Microstructure and Characteristics of Calcium Phosphate Layers on Bioactive Oxide Surfaces of Air-Sintered Titanium Foams after Immersion in Simulated Body Fluid
Abstract
:1. Introduction
2. Results and Discussion
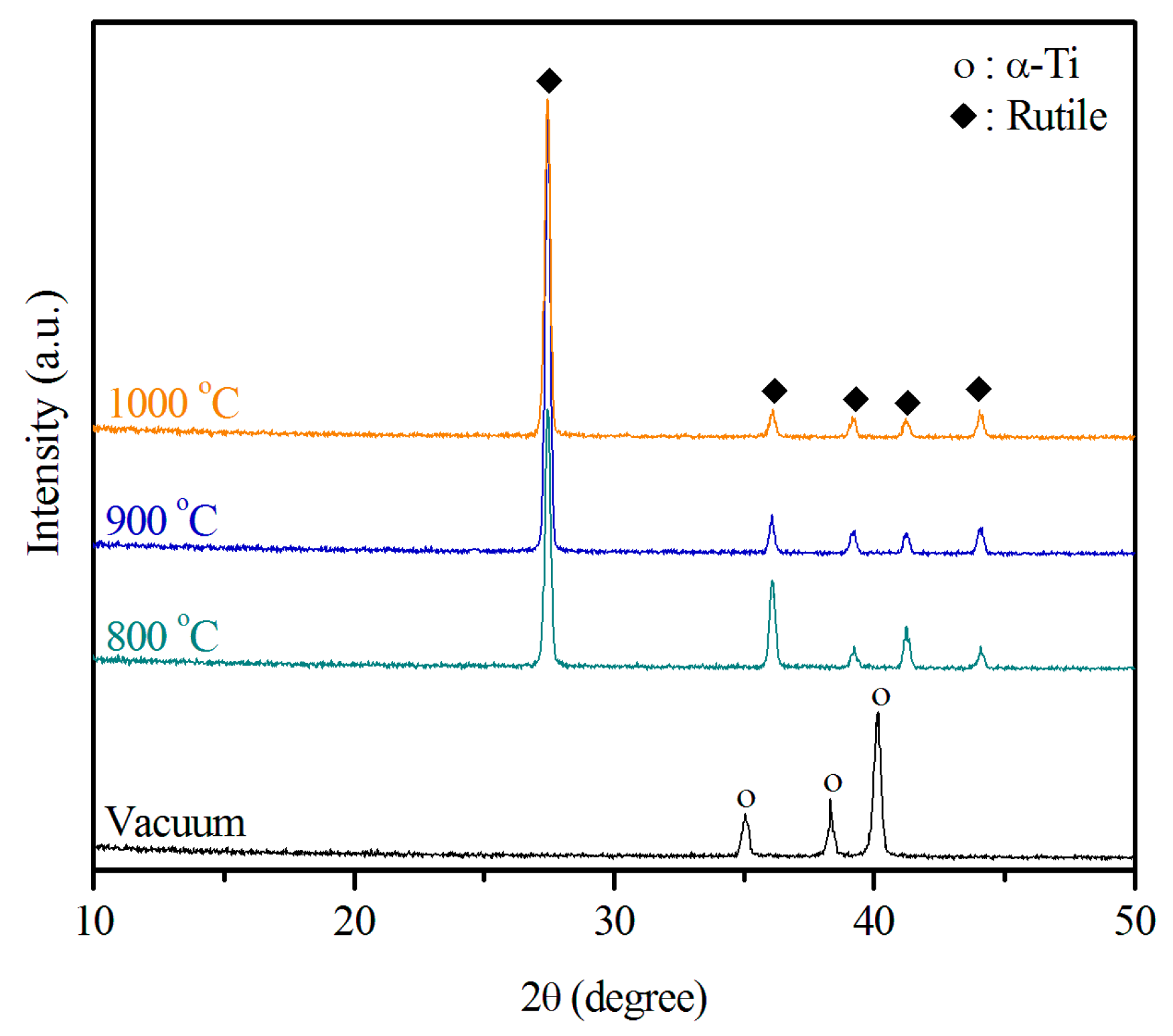
