba1−xCax)(Ti0.98Zr0.02)O3 | x = 0.00−0.04 | Solid State | 1200 (4) | 1450 (4) | – | 1100–2200/– | 110–120 | 270–375/44–32 | [84] |
| (Ba1−xCax)(Ti0.95Zr0.05)O3 | x = 0.02−0.20 | Solid State | 1200 (4) | 1450 (4) | – | 1800–2900/– | 100–110 | 200–365/32–48 | [85] |
| (Ba1−xCax)(Ti0.90Zr0.10)O3 | x = 0.12−0.18 | Solid State | 1200 (4) | 1450 (4) | 5–15 | 3000–4800/– | 60–70 | 240–328/30–38 | [86] |
| (Ba0.93Ca0.07)(Ti0.95Zr0.05)O3 | – | Solid State | 1200 (4) | 1300–1500 (4) | 10 | 1000–2300/– | 105–115 | 142–387/26–44 | [81] |
| (Ba1−xCax)(Ti0.04Zr0.96)O3 | x = 0.00−0.09 | Solid State | 1200 (4) | 1300, 1400 (4) | 24–39 | 1700–2000/2 | 123–126 | 158–392/– | [87] |
| (Ba0.85Ca0.15)(Ti1−xZrx)O3 | x =0.00−0.20 | Solid State | 1200 (3) | 1500 (2) | 10–30 | 2000–3000/1.5 | 70–130 | 50–423/20–49 | [88] |
| (Ba1−xCax)(Zr0.1Ti0.9)O3 | 0.00 ≤ x ≤ 0.20 | Solid State | 1200 (6) | 1400–1420 (4) | – | 1000–3000/– | 80–90 | 160–550/30–58 | [13] |
| (Ba0.85Ca0.15)(ZryTi1−y)O3 | 0.00 ≤ y ≤ 0.15 | Solid State | 1200 (6) | 1400–1420 (4) | – | 1000–4000/– | 60–120 | 140–580/35–58 | [13] |
| (Ba0.95Ca0.05)(ZryTi1−y)O3 | 0.00 ≤ y ≤ 0.15 | Solid State | 1200 (6) | 1350 (2) | 9–14 | 1200–2000/1–3 | 60–125 | 175–340/16–35 | [89] |
| Ba1−xCaxTi0.9Zr0.1O3 | x = 0.10, 0.15 | Solid State | 1250 (2) | 1300–1400 (2) | 0.5–10 | 2800–3400/1.2 | 75–80 | 175–410/22–50 | [65] |
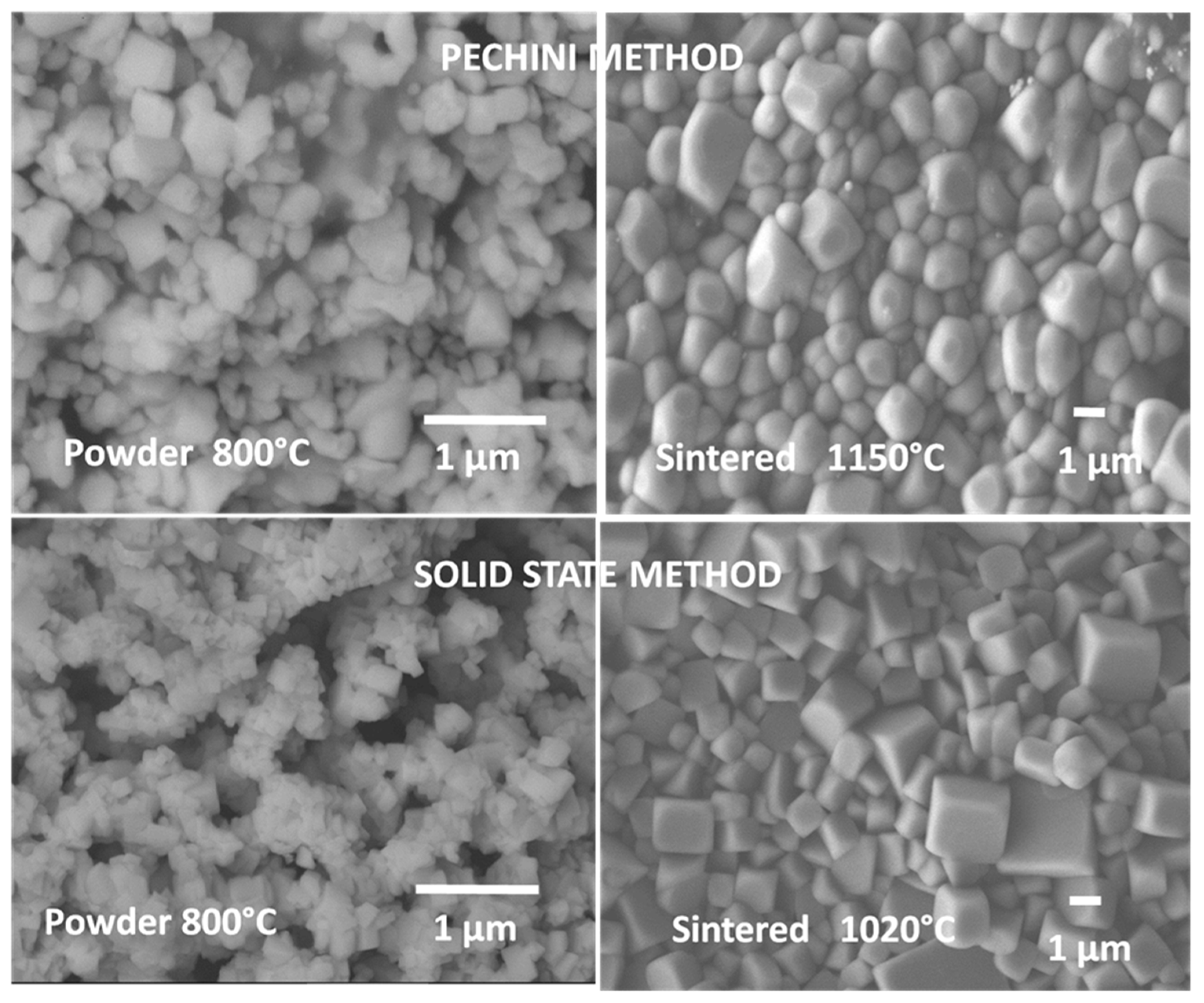
| (Ba0.95Ca0.05)(Ti0.85Zr0.15)O3 | – | Pechini | 700 (4) | 1100–1300 (2) | – | – | – | – | [15] |
| Ba0.9Ca0.1Ti0.9Zr0.1O3 | – | Pechini | 700 (1) | 1200–1275 (5) | 0.4–15.0 | 2200–2500/1.2 | 115 | 45, 240, 390/5, 49, 50 | [16] |
| Ba0.85Ca0.15Ti0.9Zr0.1O3 | – | Sol-gel | 800 (5) | 1200–1500 (10) | 0.5–32.0 | 800–2900/1–5 | 85–902 | 21–563/– | [76] |
| (1−w)Ba(Ti0.80Zr0.20)O3−w(Ba0.70 Ca0.30) TiO3 | 0.48≤ w ≤ 0.52 | Sol-gel | 600–1000 (4) | 1200–1500 (2) | 14–20 | – | – | 400–637 | [77] |
4. (Bi0.5Na0.5)TiO3-BaTiO3 (BNT-BT)
| Composition (%) | Synthesis Method | Synthesis (T (t)) (°C (h)) | Sintering (T (t)) (°C (h)) | Grain Size (μm) | εT33/tanδ (%) (at RT) | TC (°C) | d33/kp (pCN−1/%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Bi0.5Na0.5TiO3 | Solid State | 800 (5) | 1100 (5) | 4.1 | 425/3.2 | – | 94.8/– | [100] |
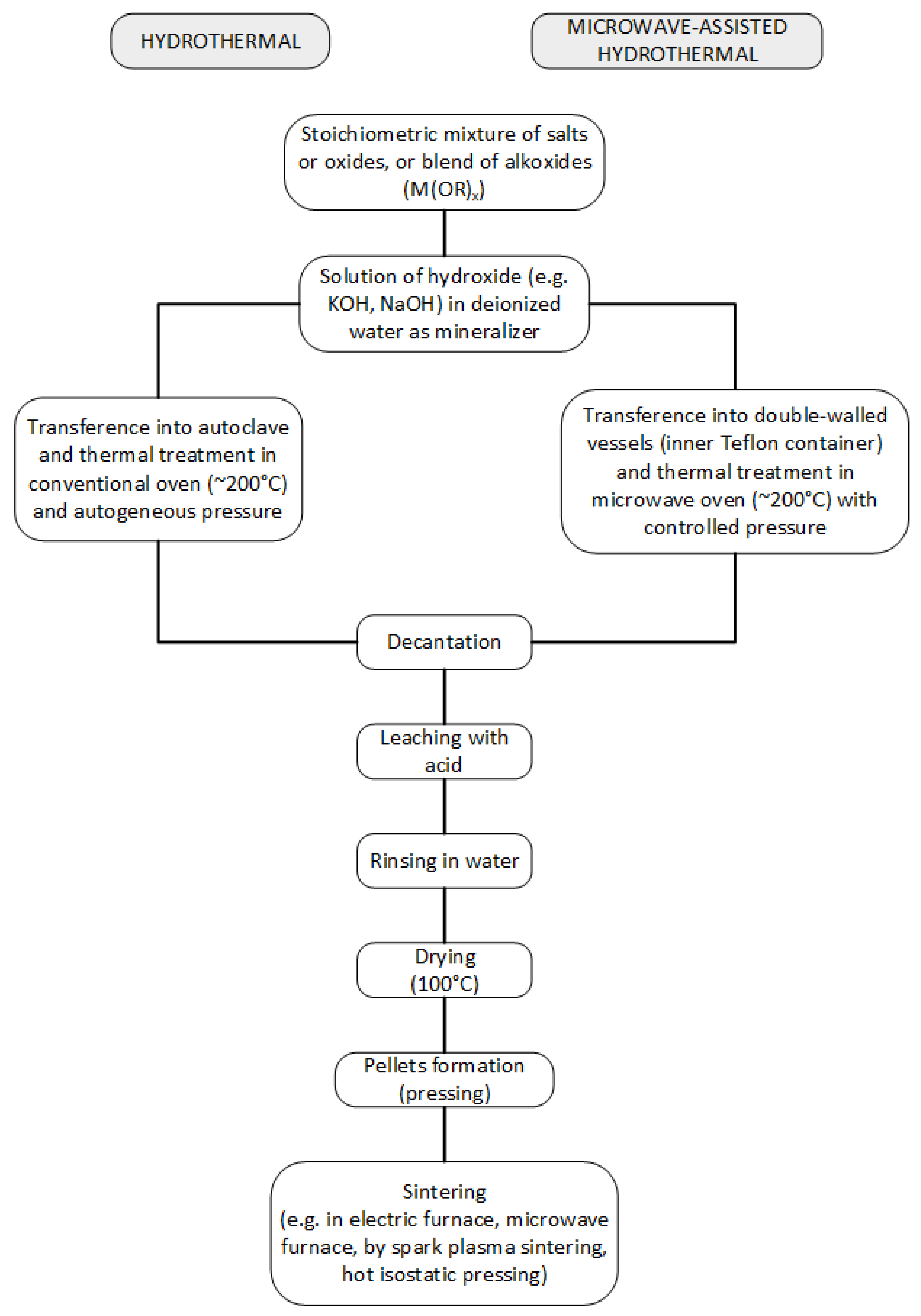
| Bi0.5Na0.5TiO3 | Hydrothermal | 700 (4) | 1010 (2) | 8.6 | 324/2.14 | – | 77.3/15.9 | [109] |
| Bi0.5Na0.5TiO3 | Hydrothermal | 200 (12) | – | 2 | – | – | – | [102] |
| Bi0.5Na0.5TiO3 | Hydrothermal | 200 (72) | – | 0.04–0.15 | – | – | – | [101] |
| Bi0.5Na0.5TiO3 | Microwave-assisted hydrothermal | 180 (8) | – | 0.04–0.15 | – | – | – | [101] |
| Bi0.5Na0.5TiO3 | Hydrothermal | 150–200 (5–20) | – | 0.05–0.2 | – | – | – | [103] |
| Bi0.5Na0.5TiO3 | Sol-gel | 600–850 (2) | 1000–1200 (2) | <0.2 | – | – | – | [104] |
| (Bi0.5Na0.5)0.95Ba0.05TiO3 | Citrate method | 700 (4) | 1050 (–) | 1 | – | – | – | [105] |
| (Bi0.5Na0.5)0.05Ba0.95TiO3 | Solid State | 850 (4) | 1200 (2) | – | 2211/17.5 | 126 | 78/– | [110] |
| (Bi0.5Na0.5)0.93Ba0.07TiO3 | Solid State | 850 (4) | 1150–1250 (2–4) | 1–2 | –/– | 246 | 148/15 | [111] |
| (Bi0.5Na0.5)0.93Ba0.07TiO3 | Citrate method | 600 (1) | 1150 (2) | – | –/– | – | 176/21.2 | [112] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 | Solid State | 800 (1) | 1200 (2) | – | 580/1.3 | 288 | 125/19 | [91] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 | Solid State | 1000 (2) | 1160–1200 (2) | – | 776/2.5 | – | 117/28 | [12] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 | Combustion | 500 (few mins) | 1100 (2) | – | 698/– | – | 125/27.2 | [106] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 | Emulsion | 700 (3) | 1200 (3) | 1–2.5 | –/– | – | 174/28 | [108] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 | Mechanosynthesis | 800–900 (2) | 1160–1180 (1) | – | –/1.7 | – | 122/29 | [92] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 | Citrate method | 600(1) | 1150 (2) | – | 600/5 | – | 180/28 | [113] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 + 3 mol % Bi2O3 | Solid State | 800( 2) | 1150–1200 (2) | – | –/2.65 | – | 165/24 | [114] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 + 0.25 at % Eu | Combustion | 600 (few mins) | 1150 (4) | – | 1658/– | – | 149/5.5 | [115] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 + La | Solid State | 1000 (2) | 1160–1200 (2) | – | 1576/4.5 | – | 125/24 | [12] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 + Co | Solid State | 1000 (2) | 1160–1200 (2) | – | 1200/2.3 | – | 139/27 | [12] |
| (Bi0.5Na0.5)0.94Ba0.06TiO3 + 0.8 wt % Nd2O3 | Solid State | 800 (–) | 1150 (2) | 2 | 1947/5.7 | – | 175/31 | [116] |
| (Bi0.5Na0.5)0.91(Ba0.07Ca0.03)0.09TiO3 | Solid State | 850 (3) | 1170 (2) | – | –/– | – | 125/33 | [98] |
| (Bi0.47Na0.47Ba0.06TiO3)0.99-(Ba0.77Ca0.23TiO3)0.01 | Solid State | 850 (2) | 1100–1200 (2) | – | –/– | – | 178/27 | [117] |
5. (K0.5Na0.5)NbO3 (KNN)
| Composition | Synthesis Method | Synthesis (T (t)) (°C (h)) | Sintering (T (t)) (°C (h)) | Grain Size/μm | εT33/tan δ (%) (at RT) | TC/°C | d33/kp pC/N/% | Reference |
|---|---|---|---|---|---|---|---|---|
| (K0.5Na0.5)NbO3 | Sol-gel | 650 (4) | 1115 (2) | 10 | 450/6 | 405 | 80/– | [138] |
