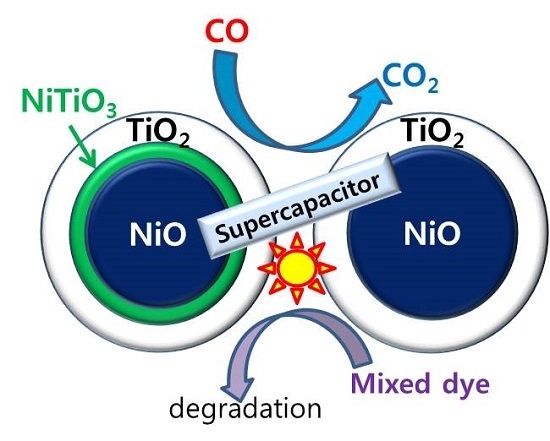
Application of Ni-Oxide@TiO2 Core-Shell Structures to Photocatalytic Mixed Dye Degradation, CO Oxidation, and Supercapacitors
Abstract
:1. Introduction
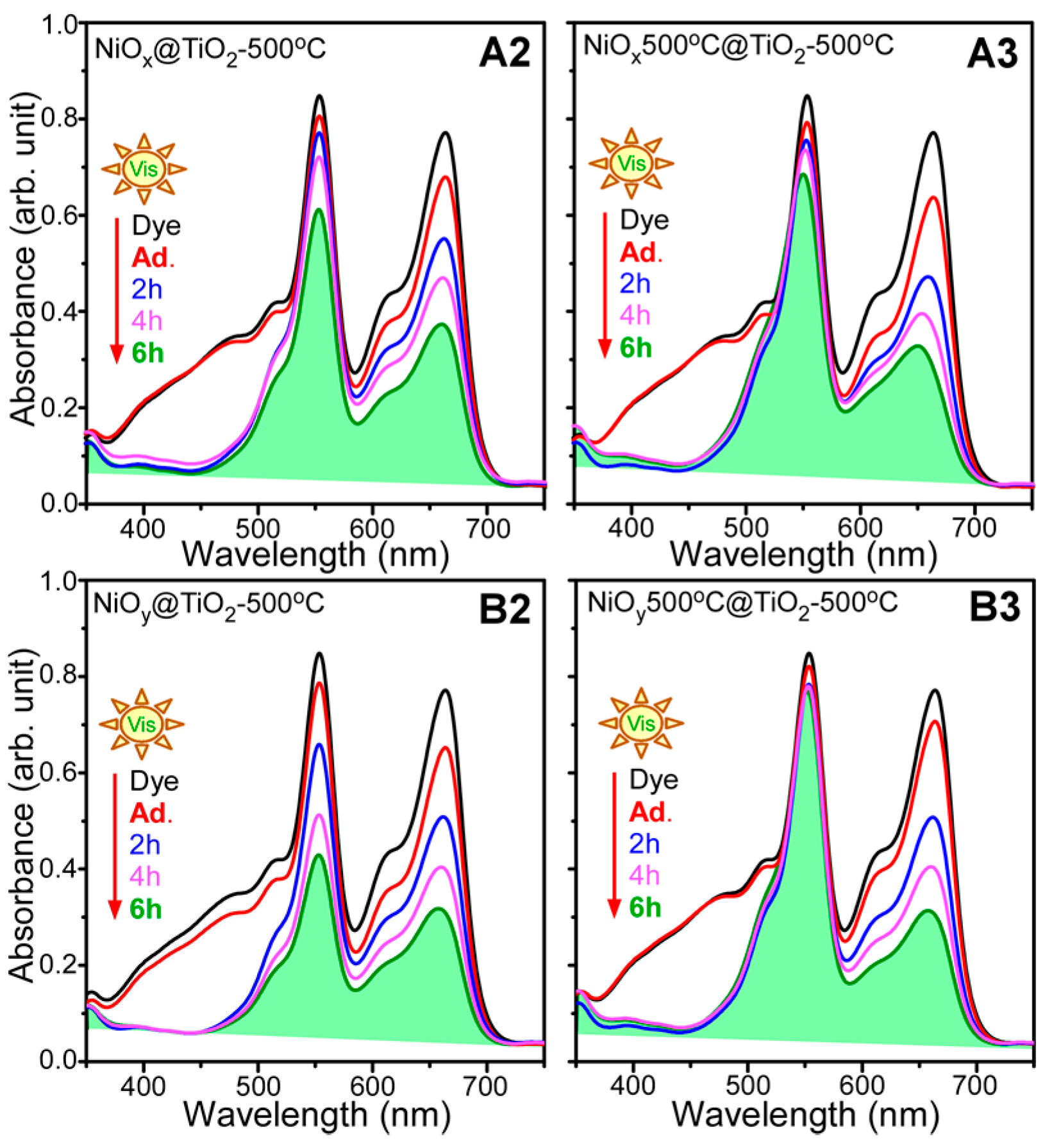