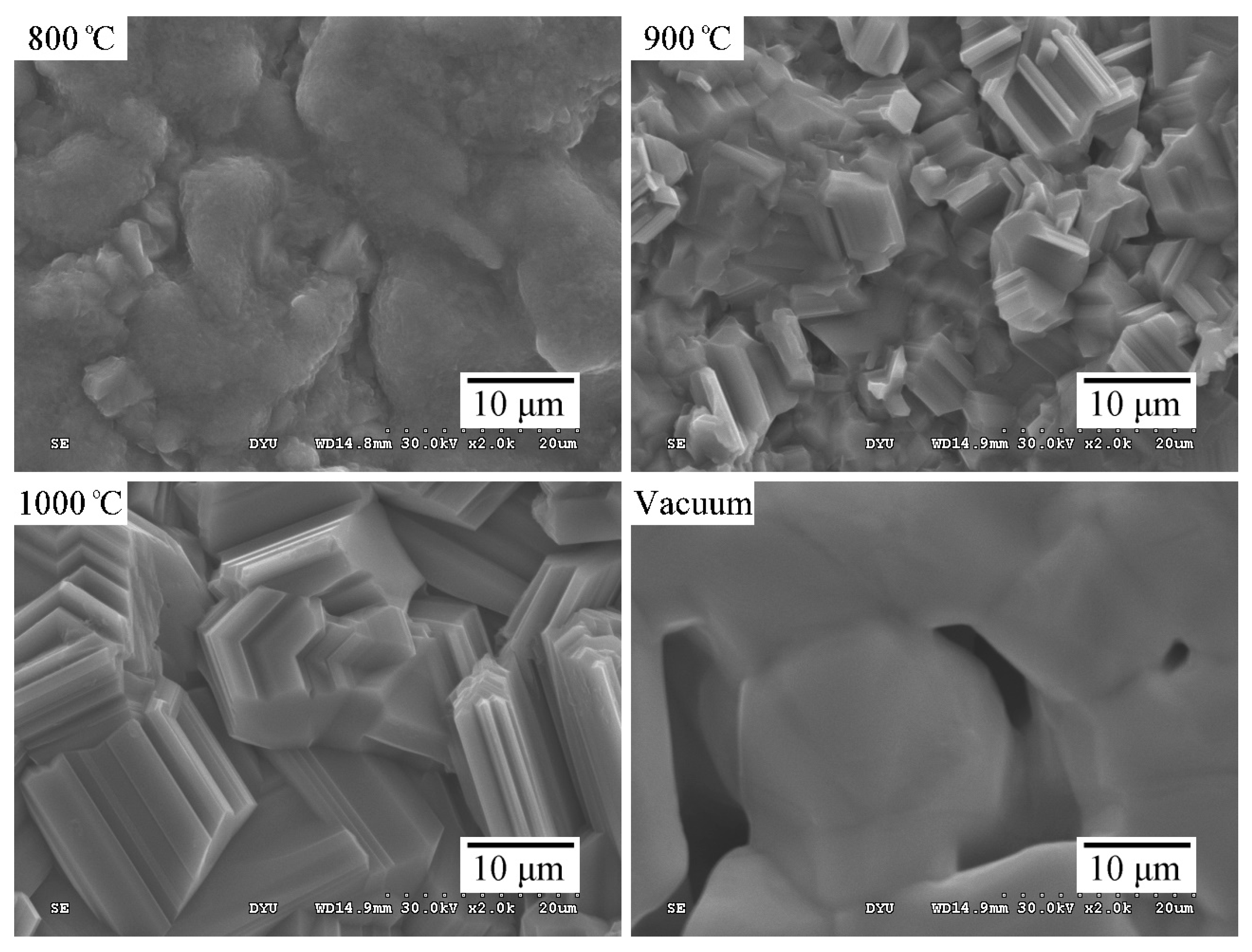
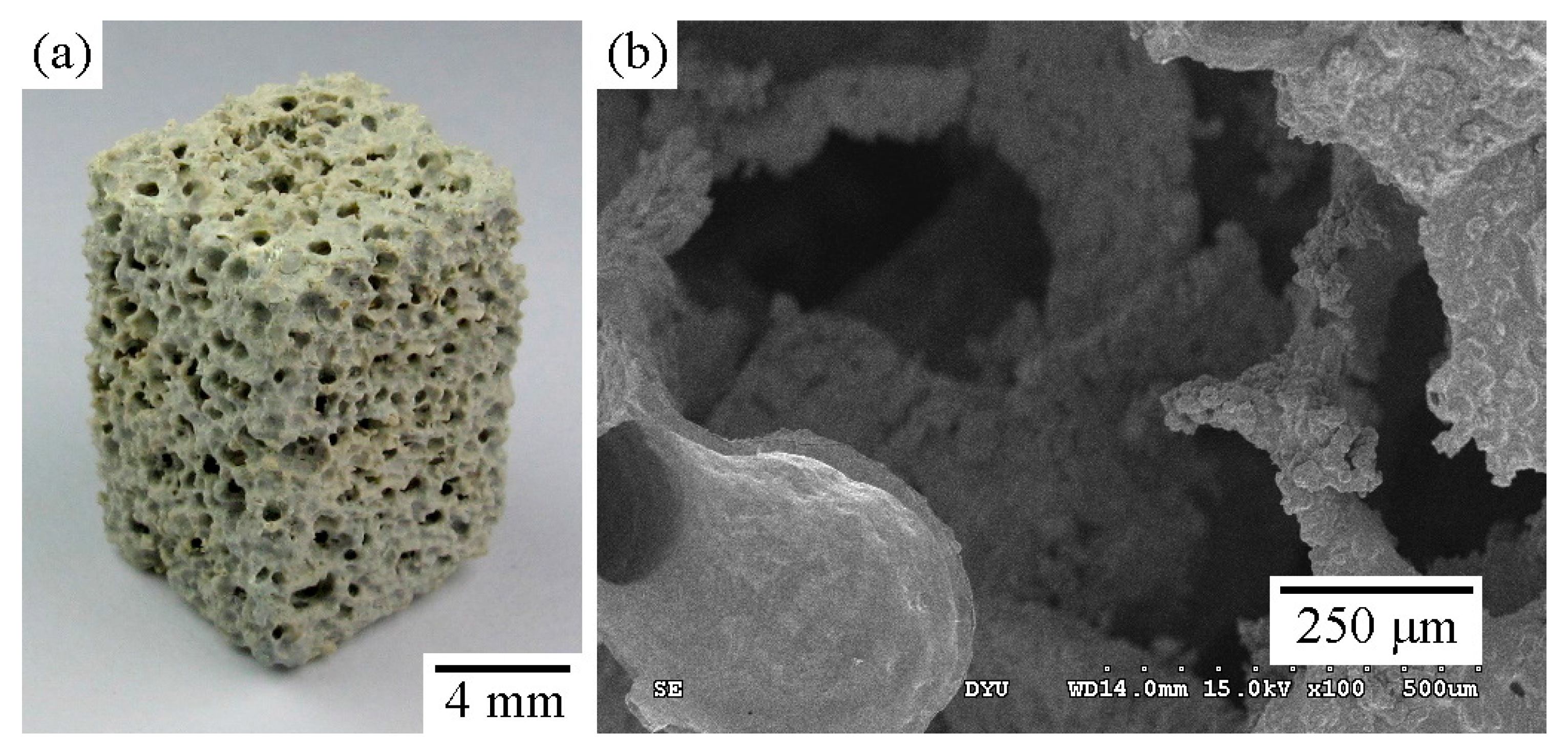
2.1. Structural Characterization