| (K0.5Na0.5)NbO3 | Solid state | 825 (4) | 1115 (2) | 7 | 925/0.1 | 395 | –/– | [139] |
| (K0.5Na0.5)NbO3 | Spray drying | 800 (1) | 1080 (2) | 10 | 380/2 | 410 | –/36 | [132] |
| (K0.5Na0.5)NbO3 | Solid state | – | – | – | 400/0.3 | 395 | 140/39 | [6] |
| (K0.5Na0.5)NbO3 | Solid state | 850 (2) | 1160 (2) | – | 496/1.5 | 420 | 127/46 | [120] |
| (K0.5Na0.5)NbO3 | Solid state | 850 (10) | 1110 (-) | – | 450/– | 420 | 95/28 | [128] |
| (K0.5Na0.5)NbO3 | Sol-gel | 500 (5) | 925 (2) | 2 | 200/4.8 | 405 | 112/20 | [140] |
| (K0.5Na0.5)NbO3 | Combustion | 450 (6) | 1120 (2) | 1.45 | 490/2.8 | 420 | 84/– | [141] |
| (K0.5Na0.5)NbO3 | Sol-gel | 650 (2) | 1110 (3) | 5 | 712/6.7 | 405 | 90/32 | [142] |
| (K0.5Na0.5)NbO3 | Mechanochemical asisted | 550 (2) | 1100 (2) | 0.3 | 680/3.5 | 420 | 95/– | [143] |
| (K0.5Na0.5)NbO3 | Microwave-assisted | 550 (2) | 1115 (2) | 3.8 | 427/3.5 | 398 | 85/– | [144] |
| (K0.5Na0.5)NbO3 | Hydrothermal | 230 (24) | 1000 (2) | 2 | –/– | – | 78/– | [145] |
| (K0.5Na0.5)0.9Li0.1NbO3 | Sol-gel | 500 (5) | 975 (2) | 2 | 400/2.8 | 485 | 170/40 | [140] |
| (K0.5Na0.5)Nb0.7Ta0.3O3 | Hydrothermal | 230 (24) | 1000 (2) | 2 | –/– | – | 210/– | [145] |
| (K0.5Na0.5)NbO3-1%GeO2 | Solid state | 650 (2) | 1000 (2) | 5 | 397/2 | – | 120/40 | [127] |
| (K0.5Na0.5)0.99Ca0.05NbO3 | Sol-gel | 650 (4) | 1115 (2) | 10 | 495/12 | 398 | 95/– | [138] |
| (K0.5Na0.5)0.99Sr0.05NbO3 | Sol-gel | 650 (4) | 1115 (2) | 10 | 500/4 | 397 | 95/– | [138] |
| (K0.5Na0.5)0.94Li0.06NbO3 | Solid state | 850 (4) | 1060–1100 (2) | – | –/– | – | 215/– | [146] |
| (K0.17Na0.83)NbO3 5%WO3 | Solid state | 825 (4) | 1160 (4) | 5 | 1250/0.1 | 425 | 45/– | [147] |
| (K0.5Na0.5)0.94Li0.06NbO3 | Solid state | 850 (4) | 1000 (2) | 4 | 560/0.8 | 410 | 215/– | [137] |
| (K0.48Na0.535)0.942Li0.058NbO3 | Solid state | 750 (4) | 1060 (2) | – | 650/– | 490 | 314/41 | [151] |
| (K0.5Na0.5)0.935Li0.065NbO3 | Solid state | 800 (4) | 1160 (1) | – | 680/0.18 | 440 | 250/44 | [119] |
| (K0.5Na0.5)NbO3-LiSbO3 | Solid state | 850 (2) | 1200 (2) | – | 1380/2 | 368 | 265/50 | [120] |
| 0.95(K0.5Na0.5)NbO3-0.05LiTaO3 | Solid state | 850 (10) | 1110 (–) | – | 570/4 | 425 | 200/36 | [128] |
| (K0.5Na0.5)0.948(LiSb)0.052Nb948O3 | Solid state | 880 (4) | 1080 (3) | – | 1100/1.9 | 385 | 286/51 | [129] |
| (K0.5Na0.5)0.94Li0.06(W0.67Bi0.33)0.008Nb0.992O3 | Solid State | 850 (4) | 1100 (3) | 7 | 950/2.5 | – | 282/45 | [148] |
| 0.92KNN-0.06BZ-0.02BLT | Molten salt & RTGG | 800 (5) | 1150 (2) | 10 | /– | 251 | 271/– | [149] |
| (Na0.5K0.5)0.975Li0.025Nb0.76 Sb0.06Ta0.18O3 | Solid state | 890 (4.5) | 1115 (3) | 2.5 | 1000/2.5 | 200 | 352/47 | [150] |
| (Na0.44K0.515Li0.045)Nb0.915 Sb0.045Ta0.05O3 | Solid state | 700 (6) | 1100 (3) | 40 | 1024/6.8 | 320 | 390/49 | [136] |
| (Na0.52K0.44Li0.04)Nb0.86 Sb0.04Ta0.1O3 | Solid state | 700 (2) | 1125 (16) | 1.2 | –/– | – | 255/– | [130] |
| 0.96(K0.4Na0.6)(Nb0.96Sb0.04)O3-0.04Bi0.5K0.5Zr0.9Sn0.1O3 | Solid state | 850 (6) | 1095 (3) | 4 | 2000/– | 250 | 460/47 | [154] |
| 0.96(K0.48Na0.52)(Nb0.97Sb0.03)O3-0.04Bi0.5(Na0.82K0.18)0.5ZrO3 | Solid state | 850 (6) | 1130 (3h) | – | 2200/– | 227 | 490/48 | [153] |

6. Conclusions



Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics; Academic Press: London, UK, 1971. [Google Scholar]
- Haertling, G.H. Ferroelectric ceramics: History and technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Bellaiche, L. Piezoelectricity of ferroelectric perovskites from first principles. Curr. Opin. Solid State Mater. Sci. 2002, 6, 19–25. [Google Scholar] [CrossRef]
- Noheda, B.; Cox, D.E.; Shirane, G.; Gonzalo, J.A.; Cross, L.E.; Park, S.-E. A monoclinic ferroelectric phase in the Pb(Zr1–xTix)O3 solid solution. Appl. Phys. Lett. 1999, 74, 2059–2061. [Google Scholar] [CrossRef]
- Heywang, W.; Lubitz, K.; Wersing, W. Piezoelectricity: Evolution and Future of a Technology; Springer: Berlin, Germany, 2008. [Google Scholar]
- Ringgaard, E.; Wurlitzer, T. Lead-free piezoceramics based on alkali niobates. J. Eur. Ceram. Soc. 2005, 25, 2701–2706. [Google Scholar] [CrossRef]
- Rödel, J.; Webber, K.G.; Dittmer, R.; Jo, W.; Kimura, M.; Damjanovic, D. Transferring lead-free piezoelectric ceramics into application. J. Eur. Ceram. Soc. 2015, 35, 1659–1681. [Google Scholar] [CrossRef]
- Takenaka, T.; Nagata, H.; Hiruma, Y. Current developments and prospective of lead-free piezoelectric ceramics. Jpn. J. Appl. Phys. 2008, 47, 3787–3801. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, R.; Shrout, T.R. Lead-free piezoelectric ceramics vs. PZT? J. Electroceram. 2007, 19, 111–124. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, H.; Chen, L.; Cao, M. Morphotropic phase boundary and piezoelectric properties of (Bi1/2Na1/2)1–x(Bi1/2K1/2)xTiO3-0.03(Na0.5K0.5)NbO3 ferroelectric ceramics. Mater. Lett. 2009, 63, 547–550. [Google Scholar] [CrossRef]
- Zhang, S.T.; Kounga, A.B.; Aulbach, E.; Granzow, T.; Jo, W.; Kleebe, H.J. Lead-free piezoceramics with giant strain in the system Bi0.5Na0.5TiO3-BaTiO3-K0.5Na0.5NbO3. I. structure and room temperature properties. J. Appl. Phys. 2008, 103, 034107. [Google Scholar] [CrossRef]