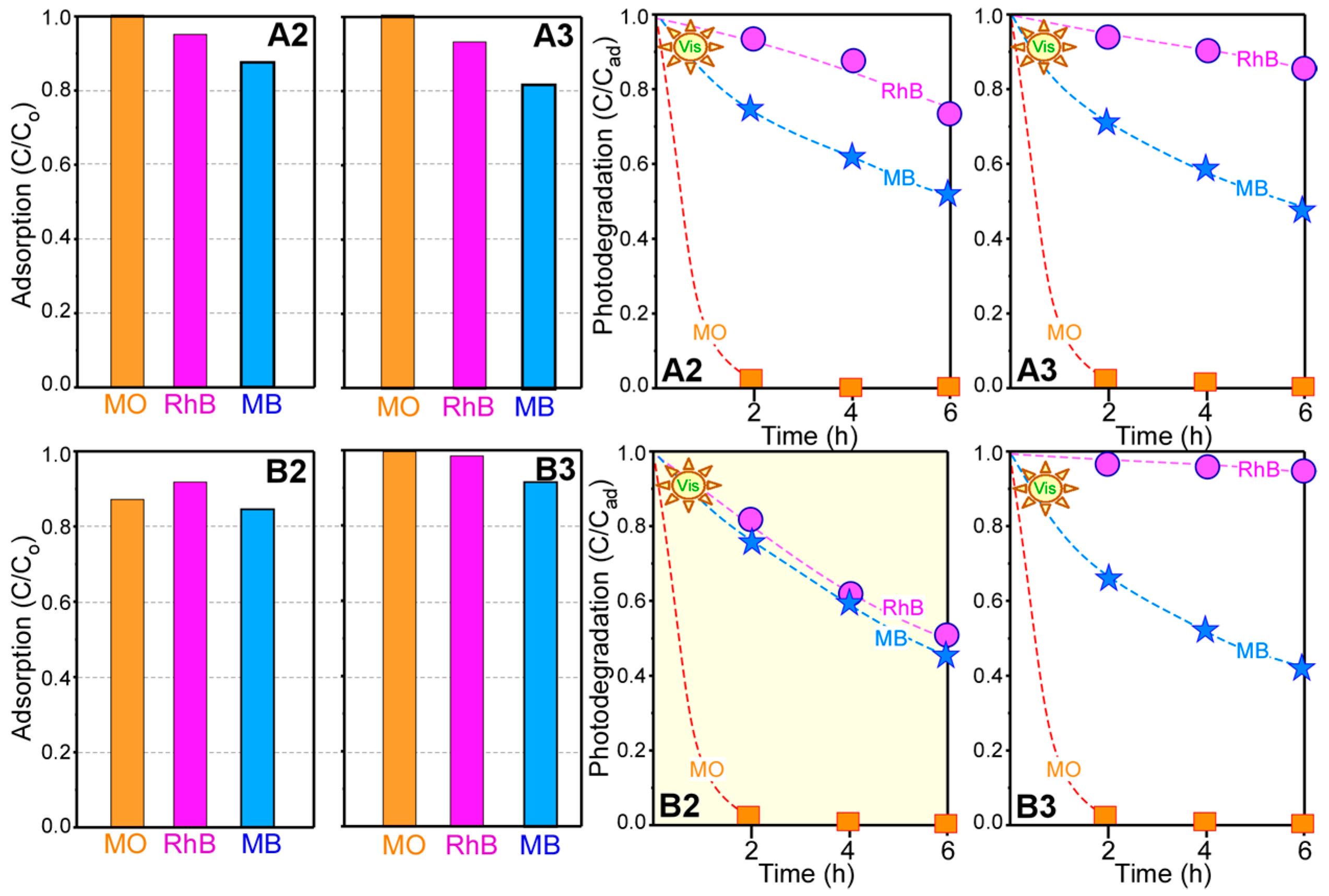
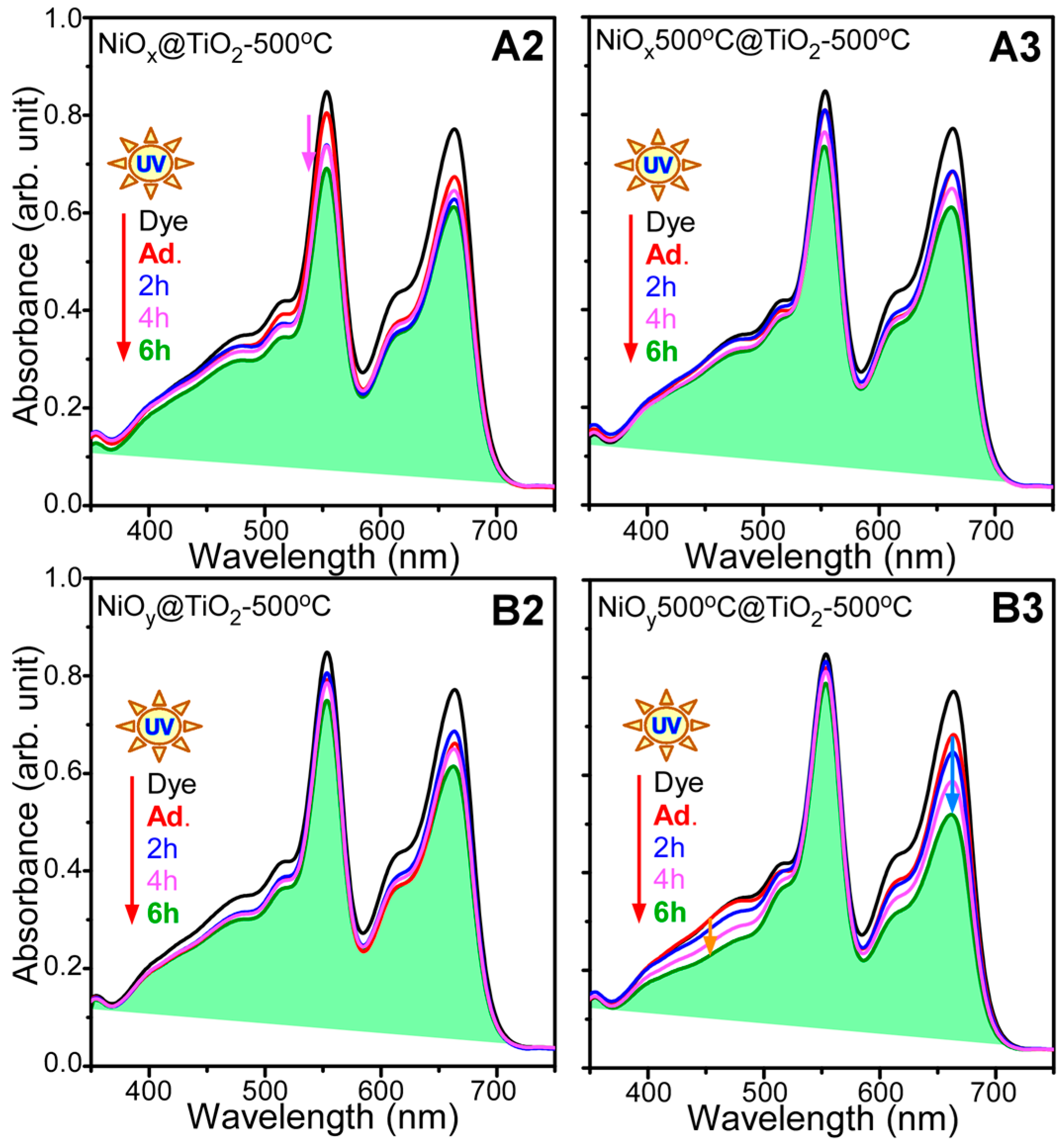
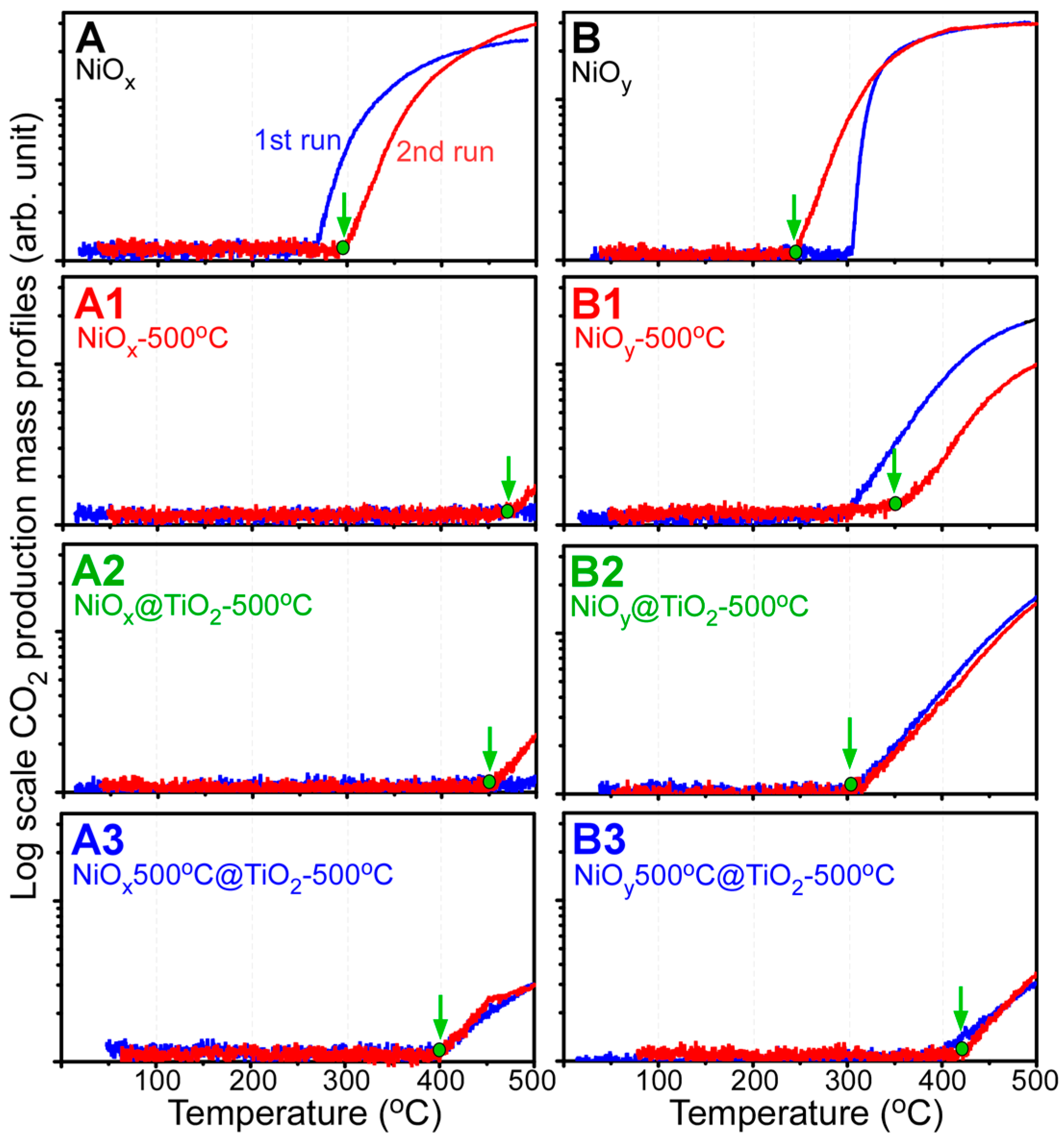
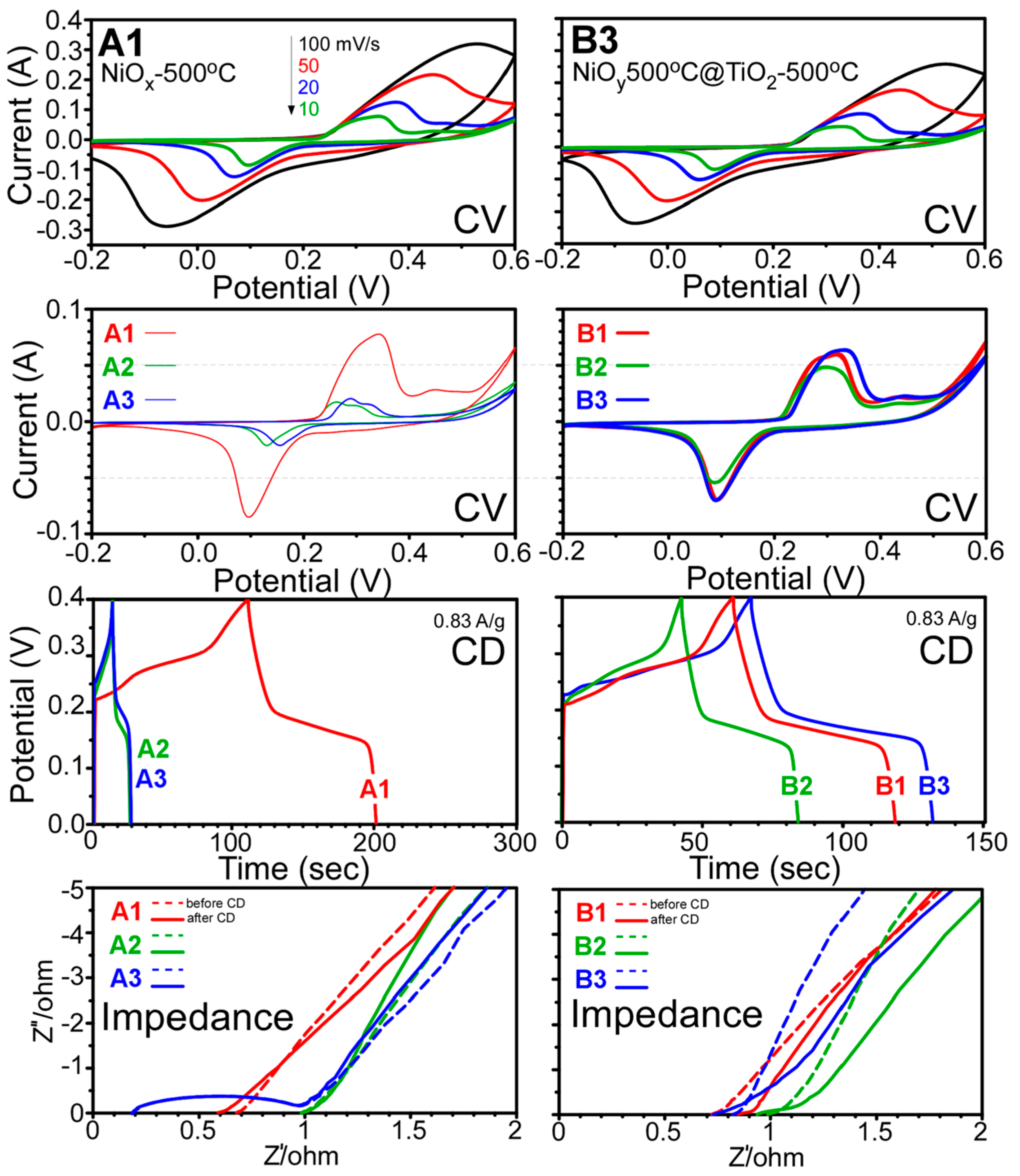
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of Ni Oxide and TiO2 Coating
