Two-Level Micro-to-Nanoscale Hierarchical TiO2 Nanolayers on Titanium Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. TiO2 Gel Layer Formation on the Titanium Surface
2.4. The Dip Coating Fabrication of Mesoporous Two-Level Micro- and Nanohierarchical TiO2 Layers on the Titanium Surface
2.4.1. TiO2 Layers Fabrication on the Substrate Surface
2.4.2. The Texturing of TiO2 Gel Layers on Titanium Surface by Fast (Shock) Drying
2.5. The Evaluation of Osteoblasts MC3T3-E1 Monolayer Formation on the Samples of TiO2-Coated Naotitanium
3. Results and Discussion
3.1. Thickness Regulation of TiO2 Gel Layer by Removal Rate Variation of the Nanotitanium Wafer from the Solution
3.2. Control of TiO2 Gel Layer Thickness Based on Repeating the Dip-Coating Process the Required Number of Times
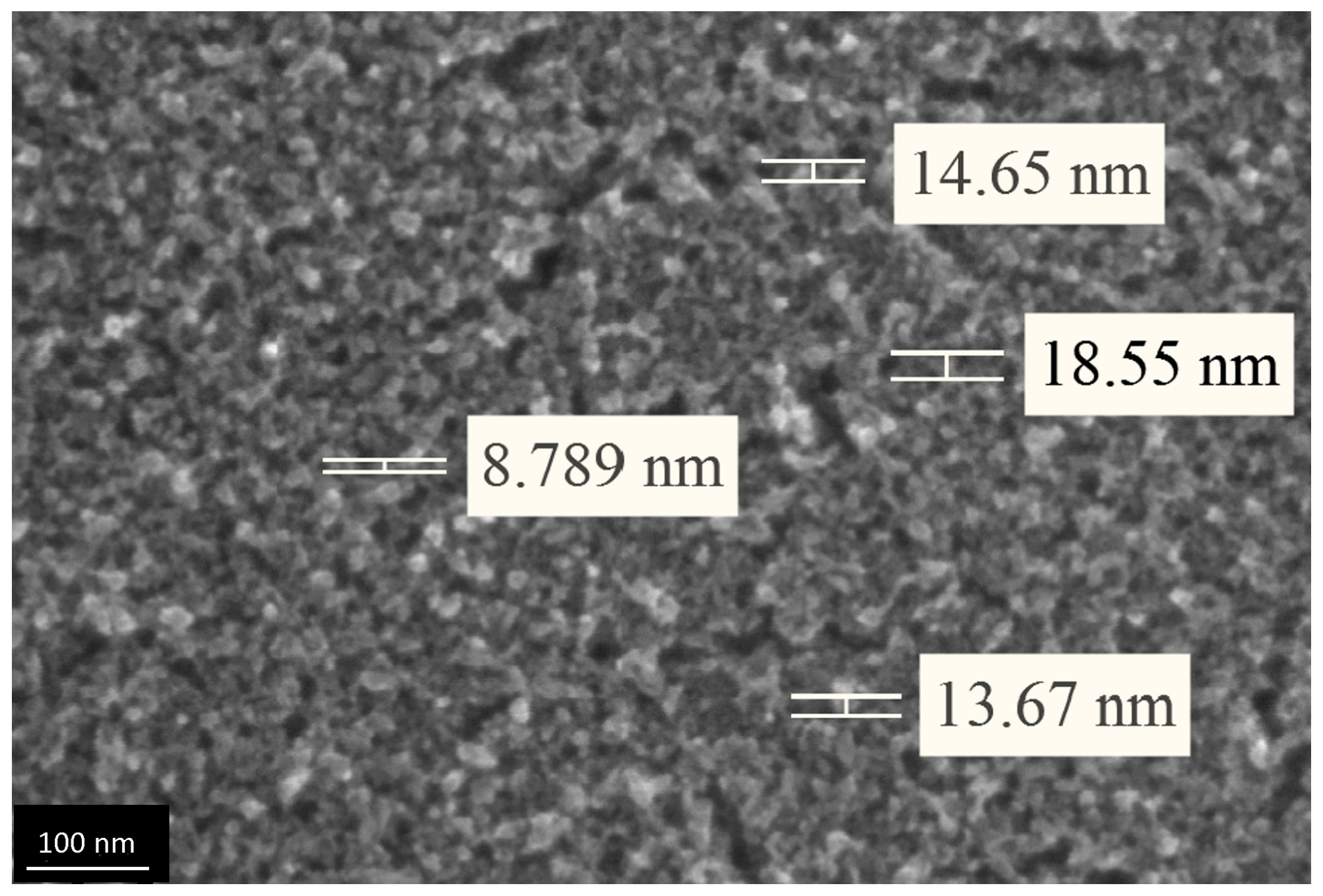
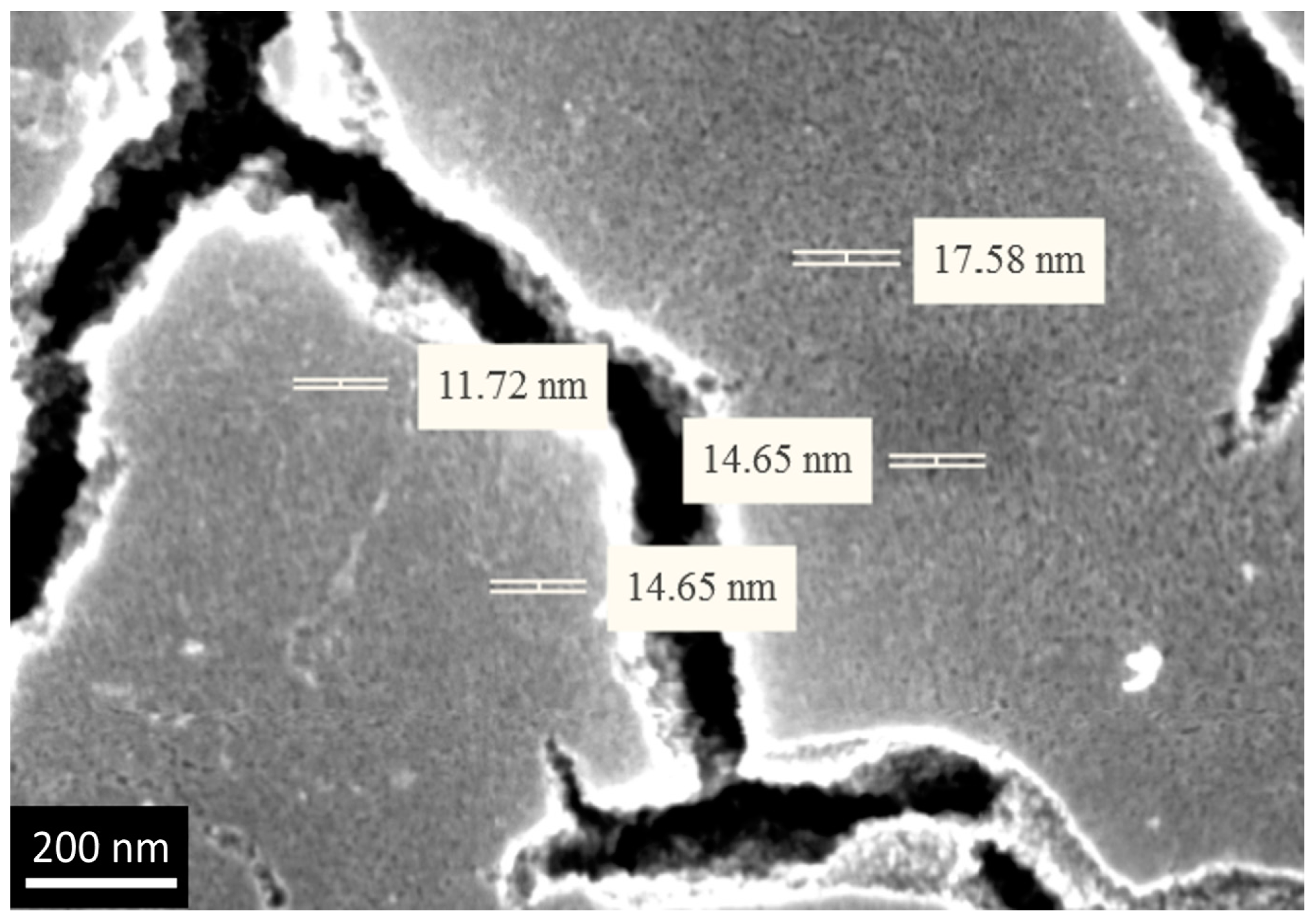
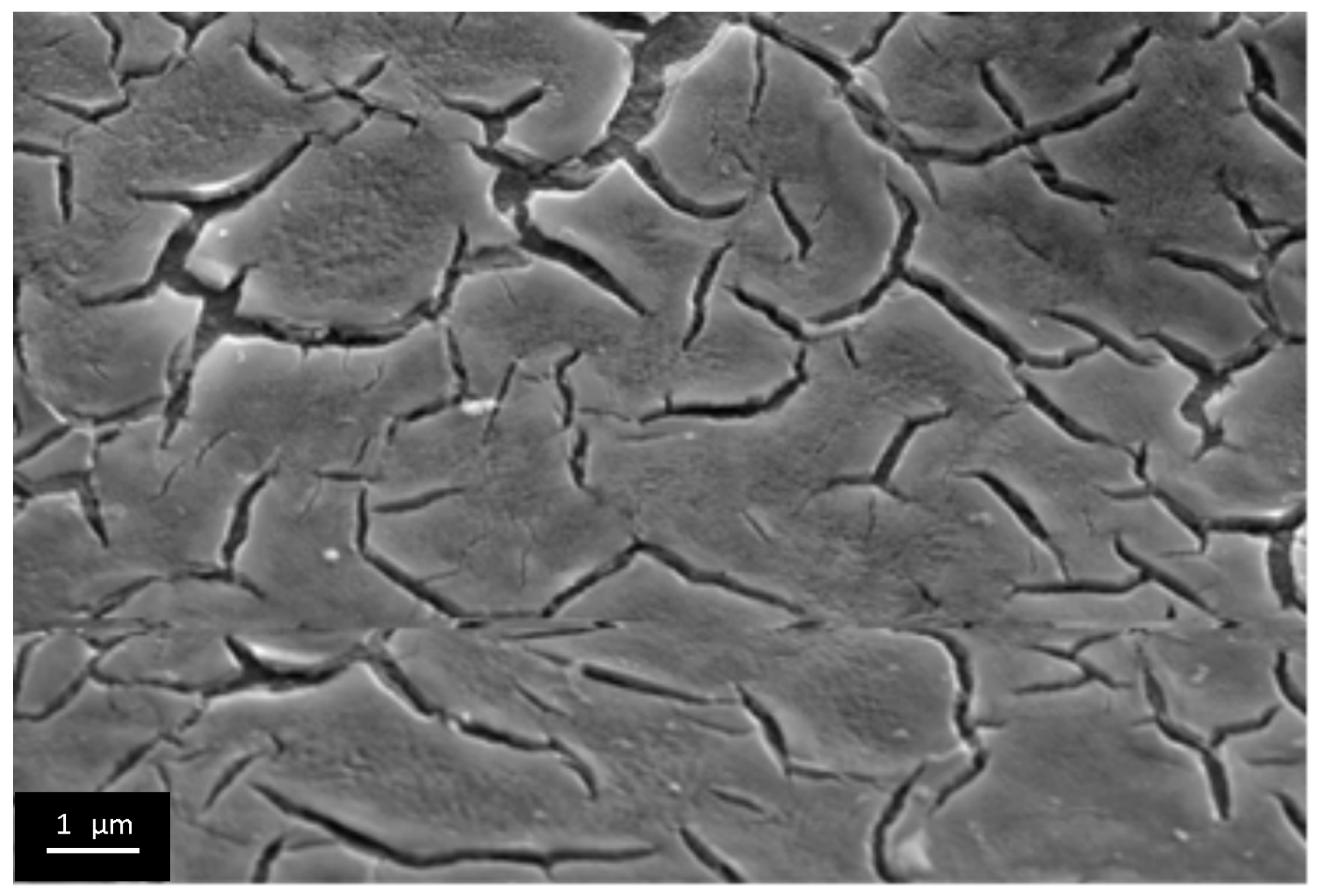
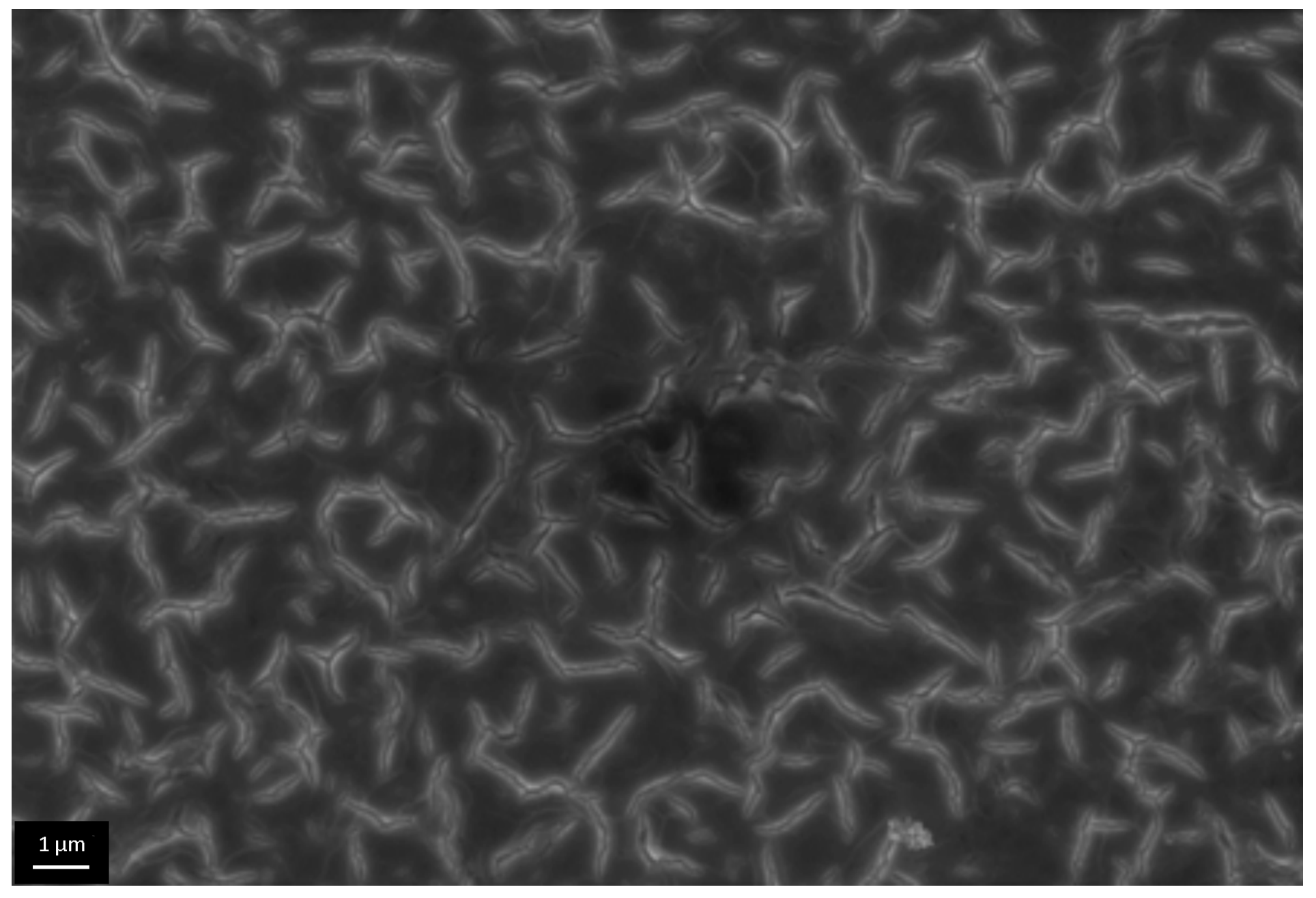
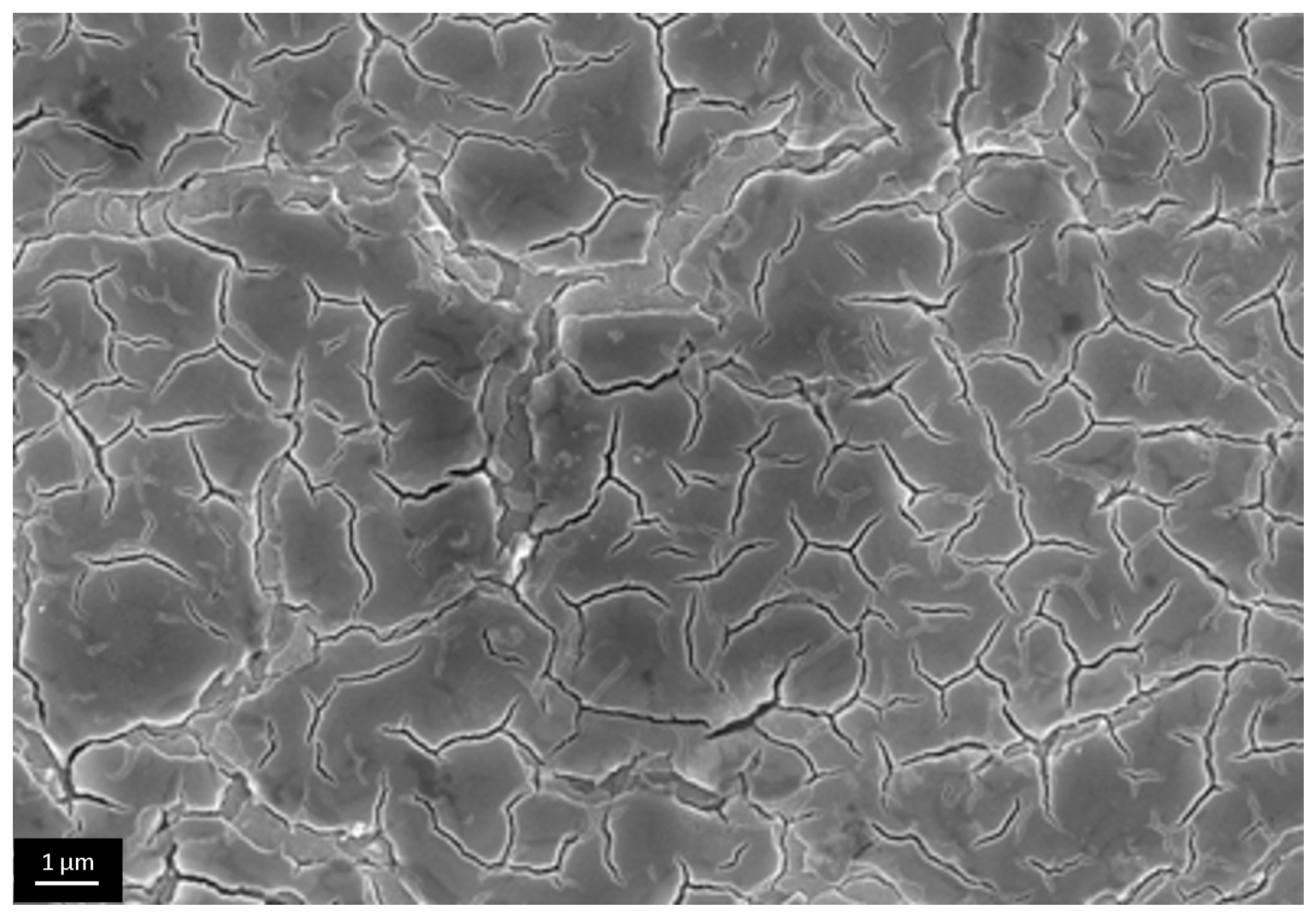
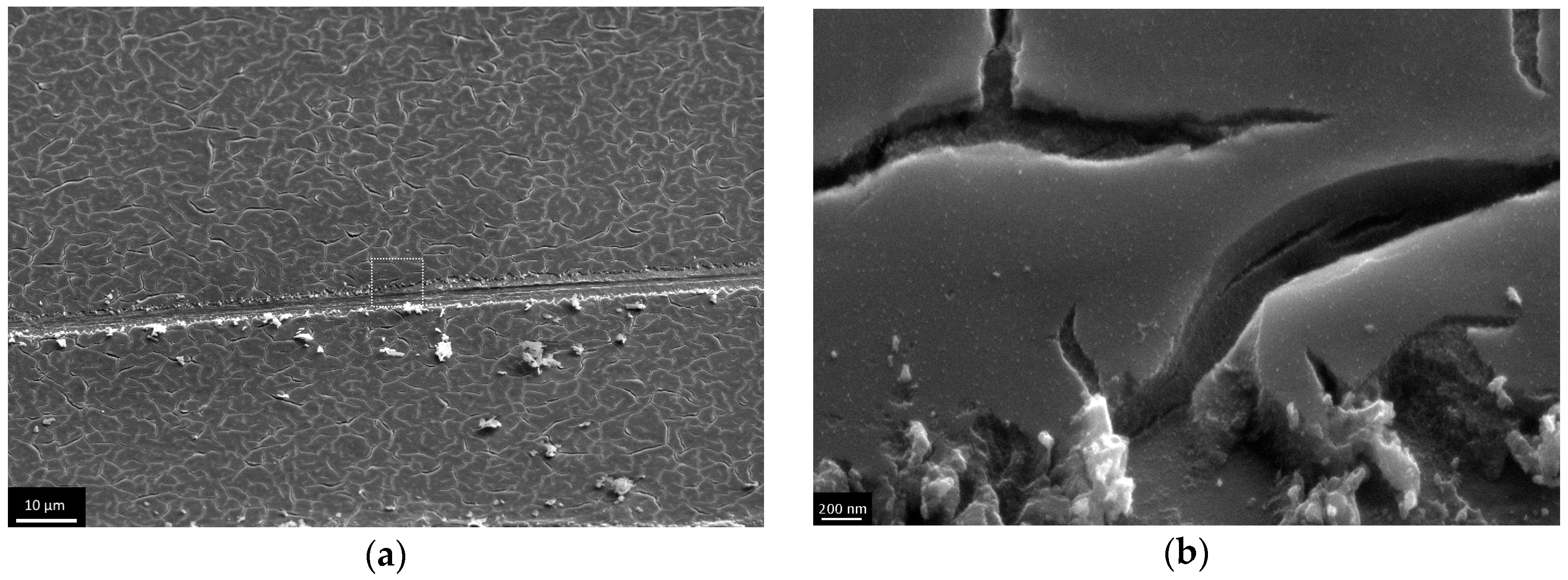
3.3. The Investigation of Morphology of TiO2 Layer, Obtained by Shock Drying
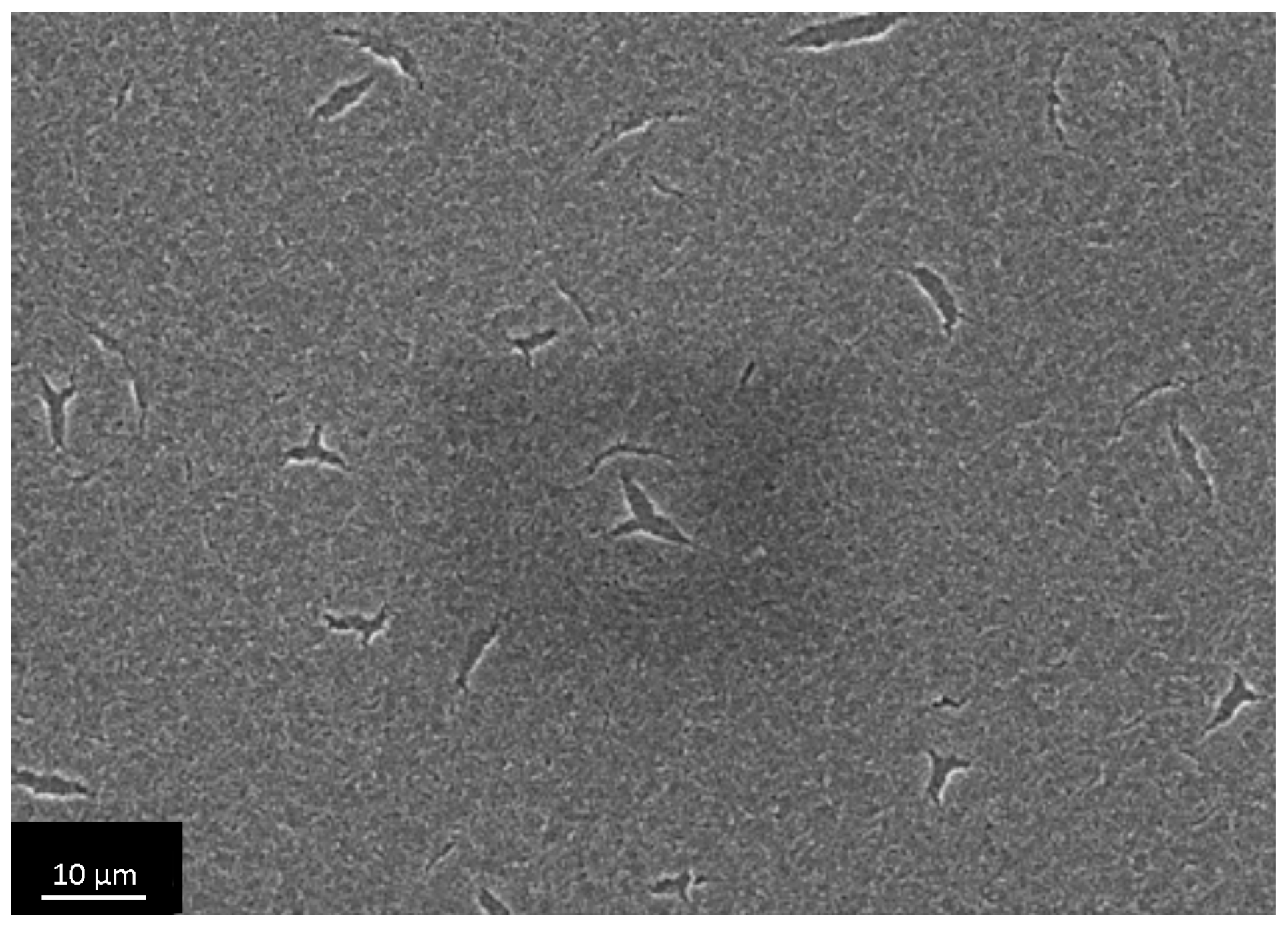