- Li, H.D.; Feng, C.D.; Yao, W.L. Some effects of different additives on dielectric and piezoelectric properties of (Bi1/2Na1/2)TiO3–BaTiO3 morphotropic-phase-boundary composition. Mater. Lett. 2004, 58, 1194–1198. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, L.; Chao, X.; Liu, Z.; Yang, Z. Phase transition behavior and large piezoelectricity near the morphotropic phase boundary of lead-free (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 ceramics. J. Am. Ceram. Soc. 2013, 96, 496–502. [Google Scholar]
- Wang, S.; Zhou, X.H.; Zhang, S.R.; Li, B.; Chen, Z. Preparation of nonreducible BCTZ ceramics by doping complex oxide dopants. J. Inorg. Mater. 2005, 20, 119–125. [Google Scholar]
- Hsieh, T.H.; Yen, S.C.; Ray, D.T. A study on the synthesis of (Ba,Ca)(Ti,Zr)O3 nano powders using Pechini polymeric precursor method. Ceram. Int. 2012, 38, 755–759. [Google Scholar] [CrossRef]
- Reyes-Montero, A.; Pardo, L.; López-Juárez, R.; González, A.M.; Cruz, M.P.; Villafuerte-Castrejón, M.E. Lead-free Ba0.9Ca0.1Ti0.9Zr0.1O3 piezoelectric ceramics processed below 1300 °C. J. Alloys Compd. 2014, 584, 28–33. [Google Scholar] [CrossRef]
- Dutta, P.K.; Asiaie, R.; Akbar, S.A.; Zhy, W. Hydrothermal synthesis and dielectric properties of tetragonal BaTiO3. Chem. Mater. 1994, 6, 1542–1548. [Google Scholar] [CrossRef]
- Hennings, D.F.K.; Metzmacher, C.; Schreinemacher, B.S. Defect chemistry and microstructure of hydrothermal barium titanate. J. Am. Ceram. Soc. 2001, 84, 179–182. [Google Scholar] [CrossRef]
- Komarneni, S.; Li, Q.; Stefansson, K.M.; Roy, R. Microwave-hydrothermal processing for synthesis of electroceramic powders. J. Mater. Res. 1993, 8, 3176–3183. [Google Scholar] [CrossRef]
- Fang, C.Y.; Wang, C.; Polotai, A.V.; Agrawal, D.K.; Lanagan, M.T. Microwave synthesis of nano-sized barium titanate. Mater. Lett. 2008, 62, 2551–2553. [Google Scholar] [CrossRef]
- Newalkar, B.L.; Komarneni, S.; Katsuki, H. Microwave-hydrothermal synthesis and characterization of barium titanate powders. Mater. Res. Bull. 2001, 36, 2347–2355. [Google Scholar] [CrossRef]
- Rørvik, P.M.; Grande, T.; Einarsrud, M.A. One-dimensional nanostructures of ferroelectric perovskites. Adv. Mater. 2011, 23, 4007–4034. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.; García, A.; Brebøl, K.; Mercadelli, E.; Galassi, C. Piezoelectric properties of lead-free submicron-structured (Bi0.5Na0.5)0.94Ba0.06TiO3 ceramics from nanopowders. Smart Mater. Struct. 2010, 19, 11507. [Google Scholar] [CrossRef]
- Rojac, T.; Bencan, A.; Malic, B.; Tutuncu, G.; Jones, J.L.; Daniels, J.E.; Damjanovic, D. BiFeO3 ceramics: Processing, electrical, and electromechanical properties. J. Am. Ceram. Soc. 2014, 97, 1993–2011. [Google Scholar] [CrossRef]
- Yu, R.; Hojo, H.; Oka, K.; Watanuki, T.; Machida, A.; Shimizu, K.; Nakano, K.; Azuma, M. New PbTiO3-type giant tetragonal compound Bi2ZnVO6 and its stability under pressure. Chem. Mater. 2015, 27, 2012–2017. [Google Scholar] [CrossRef]
- Moure, A.; Castro, A.; Pardo, L. Aurivillius-type ceramics, a class of high temperature piezoelectric materials: Drawbacks, advantages and trends. Prog. Solid State Chem. 2009, 37, 15–39. [Google Scholar] [CrossRef]
- Leonstev, S.O.; Eitel, R.E. Progress in engineering high strain lead-free piezoelectric ceramics. Sci. Technol. Adv. Mater. 2010, 11, 044302. [Google Scholar] [CrossRef]
- Taghaddos, E.; Mehdi, H.; Safari, A. Lead-free piezoelectric materials and ultrasonic transducers for medical imaging. J. Adv. Dielect. 2015, 5, 1530002. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Zhu, J. Potassium–sodium niobate lead-free piezoelectric materials: Past, present, and future of phase boundaries. Chem. Rev. 2015, 115, 2559–2595. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Nahm, S. Lead-Free Piezoelectrics; Springer: New York, NY, USA, 2011. [Google Scholar]
- Thurnauer, H.; Deaderick, J. Insulating Materials. American Lava Co. Patent 2,429,588, 2 October 1941. [Google Scholar]
- Von Hippel, A. Ferroelectricity, domain structure, and phase transitions of barium titanate. Rev. Mod. Phys. 1950, 22, 221. [Google Scholar] [CrossRef]
- Smith, M.B.; Page, K.; Siegrist, T.; Redmond, P.L.; Walter, E.C.; Seshadri, R.; Brus, L.E.; Steigerwald, M.L. Crystal structure and the paraelectric-to-ferroelectric phase transition of nanoscale BaTiO3. J. Am. Chem. Soc. 2008, 130, 6955–6963. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, R. Elastic, piezoelectric, and dielectric constants of polarized barium titanate ceramics and some applications of the piezoelectric equations. J. Acoust. Soc. Am. 1956, 28, 347–350. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, J.; Zhang, Z.; Zheng, P.; Zhao, M.; Li, J.; Wang, C. High piezoelectric properties and domain configuration in BaTiO3 ceramics obtained through the solid-state reaction route. J. Phys. D Appl. Phys. 2008, 41, 125408. [Google Scholar] [CrossRef]
- Phule, P.P.; Risbud, S.H. Low-temperature synthesis and processing of electronic materials in the BaO-TiO2 system. J. Mater. Sci. 1990, 25, 1169–1183. [Google Scholar]
- Kaur, K. Synthesis of various barium titanates. Int. J. IT Eng. Appl. Sci. Res. 2015, 4, 61–66. [Google Scholar]