3.2. Characterization of the Materials
3.3. Photocatalytic Dye Degradation, CO Oxidation, and Supercapacitor Performance Tests
4. Conclusions
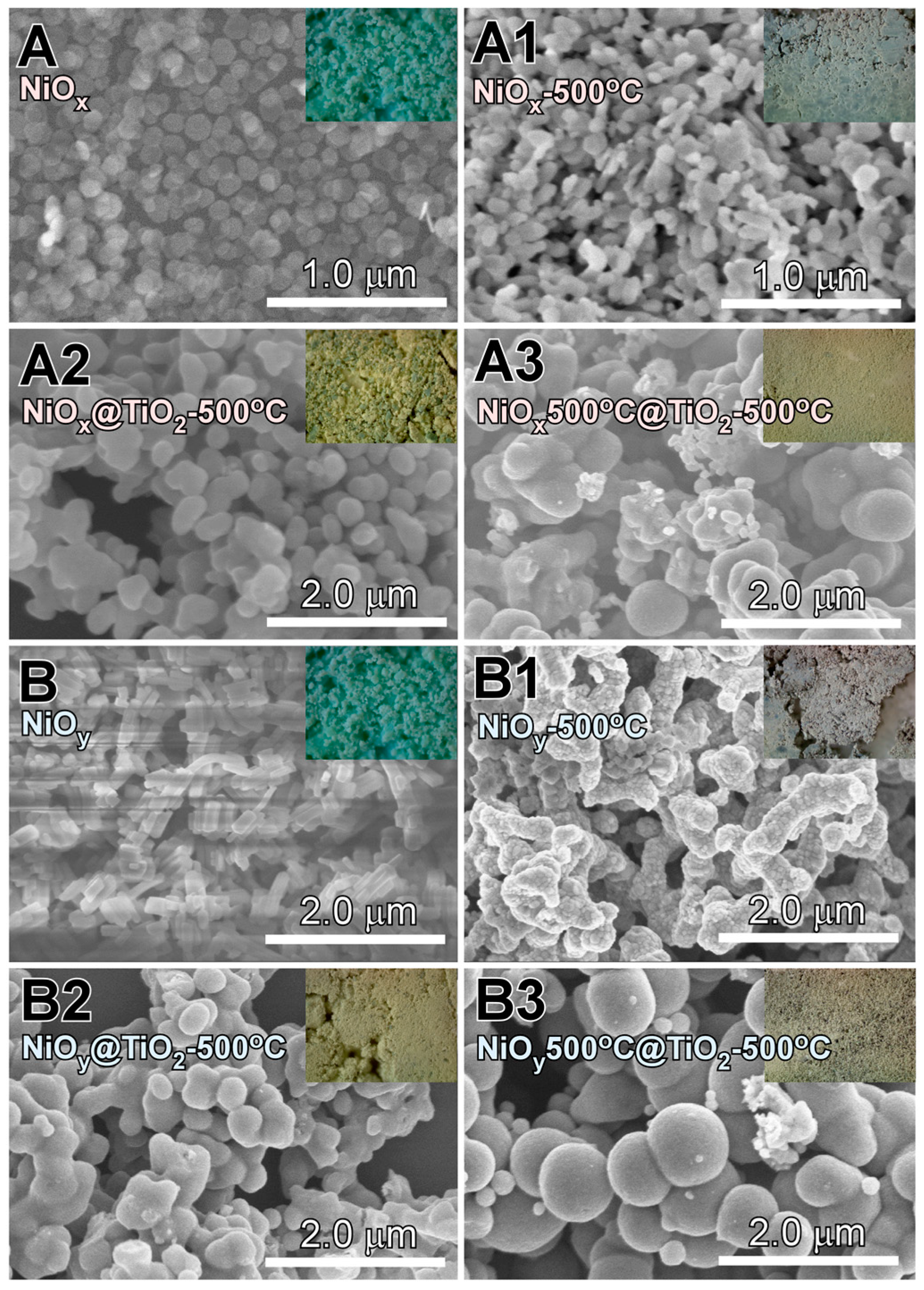
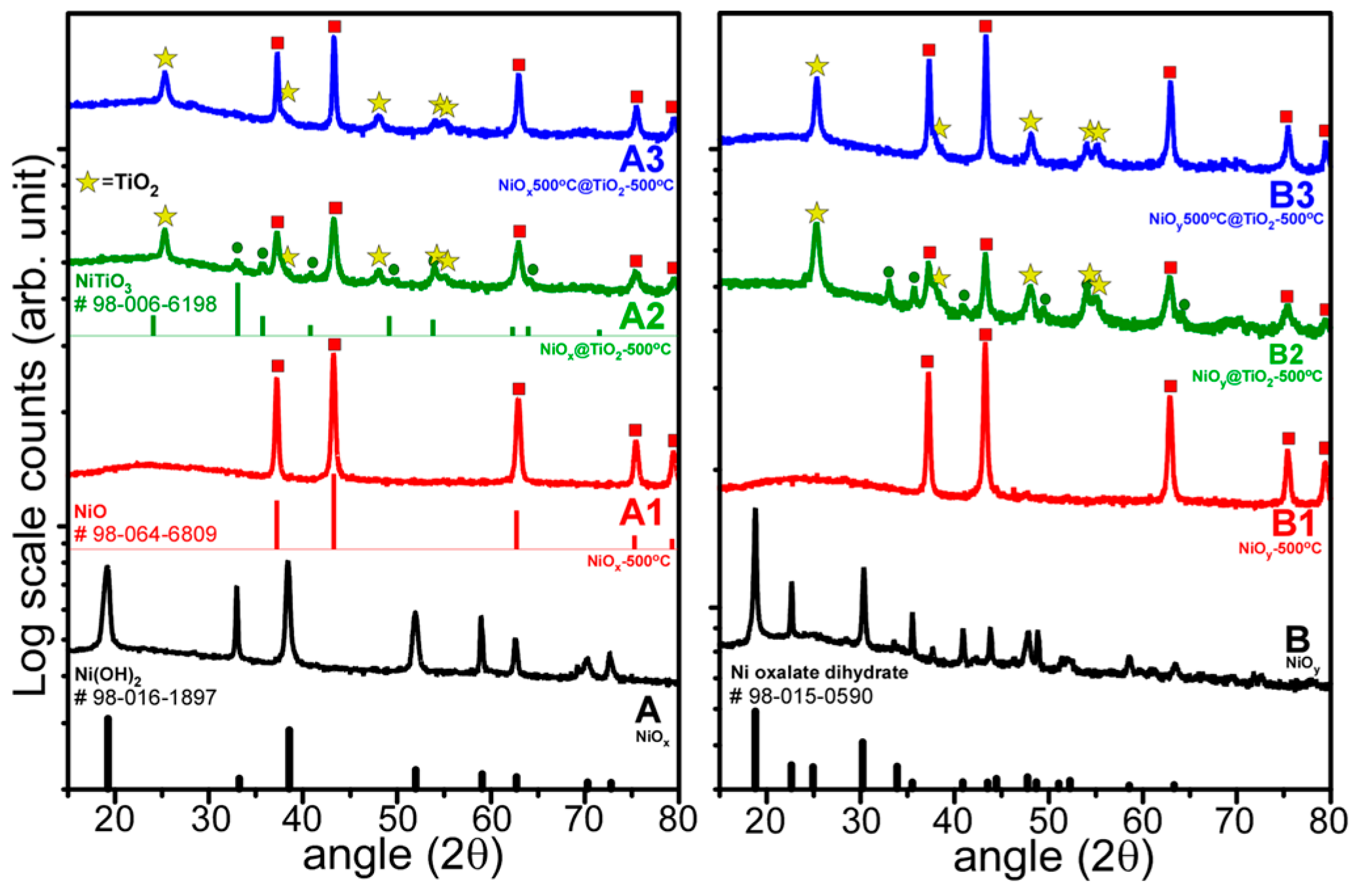
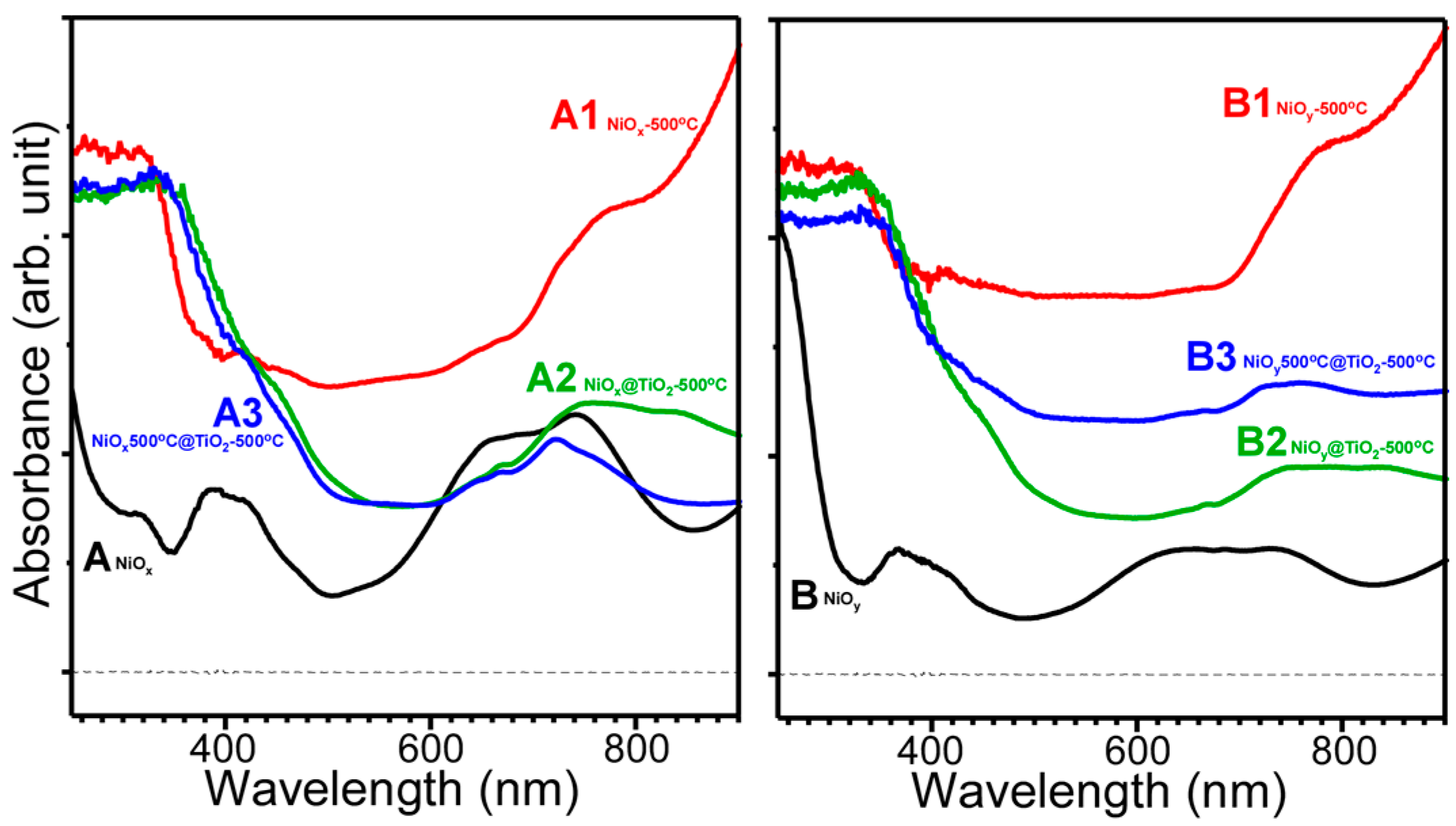
- Nanoplates and nanorods were synthesized by hydrothermal method, and showed XRD patterns of Ni(OH)2 and Ni oxalate complexes, respectively. NiO crystal phase was commonly obtained by thermal annealing at 500 °C.
- NiO@TiO2 core-shells with interfacial NiTiO3 could be prepared by TiO2 coating on as-synthesized Ni samples, followed by thermal annealing at 500 °C. TiO2 coating on annealed (500 °C) NiO, followed by thermal annealing at 500 °C, showed no XRD detectable NiTiO3 at the interface.