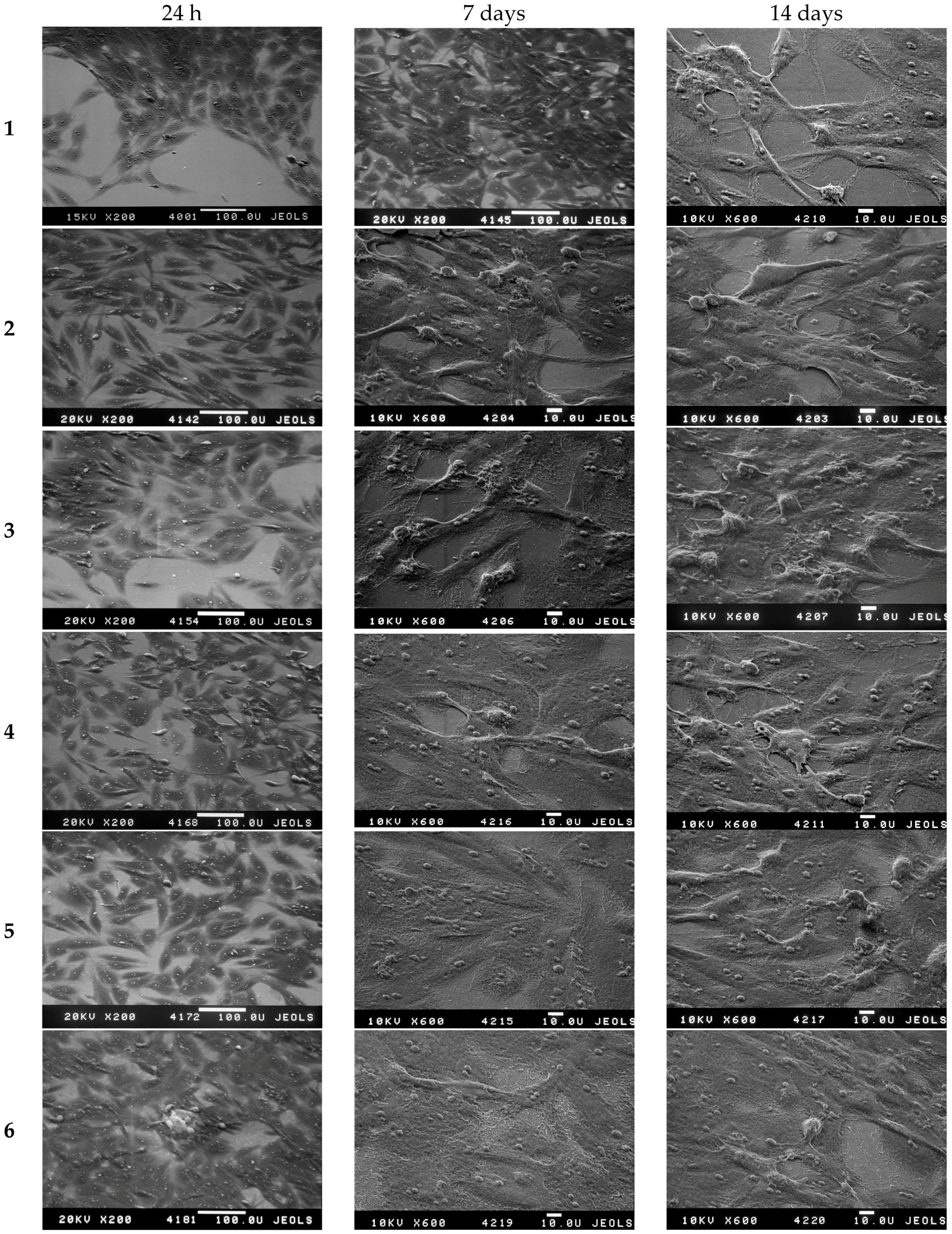
3.4. The Investigation of Osteoblasts Cell Line MC3T3-E1 State, i.e., Adhesion Character, Adhesion Time and Their Morphology on the Sample Surface
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L.; Jones, J.R. (Eds.) Biomaterials, Artificial Organs and Tissue Engineering; Woodhead Publishing Limited: Cambridge, UK, 2005; p. 304.
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. Res. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Geetha, M. Ti based biomaterials, the ultimate choice for orthopaedic implants. A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Bulk Nanostructured Materials: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 440. [Google Scholar]
- Singh, P.P. Drug Alluding Dental Implant. U.S. Patent 0,208,148-A1, 16 August 2012. [Google Scholar]
- Rammelt, S.; Schulze, E.; Bernhardt, R.; Hanisch, U.; Scharnweber, D.; Worch, H.; Zwipp, H.; Biewener, A. Coating of titanium implants with type-I collagen. J. Orthop. Res. 2004, 22, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Singh Harris, L.G.; Tosatti, S.; Wieland, M.; Textor, M.; Richards, R.G. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly (l-lysine)-grafted-poly (ethylene glycol) copolymers. Biomaterials 2004, 25, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Sabattini, V.; Cassinelli, C.; Coelho, P.G.; Minnici, A.; Trani, A.; Dohan Ehrenfest, D.M. Effect of titanium implant surface nanoroughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Maxillof. 2010, 109, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; McCloy, D.; Robertson, M.; Wilkinson, C.D.; Oreffo, R.O. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials 2006, 8, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Grigal, I.P.; Markeev, A.M.; Gudkova, S.A.; Chernikova, A.G.; Mityaev, A.S.; Alekhin, A.P. Correlation between bioactivity and structural properties of titanium dioxide coatings grown by atomic layer deposition. Appl. Surf. Sci. 2012, 258, 3415–3419. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.-M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Bento, A.C.; Almond, D.A.; Brown, S.R.; Turner, J.G. Thermal and optical characterization of the calcium phosphate biomaterial hydroxyapatite. J. Appl. Phys. 1996, 79, 6848–6852. [Google Scholar] [CrossRef]
- Boyd, D.A.; Greengard, L.; Brongersma, M.; El-Naggar, M.Y.; Goodwin, D.G. Plasmon-assisted chemical vapor deposition. Nano Lett. 2006, 6, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Synthesis of titanium dioxide (TiO2) nanomaterials. J. Nanosci. Nanotechnol. 2006, 6, 906–925. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Kim, H.-W.; Kim, H.-E.; Knowles, J.C. Fluor-hydroxyapatite sol–gel coating on titanium substrate for hard tissue implants. Biomaterials 2004, 25, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, E.M.; Søgaard, E.G. Sol–gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar] [CrossRef]
- Scriven, L.E. Physics and applications of dip coating and spin coating. In Better Ceramics through Chemistry III; Brinker, C.J., Clark, D.E., Ulrich, D.R., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1988; pp. 717–729. [Google Scholar]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Zhou, W.; Zong, X.; Wu, X.; Yuan, L.; Shu, Q.; Xia, Y. Plasmacontrolled nanocrystallinity and phase composition of TiO2: A smart way to enhance biomimetic