- Buessem, W.R.; Cross, L.E.; Goswami, A.K. Phenomenological theory of high permittivity in fine-grained barium titanate. J. Am. Ceram. Soc. 1966, 49, 33–36. [Google Scholar] [CrossRef]
- Park, S.E.; Wada, S.; Cross, L.E.; Shrout, T.R. Crystallographically engineered BaTiO3 single crystals for high-performance piezoelectrics. J. Appl. Phys. 1999, 86, 2746. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Xue, D.; Kang, H.; Liu, C. Wet routs of high purity BaTiO3 nanopowders. J. Alloys Compd. 2007, 440, 78–83. [Google Scholar] [CrossRef]
- Lu, W.; Quilitz, M.; Schmidt, H. Nanoscaled BaTiO3 powders with a large surface area synthesized by precipitation from aqueous solutions: Preparation, characterization and sintering. J. Eur. Ceram. Soc. 2007, 24, 3149–3159. [Google Scholar] [CrossRef]
- Moreno, J.; Dominguez, J.M.; Montoya, A.; Vicente, L.; Viveros, T. Synthesis and characterization of MTiO3(M = Mg, Ca, Sr, Ba) sol-gel. J. Mater. Chem. 1995, 5, 509–512. [Google Scholar] [CrossRef]
- Pfaff, G. Sol–gel synthesis of barium titanate powders of various compositions. J. Mater. Chem. 1992, 2, 591–594. [Google Scholar] [CrossRef]
- Yu, P.; Cui, B.; Shi, Q. Preparation and characterization of BaTiO3 powders and ceramics by sol–gel process using oleic acid as surfactant. Mater. Sci. Eng. A 2008, 473, 34–41. [Google Scholar] [CrossRef]
- Viviani, M.; Buscaglia, M.T.; Testino, A.; Buscaglia, V.; Bowen, P.; Nanni, P. The influence of concentration on the formation of BaTiO3 by direct reaction of TiCl4 with Ba(OH)2 in aqueous solution. J. Eur. Ceram. Soc. 2003, 23, 1383–1390. [Google Scholar] [CrossRef]
- Testino, A.; Buscaglia, M.T.; Buscaglia, V.; Viviani, M.; Bottino, C.; Nanni, P. Kinetics and mechanism of aqueous chemical synthesis of BaTiO3 particles. Chem. Mater. 2004, 16, 1536–1543. [Google Scholar] [CrossRef]
- Wang, X.; Lee, B.I.; Hu, M.; Payzant, E.A.; Blom, D.A. Nanocrystalline BaTiO3 powder via a sol process ambient conditions. J. Eur. Ceram. Soc. 2006, 26, 2319–2326. [Google Scholar] [CrossRef]
- Tao, J.; Ma, J.; Wang, Y.; Zhu, X.; Liu, J.; Jiang, X. Synthesis of barium titanate nanoparticles via a novel electrochemical route. Mater. Res. Bull. 2008, 43, 639–644. [Google Scholar] [CrossRef]
- Xu, H.; Gao, L. Hydrothermal synthesis of high-purity BaTiO3 powders: Control of powder phase and size, sintering density, and dielectric properties. Mater. Lett. 2004, 58, 1582–1586. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Schmidt, R.; Morán, E. Microwave-assisted routes for the synthesis of complex functional oxides. Inorganics 2015, 3, 101–117. [Google Scholar] [CrossRef]
- Liu, S.F.; Abothu, I.R.; Komarneni, S. Barium titanate ceramics prepared from conventional and microwave hydrothermal powders. Mater. Lett. 1999, 38, 344–350. [Google Scholar] [CrossRef]
- Komarneni, S.; Katsuki, H. Microwave-hydrothermal synthesis of barium titanate under stirring condition. Ceram. Int. 2010, 36, 1165–1169. [Google Scholar] [CrossRef]
- Sun, W.; Li, J. Microwave-hydrothermal synthesis of tetragonal barium titanate. Mater. Lett. 2006, 60, 1599–1602. [Google Scholar] [CrossRef]
- Simões, A.Z.; Moura, F.; Onofre, T.B.; Ramirez, M.A.; Varela, J.A.; Longo, E. Microwave-hydrothermal synthesis of barium strontium titanate nanoparticles. J. Alloys Compd. 2010, 508, 620–624. [Google Scholar] [CrossRef]
- Khollam, Y.B.; Deshpande, A.S.; Patil, A.J.; Potdar, H.S.; Deshpande, S.B.; Date, S. Microwave-hydrothermal synthesis of equi-axed and submicron-sized BaTiO3 powders. Mater. Chem. Phys. 2001, 71, 304–308. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Li, J. Effects of chloride ions on hydrothermal synthesis of tetragonal BaTiO3 by microwave heating and conventional heating. Powder Technol. 2006, 166, 55–59. [Google Scholar] [CrossRef]
- Kim, H.T.; Han, Y.H. Sintering of nanocrystalline BaTiO3. Ceram. Int. 2004, 30, 1719–1723. [Google Scholar] [CrossRef]
- Sadhana, K.; Krishnaveni, T.; Praveena, K.; Bharadwaj, S.; Murthy, S.R. Microwave sintering of nanobarium titanate. Scr. Mater. 2008, 59, 495–498. [Google Scholar] [CrossRef]
- Takahashi, H.; Numamoto, Y.; Tani, J.; Matsuta, K.; Qiu, J.; Tsurekawa, S. Lead-free barium titanate ceramics with large piezoelectric constant fabricated by microwave sintering. Jpn. J. Appl. Phys. Part 2 2006, 45, 43–46. [Google Scholar] [CrossRef]
- Takahashi, H.; Numamoto, Y.; Tani, J.; Tsurekawa, S. Piezoelectric properties of BaTiO3 ceramics with high performance fabricated by microwave sintering. Jpn. J. Appl. Phys. Part 1 2006, 45, 7405–7408. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X. Large piezoelectric effect in Pb-Free ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.D.; Li, W.L.; Xu, D.; Hou, Y.F.; Cao, W.P.; Feng, Y.; Fei, W.D. Piezoelectric properties of BaTiO3–CaTiO3–BaZrO3 ceramics with compositions near the morphotropic phase boundary. Ceram. Int. 2014, 40, 14907–14912. [Google Scholar] [CrossRef]
- Sun, Z.; Pu, Y.; Dong, Z.; Hu, Y.; Wang, P.; Liu, X.; Wang, Z. Impact of fast microwave sintering on the grain growth, dielectric relaxation and piezoelectric properties on Ba0.18Ca0.02Ti0.09Zr0.10O3 lead-free ceramics prepared by different methods. Mater. Sci. Eng. B 2014, 185, 114–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Glaum, J.; Groh, C.; Ehmke, M.C.; Blendell, J.E.; Bowman, K.J.; Hoffman, M.J. Correlation between piezoelectric properties and phase coexistence in (Ba,Ca)(Ti,Zr)O3 ceramics. J. Am. Ceram. Soc. 2014, 97, 2885–2891. [Google Scholar] [CrossRef]