- For photocatalytic dye degradation, TiO2 coating on Ni oxalate complex followed by thermal annealing (sample B2) showed the best photocatalytic activity for mixed dye degradation under visible light.
- For CO oxidation, the B2 sample (NiOy@TiO2-500 °C) was also more efficient for CO oxidation than other samples.
- For the case of specific capacitance, the B3 sample (NiOx500°C@TiO2-500°C) was more efficient for specific capacitance than other samples. Specific capacitances were obtained to be in the range of 50–320 F/g. When Ni(OH)2 plates were used as starting material, the TiO2 coating showed a specific capacitance of only 50 F/g. However, when rod-shaped Ni oxalate complex was used, the TiO2 coating showed specific capacitance values of 211 and 134 F/g.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, H. Multilayered metal core-shell nanostructures for inducing a large and tunable local optical field. Phys. Rev. B 2005, 72, 073405. [Google Scholar] [CrossRef]
- Su, L.; Jing, Y.; Zhou, Z. Li ion battery materials with core–shell nanostructures. Nanoscale 2011, 3, 3967–3983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Y.; Lee, K.; Ouyang, M. Nonepitaxial growth of hybrid core-shell nanostructures with large lattice mismatches. Science 2010, 327, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Zheng, M.; Liu, H.; Guan, C.; Mao, L.; Wang, J. 3D TiO2@Ni(OH)2 core-shell arrays with tunable nanostructure for hybrid supercapacitor application. Sci. Rep. 2015, 5, 13940. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Yu, J.S.; Hu, W.; Chen, Z.L.; Memon, H.; Chen, G.L. Titanate nanowire/NiO nanoflake core/shell heterostructured nanonanocomposite catalyst for methylene blue photodegradation. RSC Adv. 2016, 6, 67827–67832. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Han, J.; Guo, R.; Xiong, H.; Yin, Y. TiO2/NiO hybrid shells: p–n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. [Google Scholar] [CrossRef]
- Wu, J.B.; Guo, R.Q.; Huang, X.H.; Lin, Y. Construction of self-supported porous TiO2/NiO core/shell nanorod arrays for electrochemical capacitor application. J. Power Sources 2013, 243, 317–322. [Google Scholar] [CrossRef]
- Kim, J.-H.; Zhu, K.; Yan, Y.; Perkins, C.L.; Frank, A.J. Microstructure and pseudocapacitive properties of electrodes constructed of oriented NiO-TiO2 nanotube arrays. Nano Lett. 2010, 10, 4099–4104. [Google Scholar] [CrossRef] [PubMed]
- Balogun, M.-S.; Qiu, W.; Luo, Y.; Huang, Y.; Yang, H.; Li, M.; Yu, M.; Liang, C.; Fang, P.; Liu, P.; et al. Improving the lithium-storage properties of self-grown nickel oxide: A back-up from TiO2 nanoparticles. Chem. Electro. Chem. 2015, 2, 1243–1248. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Dong, W.; Zhang, X.; Tao, C.; Shen, L.; Zhou, J.; Fei, Y.; Ruan, S. Metal-semiconductor-metal TiO2 ultraviolet detectors with Ni electrodes. Appl. Phys. Lett. 2009, 94, 123502. [Google Scholar] [CrossRef]
- Wang, W.; Tian, M.; Abdulagatov, A.; George, S.M.; Lee, Y.-C.; Yang, R. Three-dimensional Ni/TiO2 nanowire network for high areal capacity lithium ion microbattery applications. Nano Lett. 2012, 12, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, J.-H.; Kang, Y.C. One-pot rapid synthesis of core–shell structured NiO@TiO2 nanopowders and their excellent electrochemical properties as anode materials for lithium ion batteries. Nanoscale 2013, 5, 12645–12650. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, K.; Tanpo, Y.; Nagashima, Y.; Kikuchi, R.; Satokawa, S. Effects of preparation conditions of Ni/TiO2 catalysts for selective CO methanation in the reformate gas. J. Appl. Catal. A 2103, 452, 174–178. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. CO methanation toward the production of synthetic natural gas over highly active Ni/TiO2 catalyst. AIChE J. 2014, 60, 1027–1035. [Google Scholar] [CrossRef]
- Ong, W.-J.; Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation. RSC Adv. 2013, 3, 4505–4509. [Google Scholar] [CrossRef]
- Sim, L.C.; Ng, K.W.; Ibrahim, S.; Saravanan, P. Preparation of improved p-n junction NiO/TiO2 nanotubes for solar-energy-driven light photocatalysis. Int. J. Photoenergy 2013, 2013, 659013. [Google Scholar] [CrossRef]
- Ku, Y.; Lin, C.-N.; Hou, W.-M. Characterization of coupled NiO/TiO2 photocatalyst for the photocatalytic reduction of Cr(VI) in aqueous solution. J. Mol. Catal. A 2011, 349, 20–27. [Google Scholar] [CrossRef]
- Chen, C.-J.; Liao, C.-H.; Hsu, K.-C.; Wu, Y.-T.; Wu, J.C.S. P–N junction mechanism on improved NiO/TiO2 photocatalyst. Catal. Commun. 2011, 12, 1307–1310. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A. Study of the effect of additives on the photocatalytic degradation of a triphenylmethane dye in the presence of immobilized TiO2/NiO nanoparticles: Artificial neural network modeling. Ind. Eng. Chem. Res. 2014, 53, 6881–6895. [Google Scholar] [CrossRef]
- Hang, R.; Huang, X.; Tian, L.; He, Z.; Tang, B. Preparation, characterization, corrosion behavior and bioactivity of Ni2O3-doped TiO2 nanotubes on NiTi alloy. Electrochim. Acta 2102, 70, 382–393. [Google Scholar] [CrossRef]
- Hang, R.; Zong, M.; Bai, L.; Gao, A.; Liu, Y.; Zhang, X.; Huang, X.; Tang, B.; Chu, P.K. Anodic growth of ultra-long Ni-Ti-O nanopores. Electrochem. Commun. 2016, 71, 28–32. [Google Scholar] [CrossRef]