response. J. Biomed. Mater. Res. A 2007, 81, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Schoonman, J.; Jansen, J.A. Electrostatic spray deposition (ESD) of calcium phosphate coatings. J. Biomed. Mater. Res. A 2002, 66, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, D.V.; Zemtsova, E.G.; Valiev, R.Z.; Smirnov, V.M. Formation of micro- and nanostructures on the nanotitanium surface by chemical etching and deposition of titania films by atomic layer deposition (ALD). Materials 2015, 8, 8366–8377. [Google Scholar] [CrossRef]
- Smirnov, V.M. Nanoscaled structuring as a way to constructing new solid substances and materials. Russ. J. Gen. Chem. 2002, 72, 590–612. [Google Scholar] [CrossRef]
- Smirnov, V.M.; Zemtsova, E.G.; Morozov, P.E. Forced organization of magnetic quasi-one-dimensional iron-organic nanostructures on inorganic matrices. Rev. Adv. Mater. Sci. 2009, 21, 205–210. [Google Scholar]
- Smirnov, V.; Bobrysheva, N. Magnetic properties of iron-oxygen nanostructure doped with 4f elements. J. Alloys Compd. 1998, 275–277, 533–536. [Google Scholar] [CrossRef]
- Ermakova, L.E.; Sidorova, M.P.; Smirnov, V.M. Isoelectric point of silicon oxide particles coated by monolayers of oxides of titanium and aluminum. Kolloidnyj Zhurnal 1997, 59, 563–565. [Google Scholar]
- Zemtsova, E.G.; Morozov, P.E.; Smirnov, V.M. Regulation of surface topography of nanostructured titanium using the method of ML–ALD to create bioactive nanocoatings. Mater. Phys. Mech. 2015, 24, 374–381. [Google Scholar]
- Zemtsova, E.G.; Arbenin, A.Y.; Plotnikov, A.F.; Smirnov, V.M. Pore radius fine tuning of a silica matrix (MCM-41) based on the synthesis of alumina nanolayers with different thicknesses by atomic layer deposition. J. Vacuum Sci. Technol. A 2015, 33, 021519. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press Inc.: San Diego, CA, USA, 1990; p. 908. [Google Scholar]
- Takahashi, Y.; Matsuoka, Y. Dip-coating of TiO2 films using a sol derived from Ti(O-i-Pr) 4-diethanolamine-H2O-i-PrOH system. J. Mater. Sci. 1988, 23, 2259–2266. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for 433 grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Blanchard, J.; Ribot, F.; Sanchez, C.; Bellot, P.-V.; Trokiner, A. Structural characterization of titanium-oxo-polymers synthesized in the presence of protons or complexing ligands as inhibitors. J. Non-Cryst. Solids 2000, 265, 83–97. [Google Scholar] [CrossRef]