- Reyes-Montero, A.; Pardo, L.; López-Juárez, R.; González, A.M.; Rea-López, S.O.; Cruz, M.P.; Villafuerte-Castrejón, M.E. Sub-10 μm grain size, Ba1−xCaxTi0.9Zr0.1O3 (x = 0.10 and x = 0.15) piezoceramics processed using a reduced thermal treatment. Smart Mater. Struct. 2015, 24, 065033. [Google Scholar] [CrossRef]
- Benabdallah, F.; Elissalde, C.; Seu, U.C.C.; Michau, D.; Poulon-Quintin, A.; Gayot, M.; Garreta, P.; Khemakhem, H.; Maglione, M. Structure–microstructure–property relationships in lead-free BCTZ piezoceramics processed by conventional sintering and spark plasma sintering. J. Eur. Ceram. Soc. 2015, 35, 4153–4161. [Google Scholar] [CrossRef]
- Bai, Y.; Matousek, A.; Tofel, P.; Bijalwan, V.; Nan, B.; Hughes, H.; Button, T.W. (Ba,Ca)(Zr,Ti)O3 lead-free piezoelectric ceramics—The critical role of processing on properties. J. Eur. Ceram. Soc. 2015, 35, 3445–3456. [Google Scholar] [CrossRef]
- Li, B.; Ehmke, M.C.; Blendell, J.E.; Bowman, K.J. Optimizing electrical poling for tetragonal, lead-free BZT–BCT piezoceramic alloys. J. Eur. Ceram. Soc. 2013, 33, 3037–3044. [Google Scholar] [CrossRef]
- Mitsui, T.; Westphal, W.B. Dielectric and X-ray Studies of CaxBa1−xTiO3 and CaxSr1−xTiO3. Phys. Rev. 1961, 124, 1354–1359. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J. Phase transition, microstructure, and electrical properties of Ca, Zr, and Sn-modified BaTiO3 lead-free ceramics. J. Alloys Compd. 2014, 615, 969–974. [Google Scholar] [CrossRef]
- Yu, Z.; Ang, C.; Guo, R.; Bhalla, A.S. Piezoelectric and strain properties of Ba(Ti1−xZrx)O3 ceramics. J. Appl. Phys. 2002, 92, 1489–1493. [Google Scholar] [CrossRef]
- Maiti, T.; Guo, R.; Bhalla, A.S. Structure-property phase diagram of BaZrxTi1−xO3 system. J. Am. Ceram. Soc. 2008, 91, 1769–1780. [Google Scholar] [CrossRef]
- Hao, J.; Bai, W.; Li, W.; Zhai, J. Correlation between the microstructure and electrical properties in high-performance (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free piezoelectric ceramics. J. Am. Ceram. Soc. 2012, 95, 1998–2006. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Jiang, M.; Zhao, X.; Shan, X.; Li, W.; Yuan, C.; Zhou, C. Lead-free (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3-CeO2 ceramics with high piezoelectric coefficient obtained by low-temperature sintering. Ceram. Int. 2012, 38, 4761–4764. [Google Scholar] [CrossRef]
- Jiang, M.; Lin, Q.; Lin, D.; Zheng, Q.; Fan, X.; Wu, X.; Sun, H.; Wan, Y.; Wu, L. Effects of MnO2 and sintering temperature on microstructure, ferroelectric, and piezoelectric properties of Ba0.85Ca0.15Ti0.90Zr0.10O3 lead-free ceramics. J. Mater. Sci. 2013, 48, 1035–1041. [Google Scholar] [CrossRef]
- Bharathi, P.; Varma, K.B.R. Grain and the concomitant ferroelectric domain size dependent physical properties of Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics fabricated using powders derived from oxalate precursor route. J. Appl. Phys. 2014, 116, 164107. [Google Scholar] [CrossRef]
- Praveen, J.P.; Karthik, T.; James, A.R.; Chandrakala, E.; Asthana, S.; Das, D. Effect of poling process on piezoelectric properties of sol-gel derived BZT–BCT ceramics. J. Eur. Ceram. Soc. 2015, 35, 1785–1798. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Wu, W.; Zhu, J.; Wang, J. Effect of dwell time during sintering on piezoelectric properties of (Ba0.85Ca0.15)(Ti0.90Zr0.10)O3 lead-free ceramics. J. Alloys Compd. 2011, 509, L359–L361. [Google Scholar] [CrossRef]
- Sun, Z.; Pu, Y.; Dong, Z.; Hu, Y.; Liu, X.; Wang, P. The effects of soaking time on the grain growth, dielectric and ferroelectric properties of BaTi0.95Zr0.05O3 ceramics prepared by microwave sintering. Vacuum 2014, 101, 228–232. [Google Scholar] [CrossRef]
- Mishra, P.; Sonia; Kumar, P. Enhanced dielectric and piezoelectric properties of BZT–BCT system near MPB. Ceram. Int. 2014, 40, 14149–14157. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. High piezoelectric d33 coefficient of lead-free (Ba0.93Ca0.07)(Ti0.95Zr0.05)O3 ceramics sintered at optimal temperature. Mater. Sci. Eng. B 2011, 176, 65–67. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Wu, B.; Wu, W.; Zhu, J.; Yang, Z.; Wang, J. Sintering temperature-induced electrical properties of (Ba0.90Ca0.10)(Ti0.85Zr0.15)O3 lead-free ceramics. Mater. Res. Bull. 2012, 47, 1281–1284. [Google Scholar] [CrossRef]
- Bao, H.; Zhou, C.; Xue, D.; Gao, J.; Ren, X. A modified lead-free piezoelectric BZT–xBCT system with higher TC. J. Phys. D Appl. Phys. 2010, 43, 465401. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. High piezoelectric d33 coefficient in (Ba1−xCax)(Ti0.98Zr0.02)O3 lead-free ceramics with relative high Curie temperature. Mater. Lett. 2010, 64, 2325–2327. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. Piezoelectric and dielectric properties of (Ba1−xCax)(Ti0.95Zr0.05)O3 lead-free ceramics. J. Am. Ceram. Soc. 2010, 93, 2942–2944. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. Polymorphic phase transition and piezoelectric properties of (Ba1−xCax)(Ti0.9Zr0.1)O3 lead-free ceramics. Phys. B 2010, 405, 4513–4516. [Google Scholar] [CrossRef]