- Hou, G.-Y.; Xie, Y.-Y.; Wu, L.-K.; Cao, H.-Z.; Tang, Y.-P.; Zheng, G.-Q. Electrocatalytic performance of Ni-Ti-O nanotube arrays/NiTi alloy electrode annealed under H2 atmosphere for electro-oxidation of methanol. Int. J. Hydrogen Energy 2016, 41, 9295–9302. [Google Scholar] [CrossRef]
- Hou, L.; Li, S.; Lin, Y.; Wang, D.; Xie, T. Photogenerated charges transfer across the interface between NiO and TiO2 nanotube arrays for photocatalytic degradation: A surface photovoltage study. J. Colloid Interface Sci. 2016, 464, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, W.; Cheng, B. Synthesis and enhanced photocatalytic activity of a hierarchical porous flowerlike p–n junction NiO/TiO2 photocatalyst. Chem. Asian J. 2010, 5, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, K.; Ganapathy, A.; Paramasivan, G.; Govindasamy, M.; Viswanathan, A. NiO/TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Am. Ceram. Soc. 2011, 94, 2499–2505. [Google Scholar] [CrossRef]
- Gao, Z.-D.; Han, Y.; Wang, Y.; Xu, J.; Song, Y.-Y. One-Step to prepare self-organized nanoporous NiO/TiO2 layers and its use in non-enzymatic glucose sensing. Sci. Rep. 2013, 3, 3323. [Google Scholar] [CrossRef] [PubMed]
- Salmistraro, M.; Schwartzberg, A.; Bao, W.; Depero, L.E.; Weber-Bargioni, A.; Cabrini, S.; Alessandri, I. Triggering and monitoring plasmon-enhanced reactions by optical nanoantennas coupled to photocatalytic beads. Small 2013, 9, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Ferroni, M.; Depero, L.E. In situ plasmon-heating-induced generation of Au/TiO2 “hot spots” on colloidal crystals. Chem. Phys. Chem. 2009, 10, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I. Enhancing Raman scattering without plasmons: Unprecedented sensitivity achieved by TiO2 shell-based resonators. J. Am. Chem. Soc. 2013, 135, 5541–5544. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Depero, L.E. All-oxide Raman-active traps for light and matter: Probing redox homeostasis model reactions in aqueous environment. Small 2014, 10, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman scattering with dielectrics. Chem. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, L.; Liao, M.; Hu, X.; Fang, X. Electrical transport properties of large, individual NiCo2O4 nanoplates. Adv. Funct. Mater. 2012, 22, 998–1004. [Google Scholar] [CrossRef]
- Lee, S.; Kang, J.S.; Leung, K.T.; Kim, S.K.; Sohn, Y. Magnetic Ni-Co alloys induced by water gas shift reaction, Ni-Co oxides by CO oxidation and their supercapacitor applications. Appl. Suf. Sci. 2016, 386, 393–404. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, C.; Wu, S.; Liu, G.; Zhang, F.; Wang, T. Lotus-root-like NiO nanosheets and flower-like NiO microspheres: Synthesis and magnetic properties. CrystEngComm 2011, 13, 4930–4934. [Google Scholar] [CrossRef]
- Harvey, A.; He, X.; Godwin, I.J.; Backes, C.; McAteer, D.; Berner, N.C.; McEvoy, N.; Ferguson, A.; Shmeliov, A.; Lyons, M.E.G.; et al. Production of Ni(OH)2 nanosheets by liquid phase exfoliation: From optical properties to electrochemical applications. J. Mater. Chem. A 2016, 4, 11046–11059. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, D.; Chen, D.; Waller, G.; Bu, Y.; Wang, Z.; Liu, M. Three-dimensional ultrathin Ni(OH)2 nanosheets grown on nickel foam for high-performance supercapacitors. Nano Energy 2015, 11, 154–161. [Google Scholar] [CrossRef]
- Sun, W.; Rui, X.; Ulaganathan, M.; Madhavi, S.; Yan, Q. Few-layered Ni(OH)2 nanosheets for high-performance supercapacitors. J. Power Sources 2015, 295, 323–328. [Google Scholar] [CrossRef]
- Gao, H.; Wang, G.; Yang, M.; Tan, L.; Yu, J. Novel tunable hierarchical Ni–Co hydroxide and oxide assembled from two-wheeled units. Nanotechnology 2012, 23, 015607. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, P.A.; Burgess, K.; Wang, L.; Chowdhury, R.R.; Lotus, A.F.; Moula, G. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology 2012, 23, 425606. [Google Scholar] [CrossRef] [PubMed]
- Wezynfeld, N.E.; Goch, W.; Bal, W.; Frączyk, T. cis-Urocanic acid as a potential nickel(II) binding molecule in the human skin. Dalton Trans. 2014, 43, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.I.; Lee, S.; Kim, S.K.; Kim, Y.I.; Cho, D.W.; Khan, M.M.; Sohn, Y. Fabrication of ZnO, ZnS, Ag-ZnS, and Au-ZnS microspheres for photocatalytic activities, CO oxidation and 2-hydroxyterephthalic acid synthesis. J. Alloys Compd. 2016, 675, 46–56. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Pradhan, D.; Sohn, Y. AgX (X = Cl, Br, I)/BiOX nanoplates and microspheres for pure and mixed (methyl orange, rhodamine B and methylene blue) dyes. J. Ind. Eng. Chem. 2016, 35, 231–252. [Google Scholar] [CrossRef]
- Choi, Y.I.; Jeon, K.H.; Kim, H.S.; Lee, J.H.; Park, S.J.; Roh, J.E.; Khan, M.M.; Sohn, Y. TiO2/BiOX (X = Cl, Br, I) hybrid microspheres for artificial waste water and real sample treatment under visible light irradiation. Sep. Purif. Technol. 2016, 160, 28–42. [Google Scholar] [CrossRef]
- Yoon, H.J.; Choi, Y.I.; Jang, E.S.; Sohn, Y. Graphene, charcoal, ZnO, and ZnS/BiOX (X = Cl, Br, and I) hybrid microspheres for photocatalytic simulated real mixed dye treatments. J. Ind. Eng. Chem. 2015, 32, 137–152. [Google Scholar] [CrossRef]
- Choi, Y.I.; Kim, Y.I.; Cho, D.W.; Kang, J.S.; Leung, K.T.; Sohn, Y. Recyclable magnetic CoFe2O4/BiOX (X = Cl, Br and I) microflowers for photocatalytic treatment of water contaminated with methyl orange, rhodamine B, methylene blue, and a mixed dye. RSC Adv. 2015, 5, 79624–79634. [Google Scholar] [CrossRef]