- Arbenin, A.Y.; Zemtsova, E.G.; Valiev, R.Z.; Smirnov, V.M. Characteristics of the synthesis of TiO2 films on a titanium surface by the sol-gel technique. Russ. J. Gen. Chem. 2014, 84, 2453–2454. [Google Scholar] [CrossRef]
- Raj, V.; Raj, R.M.; Sasireka, A.; Priya, P. Fabrication of TiO2-strontium loaded CaSiO3/biopolymer coatings with enhanced biocompatibility and corrosion resistance by controlled release of minerals for improved orthopedic applications. J. Mech. Behav. Biomed. Mater. 2016, 60, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.X.; Du, J.; Zhao, J.M.; Si, M.S.; Mo, J.J.; Lai, H.C. Characterization and preosteoblastic behavior of hydroxyapatite-deposited nanotube surface of titanium prepared by anodization coupled with alternative immersion method. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
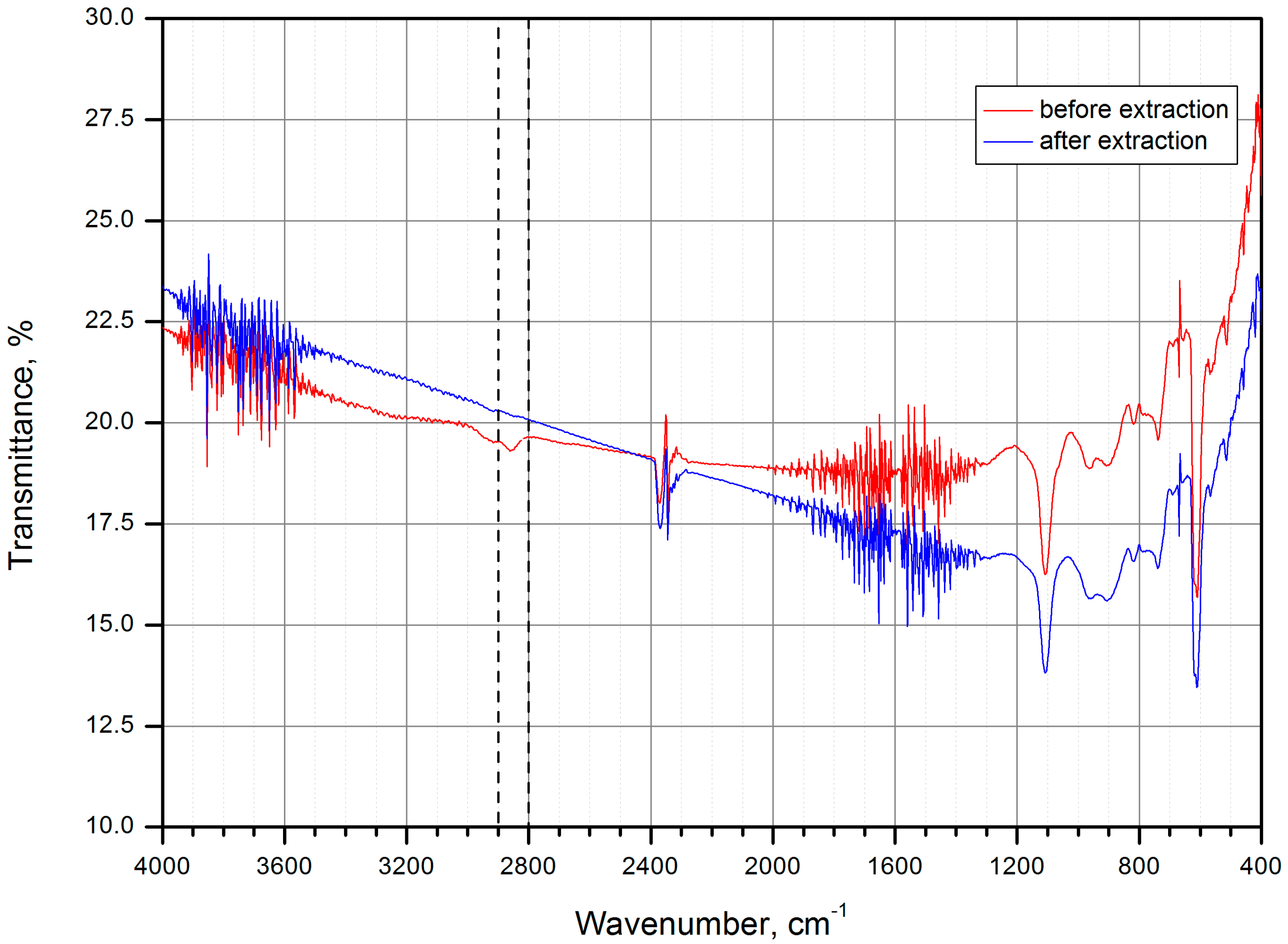

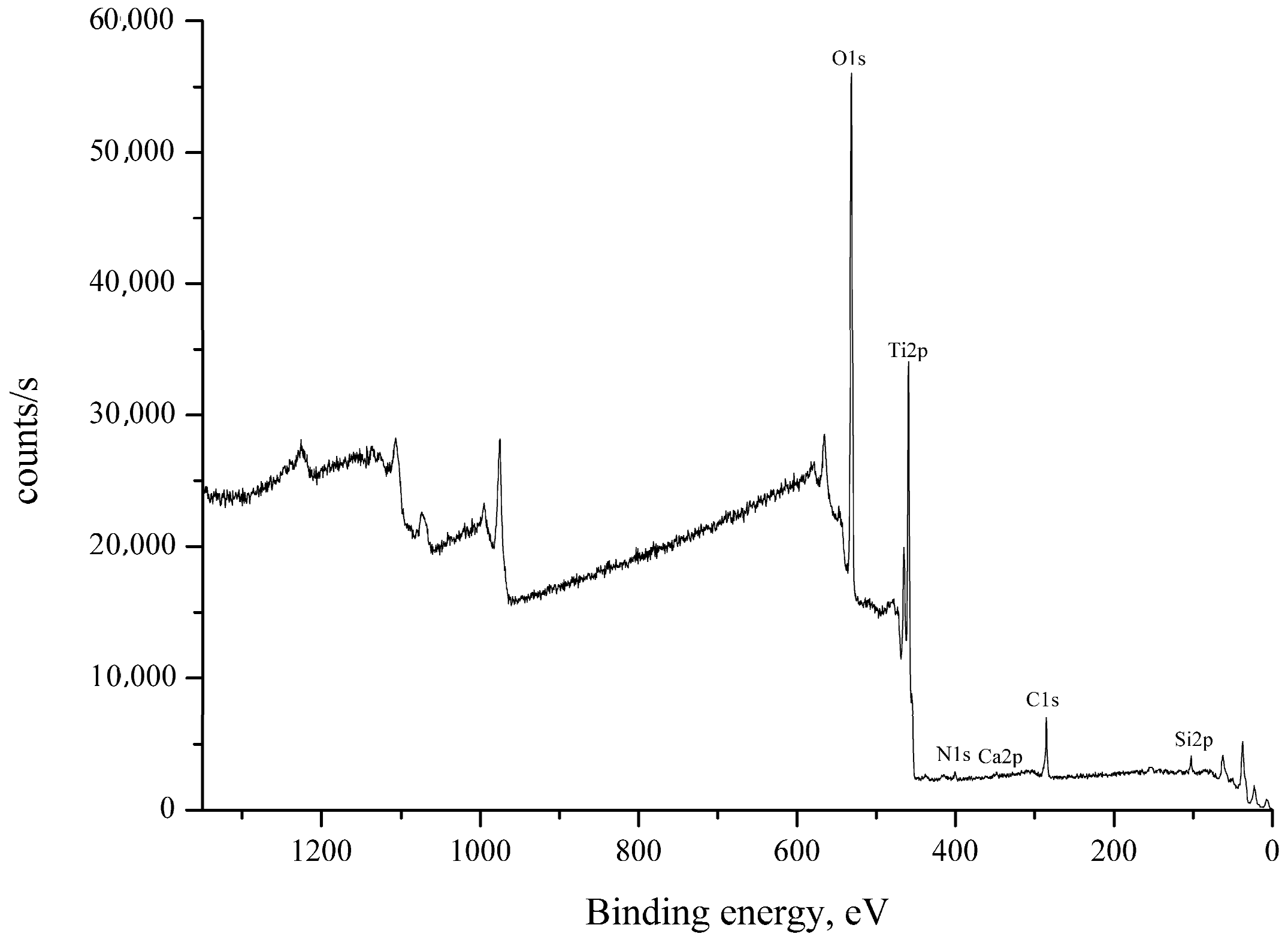
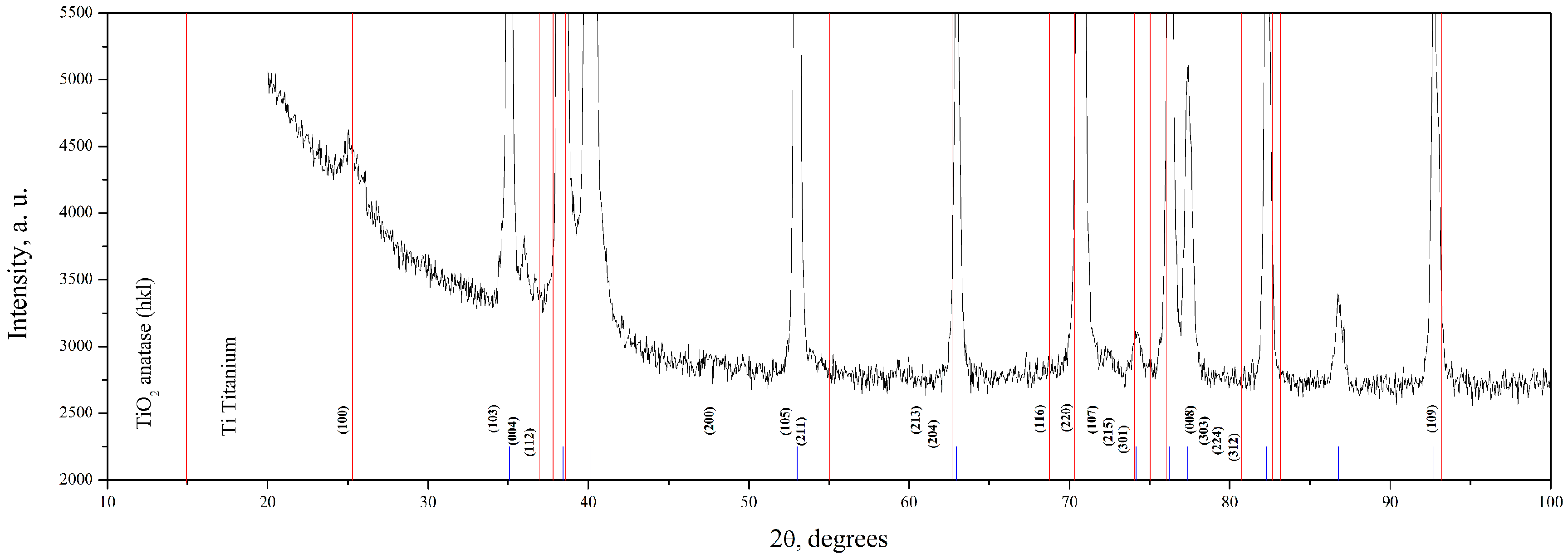

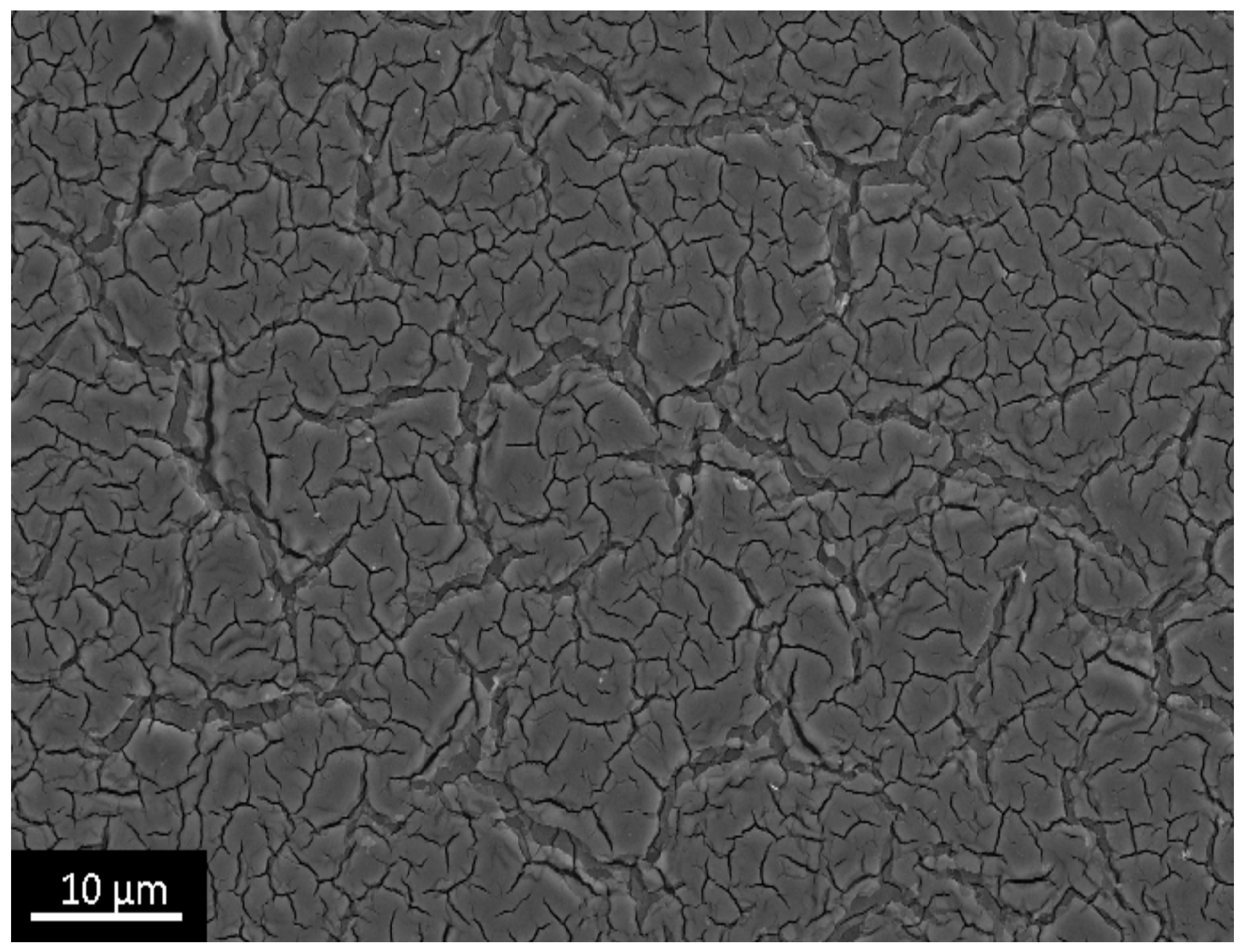