- Pisitpipathsin, N.; Kantha, P.; Pengpat, K.; Rujijanagul, G. Influence of Ca substitution on microstructure and electrical properties of Ba(Zr,Ti)O3 ceramics. Ceram. Int. 2013, 39, S35–S39. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Wu, W.; Chen, Q.; Zhu, J.; Yang, Z.; Wang, J. Composition and poling condition-induced electrical behavior of (Ba0.85Ca0.15)(Ti1−xZrx)O3 lead-free piezoelectric ceramics. J. Eur. Ceram. Soc. 2012, 32, 891–898. [Google Scholar] [CrossRef]
- Zhang, S.W.; Zhang, H.; Zhang, B.P.; Yang, S. Phase-transition behavior and piezoelectric properties of lead-free (Ba0.95Ca0.05)(Ti1−xZrx)O3 ceramics. J. Alloys Compd. 2010, 506, 131–135. [Google Scholar] [CrossRef]
- Smolensky, G.A.; Isupov, V.A.; Agranovskaya, A.I.; Krainik, N.N. New ferroelectrics of complex composition. Sov. Phys. Solid State 1961, 2, 2651–2654. [Google Scholar]
- Takenaka, T.; Maruyama, K.I.; Sakata, K. (Bi1/2Na1/2)TiO3-BaTiO3 system for lead-free piezoelectric ceramics. Jap. J. Appl. Phys. 1991, 30, 2236–2239. [Google Scholar] [CrossRef]
- Chu, B.J.; Chen, D.R.; Li, G.R.; Yin, Q.R. Electrical properties of Na1/2Bi1/2TiO3–BaTiO3 ceramics. J. Eur. Ceram. Soc. 2002, 22, 2115–2121. [Google Scholar] [CrossRef]
- Jo, W.; Dittmer, R.; Acosta, M.; Zang, J.; Groh, C.; Sapper, E.; Wang, K.; Rödel, J. Giant electric-field-induced strains in lead-free ceramics for actuator applications—Status and perspective. J. Electroceram. 2012, 29, 71–93. [Google Scholar] [CrossRef]
- Vakhrushev, S.B.; Isupov, V.A.; Kvyatkovsky, B.E.; Okuneva, N.M.; Pronin, I.P.; Smolensky, G.A.; Syrnikov, P.P. Phase transitions and soft modes in sodium bismuth titanate. Ferroelectrics 1985, 63, 153–160. [Google Scholar] [CrossRef]
- Hiruma, Y.; Nagata, H.; Takenaka, T. Thermal depoling process and piezoelectric properties of bismuth sodium titanate ceramics. J. Appl. Phys. 2009, 105, 084112. [Google Scholar] [CrossRef]
- Eerd, B.W.; Damjanovic, D.; Klein, N.; Setter, N.; Trodahl, J. Structural complexity of (Na0.5Bi0.5)TiO3-BaTiO3 as revealed by Raman spectroscopy. Phys. Rev. B. 2010, 82, 104112. [Google Scholar] [CrossRef]
- Pardo, L.; Mercadelli, E.; García, Á.; Brebøl, K.; Galassi, C. Field-induced phase transition and relaxor character in submicron structured lead-free (Bi0.5Na0.5)0.94Ba0.06TiO3 piezoceramics at the morphotropic phase boundary. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, P.; Bian, X.; Jing, H.; Wang, Y.; Zhang, Y.; Wu, Y.; Song, W. Dielectric, ferroelectric and piezoelectric properties of Bi0.5Na0.5TiO3-(Ba0.7Ca0.3)TiO3 ceramics at morphotropic phase boundary composition. Mater. Sci. Eng. B 2011, 176, 260–265. [Google Scholar] [CrossRef]
- Takenaka, T.; Nagata, H.; Hiruma, Y. Phase transition temperatures and piezoelectric properties of (Bi1/2Na1/2)TiO3-and (Bi1/2K1/2)TiO3-based bismuth perovskite lead-free ferroelectric ceramics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Naderer, M.; Kainz, T.; Schütz, D.; Reichmann, K. The influence of Ti-nonstoichiometry in Bi0.5Na0.5TiO3. J. Eur. Ceram. Soc. 2014, 34, 663–667. [Google Scholar] [CrossRef]
- Lencka, M.M.; Oledzka, M.; Riman, R.E. Hydrothermal synthesis of sodium and potassium bismuth titanates. Chem. Mater. 2000, 12, 1323–1330. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Dai, S. Hydrothermal synthesis of monosized Bi0.5Na0.5TiO3 spherical particles under low alkaline solution concentration. J. Alloys Compd. 2009, 484, 801–805. [Google Scholar] [CrossRef]
- Pookmanee, P.; Phanichphant, S. Low temperature hydrothermal synthesis of bismuth sodium titanate nanopowders. Int. J. Nanosci. 2005, 4, 637–641. [Google Scholar] [CrossRef]
- Zhao, M.L.; Wang, C.L.; Zhong, W.L.; Wang, J.F.; Li, Z.F. Grain-size effect on the dielectric properties of Bi0.5Na0.5TiO3. Chin. Phys. Lett. 2003, 20, 290–292. [Google Scholar]
- West, D.L.; Payne, D.A. Preparation of 0.95Bi1/2Na1/2TiO3·0.05BaTiO3 ceramics by an aqueous citrate-gel route. J. Am. Ceram. Soc. 2003, 86, 192–194. [Google Scholar] [CrossRef]
- Mercadelli, E.; Galassi, C.; Costa, A.L.; Albonetti, S.; Sanson, A. Sol-gel combustion synthesis of BNBT powders. J. Sol Gel Sci. Technol. 2008, 46, 39–45. [Google Scholar] [CrossRef]
- Alonso-Sanjosé, D.; Jiménez, R.; Bretos, I.; Calzada, M.L. Lead-free ferroelectric (Na1/2Bi1/2)TiO3-BaTiO3 thin films in the morphotropic phase boundary composition: Solution processing and properties. J. Am. Ceram. Soc. 2009, 92, 2218–2225. [Google Scholar] [CrossRef]
- Kim, B.H.; Han, S.J.; Kim, J.H.; Lee, J.H.; Ahn, B.K.; Xu, Q. Electrical properties of (1−x)(Bi0.5Na0.5)TiO3-xBaTiO3 synthesized by emulsion method. Ceram. Int. 2007, 33, 447–452. [Google Scholar] [CrossRef]
- Kanie, K.; Numamoto, Y.; Tsukamoto, S.; Sasaki, T.; Nakaya, M.; Tani, J.; Takahashi, H.; Muramatsu, A. Size-controlled hydrothermal synthesis of bismuth sodium and bismuth potassium titanates fine particles and application to lead-free piezoelectric ceramics. Mater. Trans. JIM 2011, 52, 1396–1401. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, L.; Hu, Y.; Du, H. Compositional effects on the properties of (1–x)BaTiO3-xBi0.5Na0.5TiO3 ceramics. J. Mater. Sci. Mater. Electron. 2007, 18, 605–609. [Google Scholar] [CrossRef]