- Shifu, C.; Sujuan, Z.; Wei, L.; Wei, Z. Preparation and activity evaluation of p–n junction photocatalyst NiO/TiO2. J. Hazard. Mater. 2008, 155, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Kim, Y.; Cho, D.W.; Pradhan, D.; Sohn, Y. Adsorption/photocatalytic performances of hierarchical flowerlike BiOBrxCl1−x nanostructures for methyl orange, Rhodamine B and methylene blue. Mater. Sci. Semicond. Process. 2104, 27, 181–190. [Google Scholar] [CrossRef]
- Na, Y.; Lee, S.W.; Roy, N.; Pradhan, D.; Sohn, Y. Room temperature light-induced recrystallization of Cu2O cubes to CuO nanoribbons in water. CrystEngComm 2014, 16, 8546–8554. [Google Scholar] [CrossRef]
- Park, Y.; Na, Y.; Pradhan, D.; Min, B.-K.; Sohn, Y. Adsorption and UV/Visible photocatalytic performance of echinoid-like BiOI for methyl orange, Rhodamine B and methylene blue: Ag and Ti-loading effects. CrystEngComm 2014, 16, 3155–3167. [Google Scholar] [CrossRef]
- Kim, W.J.; Pradhan, D.; Min, B.-K.; Sohn, Y. Adsorption/photocatalytic activity and fundamental natures of BiOCl and BiOClxI1−x prepared in water and ethylene glycol environments, and Ag and Au-doping effects. Appl. Catal. B 2014, 147, 711–725. [Google Scholar] [CrossRef]
- Lee, S.; Cho, I.; Sohn, Y. Hierarchical BiOBr, AgBr/BiOBr and BiOBrxI1-x nano-assembled microspheres for photocatalytic methyl orange treatment. J. Nanosci. Nanotechnol. 2015, 15, 8362–8369. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.I.; Jung, H.J.; Shin, W.G.; Sohn, Y. Band gap-engineered ZnO and Ag/ZnO by ball-milling method and their photocatalytic and Fenton-like photocatalytic activities. Appl. Surf. Sci. 2015, 356, 615–625. [Google Scholar] [CrossRef]
- Lee, S.; Kang, J.-S.; Leung, K.T.; Lee, W.; Kim, D.; Han, S.; Yoo, W.; Yoon, H.J.; Nam, K.; Sohn, Y. Unique multi-phase Co/Fe/CoFe2O4 by water-gas shift reaction, CO oxidation and enhanced supercapacitor performances. J. Ind. Eng. Chem. 2016, 43, 69–77. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, S.W.; Sohn, Y. Metallic Sn spheres and SnO2@C core-shells by anaerobic and aerobic catalytic ethanol and CO oxidation reactions over SnO2 nanoparticles. Sci. Rep. 2015, 5, 13448. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, S.W.; Kim, K.H.; Min, B.-K.; Nayak, A.K.; Pradhan, D.; Sohn, Y. Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts, and single crystalline ultra-long Na4Mn9O18 nanowires. Sci. Rep. 2015, 5, 18275. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, S.K.; Pradhan, D.; Sohn, Y. Thermal H2-treatment effects on CO/CO2 conversion over Pd-doped CeO2 comparison with Au and Ag-doped CeO2. React. Kinet. Mech. Cat. 2014, 113, 85–100. [Google Scholar] [CrossRef]
- Ci, S.; Wen, Z.; Qian, Y.; Mao, S.; Cui, S.; Chen, J. NiO-microflower formed by nanowire-weaving nanosheets with interconnected Ni-network decoration as supercapacitor electrode. Sci. Rep. 2015, 5, 11919. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, C.; Zhou, L.; Liu, Y.; Huang, H. Supercapacitor application of nickel oxide-titania nanocomposites. Compos. Sci. Technol. 2009, 69, 2108–2114. [Google Scholar] [CrossRef]
- Cui, L.H.; Wang, Y.; Shu, X.; Zhang, J.F.; Yu, C.P.; Cui, J.W.; Zheng, H.M.; Zhang, Y.; Wu, Y.C. Supercapacitive performance of hydrogenated TiO2 nanotube arrays decorated with nickel oxide nanoparticles. RSC Adv. 2016, 6, 12185–12192. [Google Scholar] [CrossRef]

| Catalysts | Order of Dye Degradation under Visible Light | Reference |
|---|---|---|
| ZnO, ZnS, Au-ZnS, Ag-ZnS | RhB < MB << MO | 41 |
| BiOX, AgX/BiOX (X = Cl, Br, I) | RhB < MB << MO | 42 |
| TiO2/BiOX (X = Cl, Br, I) | RhB < MB << MO | 43 |
| Graphene, Charcoal, ZnO, and ZnS/BiOX (X = Cl, Br, I) | RhB < MB < MO | 44 |
| Ag/ZnO by wet-milling method | MB < RhB < MO | 52 |
| NiO@TiO2 core-shells | RhB < MB << MO | This study |
| Samples | Preparation Methods | Specific Capacitance | Reference |
|---|---|---|---|
| TiO2@Ni(OH)2 nanowire arrays | Hydrothermal synthesis and chemical bath deposition | 181 F/g at 5 mV/s | 4 |
| TiO2/NiO nanorod arrays | Hydrothermal synthesis and electro-deposition methods | 611 F/g at 2 A/g | 7 |
| flower-like NiO–TiO2 nanocomposite | One (or multi)-cycle alternate electrodeposition-oxidation and thermal dehydration | 46.3 mF·cm−2 | 58 |
| NiOx decorated TiO2 nanotubes | Cyclic voltammetry electrochemical deposition process | 689.28 F/g at 1.5 A/g | 59 |
| NiO-TiO2 nanotubes | Electrochemical anodization and thermal annealing | 40–300 F/g | 8 |
| NiO@TiO2 core-shells | Wet chemical and thermal annealing | 50–211 F/g at 0.83 A/g | This study |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, J.; Nam, K.; Shin, W.G.; Sohn, Y. Application of Ni-Oxide@TiO2 Core-Shell Structures to Photocatalytic Mixed Dye Degradation, CO Oxidation, and Supercapacitors. Materials 2016, 9, 1024. https://doi.org/10.3390/ma9121024
Lee S, Lee J, Nam K, Shin WG, Sohn Y. Application of Ni-Oxide@TiO2 Core-Shell Structures to Photocatalytic Mixed Dye Degradation, CO Oxidation, and Supercapacitors. Materials. 2016; 9(12):1024. https://doi.org/10.3390/ma9121024
Chicago/Turabian StyleLee, Seungwon, Jisuk Lee, Kyusuk Nam, Weon Gyu Shin, and Youngku Sohn. 2016. "Application of Ni-Oxide@TiO2 Core-Shell Structures to Photocatalytic Mixed Dye Degradation, CO Oxidation, and Supercapacitors" Materials 9, no. 12: 1024. https://doi.org/10.3390/ma9121024