| Name | Peak BE | Atomic % |
|---|---|---|
| O1s | 530.81 | 63.03 |
| Ti2p | 459.27 | 31.57 |
| C1s | 285.07 | 2.52 |
| Si2p | 102.87 | 2.23 |
| N1s | 399.95 | 0.43 |
| Ca2p | 347.87 | 0.22 |
| The number of cycles | 1 | 2 | 3 | 4 | 5 |
| Film thickness (nm) | 33 | 72 | 124 | 173 | 204 |
| The thickness increment (nm) | 33 | 39 | 52 | 49 | 31 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemtsova, E.G.; Arbenin, A.Y.; Valiev, R.Z.; Orekhov, E.V.; Semenov, V.G.; Smirnov, V.M. Two-Level Micro-to-Nanoscale Hierarchical TiO2 Nanolayers on Titanium Surface. Materials 2016, 9, 1010. https://doi.org/10.3390/ma9121010
Zemtsova EG, Arbenin AY, Valiev RZ, Orekhov EV, Semenov VG, Smirnov VM. Two-Level Micro-to-Nanoscale Hierarchical TiO2 Nanolayers on Titanium Surface. Materials. 2016; 9(12):1010. https://doi.org/10.3390/ma9121010
Chicago/Turabian StyleZemtsova, Elena G., Andrei Yu. Arbenin, Ruslan Z. Valiev, Evgeny V. Orekhov, Valentin G. Semenov, and Vladimir M. Smirnov. 2016. "Two-Level Micro-to-Nanoscale Hierarchical TiO2 Nanolayers on Titanium Surface" Materials 9, no. 12: 1010. https://doi.org/10.3390/ma9121010