- Gao, L.; Huang, Y.; Liu, L.; Liu, T.; Liu, C.; Zhou, F.; Wan, X. Crystal structure and properties of BaTiO3-(Bi0.5Na0.5)TiO3 ceramic system. J. Mater. Sci. 2008, 43, 6267–6271. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Q.; Kim, B.H.; Ahn, B.K.; Ko, J.H.; Kang, W.J.; Nam, O.J. Structure and electrical properties of (Na0.5Bi0.5)1−xBaxTiO3 piezoelectric ceramics. J. Eur. Ceram. Soc. 2008, 28, 843–849. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, X.L.; Chen, W.; Kim, B.H.; Xu, S.L.; Chen, M. Structure and electrical properties of (Na0.5Bi0.5)1−xBaxTiO3 ceramics made by a citrate method. J. Electroceram. 2008, 21, 617–620. [Google Scholar] [CrossRef]
- Hu, J.Q.; Chen, Z.W. Piezoelectric and dielectric properties of Bi2O3-Doped (Bi0.5Na0.5)0.94Ba0.06TiO3 lead-free piezoelectric ceramics. Key Eng. Mater. 2008, 368–372, 1915–1918. [Google Scholar] [CrossRef]
- Ma, X.; Yin, J.; Zhou, Q.; Xue, L.; Yan, Y. Effect of Eu doping on structure and electrical properties of lead-free (Bi0.5Na0.5)0.94Ba0.06 TiO3 ceramics. Ceram. Int. 2014, 40, 7007–7013. [Google Scholar] [CrossRef]
- Fu, P.; Xu, Z.; Chu, R.; Li, W.; Zang, G.; Hao, J. Piezoelectric, ferroelectric and dielectric properties of Nd2O3-doped (Bi0.5Na0.5)0.94Ba0.06 TiO3 lead-free ceramics. Mater. Sci. Eng. B 2010, 167, 161–166. [Google Scholar] [CrossRef]
- Luo, L.; Ni, F.; Zhang, H.; Chen, H. The enhanced piezoelectric performance of Bi1/2Na1/2TiO3–BaTiO3 ceramics by Ba0.77Ca0.23TiO3 substitution. J. Alloys Compd. 2012, 536, 113–118. [Google Scholar] [CrossRef]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, E.; Davis, M.; Damjanovic, D.; Setter, N. Piezoelectric properties of Li- and Ta-modified (K0.5Na0.5)NbO3 ceramics. Appl. Phys. Lett. 2005, 87, 182905. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, R.; Shrout, T.R.; Zang, G.; Wang, J. Characterization of lead free (K0.5Na0.5)NbO3-LiSbO3 piezoceramic. Solid State Commun. 2007, 141, 675–679. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, K.; Zhu, F.Y.; Cheng, L.Q.; Yao, F.Z. (K, Na)NbO3-based lead-free piezoceramics: Fundamental aspects, processing technologies, and remaining challenges. J. Am. Ceram. Soc. 2013, 96, 3677–3696. [Google Scholar] [CrossRef]
- Jaeger, R.E.; Egerton, L. Hot pressing of potassium-sodium niobates. J. Am. Ceram. Soc. 1962, 45, 209–213. [Google Scholar] [CrossRef]
- Egerton, L.; Bieling, C.A. Isostatically hot-pressed sodium-potassium niobate transducer material for ultrasonic devices. Am. Ceram. Soc. Bull. 1968, 47, 1151–1156. [Google Scholar]
- Kosec, M.; Kolar, D. On activated sintering and electrical properties of NaKNbO3. Mater. Res. Bull. 1975, 10, 335–339. [Google Scholar] [CrossRef]
- Tennery, V.J.; Hang, K. Thermal and X-ray diffraction studies of the NaNbO3–KNbO3 system. J. Appl. Phys. 1968, 39, 4749. [Google Scholar] [CrossRef]
- Senna, M.; Pavlic, J.; Rojac, T.; Malic, B.; Kosec, M. Preparation of phase-pure K0.5Na0.5NbO3 fine powders by a solid-state reaction at 625 °C from a precursor comprising Nb2O5 and K, Na acetates. J. Am. Ceram. Soc. 2014, 97, 413–419. [Google Scholar] [CrossRef]
- Bernard, J.; Bencan, A.; Rojac, T.; Holc, J.; Malic, B.; Kosec, M. Low-temperature sintering of K0.5Na0.5NbO3 ceramics. J. Am. Ceram. Soc. 2008, 91, 2409–2411. [Google Scholar] [CrossRef]
- Guo, Y.; Kakimoto, K.I.; Ohsato, H. (Na0.5K0.5)NbO3–LiTaO3 lead-free piezoelectric ceramic. Mater. Lett. 2005, 59, 241–244. [Google Scholar] [CrossRef]
- Zang, G.Z.; Wang, J.F.; Chen, H.C.; Su, W.B.; Wang, C.M.; Qi, P.; Ming, B.Q.; Du, J.; Zheng, L.M.; Zhang, S.; et al. Perovskite (Na0.5K0.5)1−x(LiSb)xNb1−xO3 lead-free piezoceramics. Appl. Phys. Lett. 2006, 88, 212908. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Martín-González, M.S.; Fernández, J.F. Effect of stoichiometry and milling processes in the synthesis and the piezoelectric properties of modified KNN nanoparticles by solid state reaction. J. Eur. Ceram. Soc. 2010, 30, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- López, R.; González, F.; Villafuerte-Castrejón, M.E. Structural and electrical characterization of (K0.48Na0.52)0.96Li0.04Nb0.85Ta0.15O3 synthesized by spray drying. J. Eur. Ceram. Soc. 2010, 30, 1549–1553. [Google Scholar] [CrossRef]
- López, R.; González, F.; Cruz, M.P.; Villafuerte-Castrejón, M.E. Piezoelectric and ferroelectric properties of K0.5Na0.5NbO3 ceramics synthesized by spray drying method. Mater. Res. Bull. 2011, 46, 70–74. [Google Scholar] [CrossRef]
- López-Juárez, R.; González-García, F.; Zárate-Medina, J.; Escalona-González, R.; de la Torre, S.D.; Villafuerte-Castrejón, M.E. Piezoelectric properties of Li–Ta co-doped potassium–sodium niobate ceramics prepared by spark plasma and conventional sintering. J. Alloys Compd. 2011, 509, 3837–3842. [Google Scholar] [CrossRef]
- López-Juárez, R.; Castañeda-Guzmán, R.; Villafuerte-Castrejón, M.E. Fast synthesis of NaNbO3 and K0.5Na0.5NbO3 by microwave hydrothermal method. Ceram. Int. 2014, 40, 14757–14764. [Google Scholar] [CrossRef]
- López-Juárez, R.; Novelo-Peralta, O.; González-García, F.; Rubio-Marcos, F.; Villafuerte-Castrejón, M.E. Ferroelectric domain structure of lead-free potassium-sodium niobate ceramics. J. Eur. Ceram. Soc. 2011, 31, 1861–1864. [Google Scholar] [CrossRef]
- Zhengfa, L.; Yongxiang, L.; Jiwei, Z. Grain growth and piezoelectric property of KNN-based lead-free ceramics. Curr. Appl. Phys. 2011, 11, S2–S13. [Google Scholar] [CrossRef]
- Saeri, M.R.; Barzegar, A.; Moghadam, H.A. Investigation of nano particle additives on lithium doped KNN lead free piezoelectric ceramics. Ceram. Int. 2011, 37, 3083–3087. [Google Scholar] [CrossRef]
- Malic, B.; Bernard, J.; Holc, J.; Jenko, D.; Kosec, M. Alkaline-earth doping in (K,Na)NbO3 based piezoceramics. J. Eur. Ceram. Soc. 2005, 25, 2707–2711. [Google Scholar] [CrossRef]
- Rani, J.; Patel, P.K.; Adhlakha, N.; Singh, H.; Yadav, K.L.; Prakash, S. Mo6+ modified (K0.5Na0.5)NbO3 lead free ceramics: Structural, electrical and optical properties. J. Mater. Sci. Technol. 2014, 30, 459–465. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, C.; Zhao, J.; Ge, H.; Zhu, M.; Yan, H. The fine-grained KNN–LN ceramics densified from nanoparticles obtained by an economical sol-gel route. Mater. Chem. Phys. 2012, 134, 518–522. [Google Scholar] [CrossRef]
- Palei, P.; Pattanaik, M.; Kumar, P. Effect of oxygen sintering on the structural and electrical properties of KNN ceramics. Ceram. Int. 2012, 38, 851–854. [Google Scholar] [CrossRef]
- Hao, J.; Xu, Z.; Chu, R.; Zhang, Y.; Chen, Q.; Fu, P.; Li, W.; Li, G.; Yin, Q. Characterization of (K0.5Na0.5)NbO3 powders and ceramics prepared by a novel hybrid method of sol-gel and ultrasonic atomization. Mater. Des. 2010, 31, 3146–3150. [Google Scholar] [CrossRef]
- Singh, R.; Patro, P.K.; Kulkarni, A.R.; Harendranath, C.S. Synthesis of nano-crystalline potassium sodium niobate ceramic using mechanochemical activation. Ceram. Int. 2014, 40, 10641–10647. [Google Scholar] [CrossRef]
- Feizpour, M.; Bafrooei, H.B.; Hayati, R.; Ebadzadeh, T. Microwave-assisted synthesis and sintering of potassium sodium niobate lead-free piezoelectric ceramics. Ceram. Int. 2014, 40, 871–877. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, M.; Zhang, C.; Zhang, M. Hydrothermal synthesis and piezoelectric property of Ta-doping K0.5Na0.5NbO3 lead-free piezoelectric ceramic. Ceram. Int. 2009, 35, 3253–3258. [Google Scholar] [CrossRef]
- Wang, K.; Li, J.F. Analysis of crystallographic evolution in (Na,K)NbO3-based lead-free piezoceramics by X-ray diffraction. Appl. Phys. Lett. 2007, 91, 262902. [Google Scholar] [CrossRef]
- Rani, J.; Yadav, K.L.; Prakash, S. Enhanced dielectric, ferroelectric and optical properties of lead free (K0.17Na0.83)NbO3 ceramic with WO3 addition. Mater. Sci. Eng. B 2013, 178, 1469–1475. [Google Scholar] [CrossRef]
- Du, J.; Zang, G.Z.; Yi, X.J.; Zhang, D.F.; Huang, Z.F. Effects of W2/3Bi1/3 substitute on piezoelectric properties of KNN-based ceramics. Mater. Lett. 2012, 70, 23–25. [Google Scholar] [CrossRef]
- Li, L.; Bai, W.; Zhang, Y.; Shen, B.; Zhai, J. The preparation and piezoelectric property of textured KNN-based ceramics with plate-like NaNbO3 powders as template. J. Alloys Compd. 2015, 622, 137–142. [Google Scholar] [CrossRef]
- Du, J.; Wang, J.; Zang, G.; Yi, X. Phase transition behavior and piezoelectric properties of low-Li and high-Sb modified KNN based piezoceramics. Phys. B 2011, 406, 4077–4079. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, B.P.; Li, J.F. High piezoelectric d33 coefficient in Li-modified lead-free (Na,K)NbO3 ceramics sintered at optimal temperature. Appl. Phys. Lett. 2007, 20, 242904. [Google Scholar] [CrossRef]
- Park, S.U.; Koh, J.H. Dielectric properties of Ag-doped 0.94(K0.5Na0.5)NbO3–0.06LiNbO3 ceramics prepared by templated grain growth. Mater. Res. Bull. 2014, 58, 69–73. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Xiao, D.; Zhu, J.; Cheng, X.; Zheng, T.; Zhang, B.; Lou, X.; Wang, X. Giant piezoelectricity in potassium–sodium niobate lead-free ceramics. J. Am. Chem. Soc. 2014, 136, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wu, J.; Xiao, D.; Zhu, J.; Wang, X.; Xin, L.; Lou, X. Strong piezoelectricity in (1−x)(K0.4Na0.6)(Nb0.96Sb0.04)O3–xBi0.5K0.5Zr1–ySnyO3 lead-free binary system: Identification and role of multiphase coexistence. ACS Appl. Mater. Interfaces 2015, 7, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafuerte-Castrejón, M.E.; Morán, E.; Reyes-Montero, A.; Vivar-Ocampo, R.; Peña-Jiménez, J.-A.; Rea-López, S.-O.; Pardo, L. Towards Lead-Free Piezoceramics: Facing a Synthesis Challenge. Materials 2016, 9, 21. https://doi.org/10.3390/ma9010021
Villafuerte-Castrejón ME, Morán E, Reyes-Montero A, Vivar-Ocampo R, Peña-Jiménez J-A, Rea-López S-O, Pardo L. Towards Lead-Free Piezoceramics: Facing a Synthesis Challenge. Materials. 2016; 9(1):21. https://doi.org/10.3390/ma9010021
Chicago/Turabian StyleVillafuerte-Castrejón, María Elena, Emilio Morán, Armando Reyes-Montero, Rodrigo Vivar-Ocampo, Jesús-Alejandro Peña-Jiménez, Salvador-Oliver Rea-López, and Lorena Pardo. 2016. "Towards Lead-Free Piezoceramics: Facing a Synthesis Challenge" Materials 9, no. 1: 21. https://doi.org/10.3390/ma9010021