of the Sintered Ti Foam
2.2. Wettability
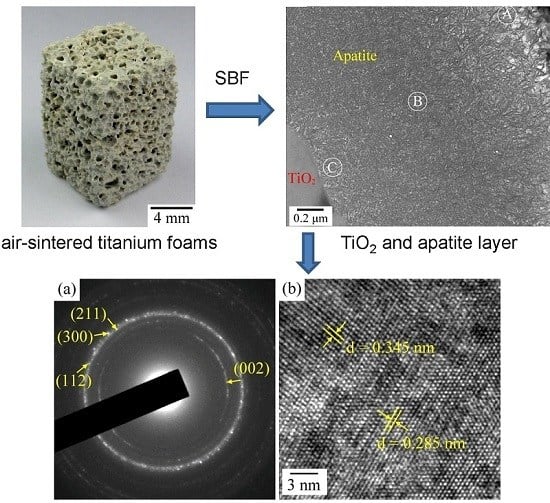
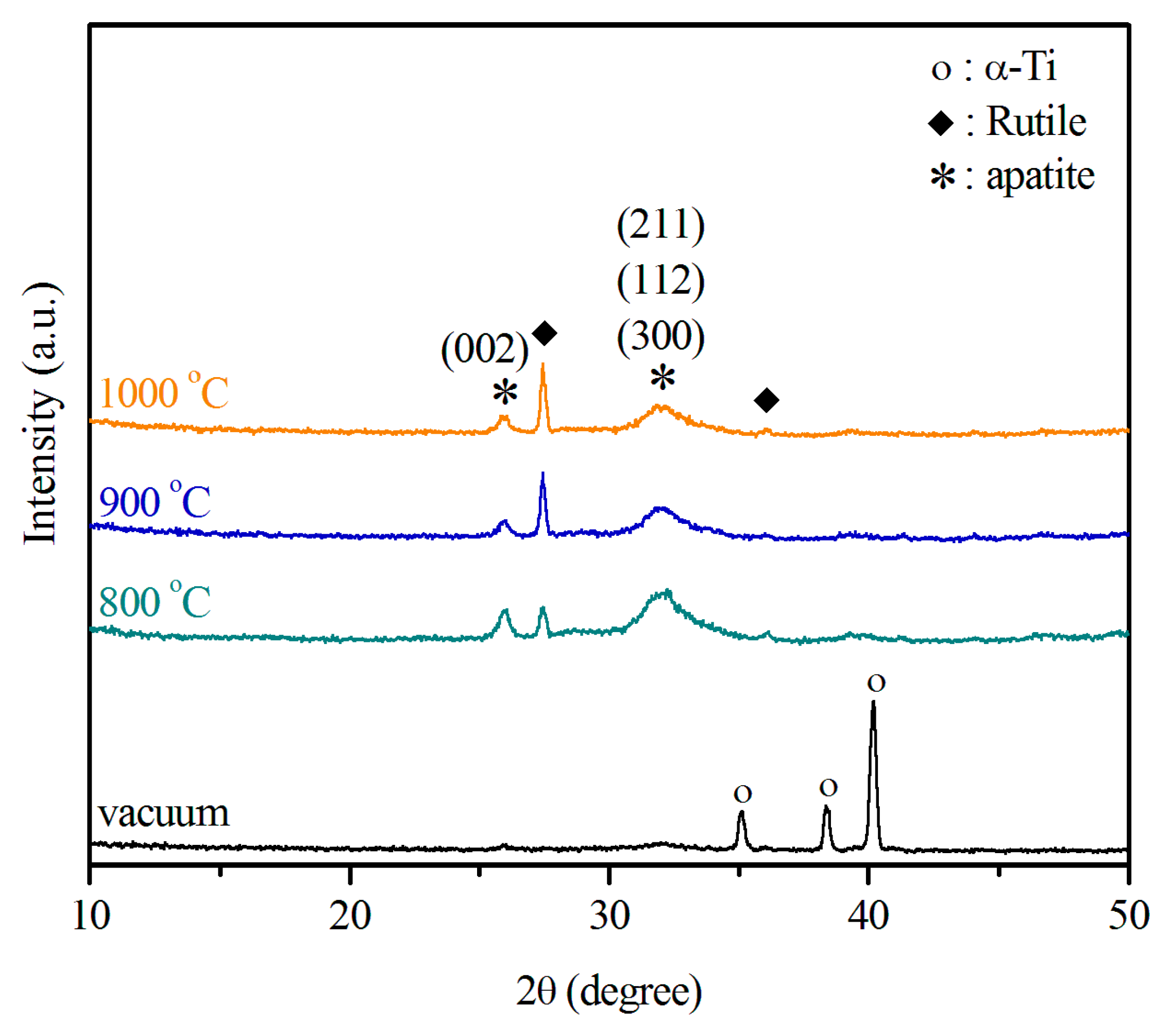
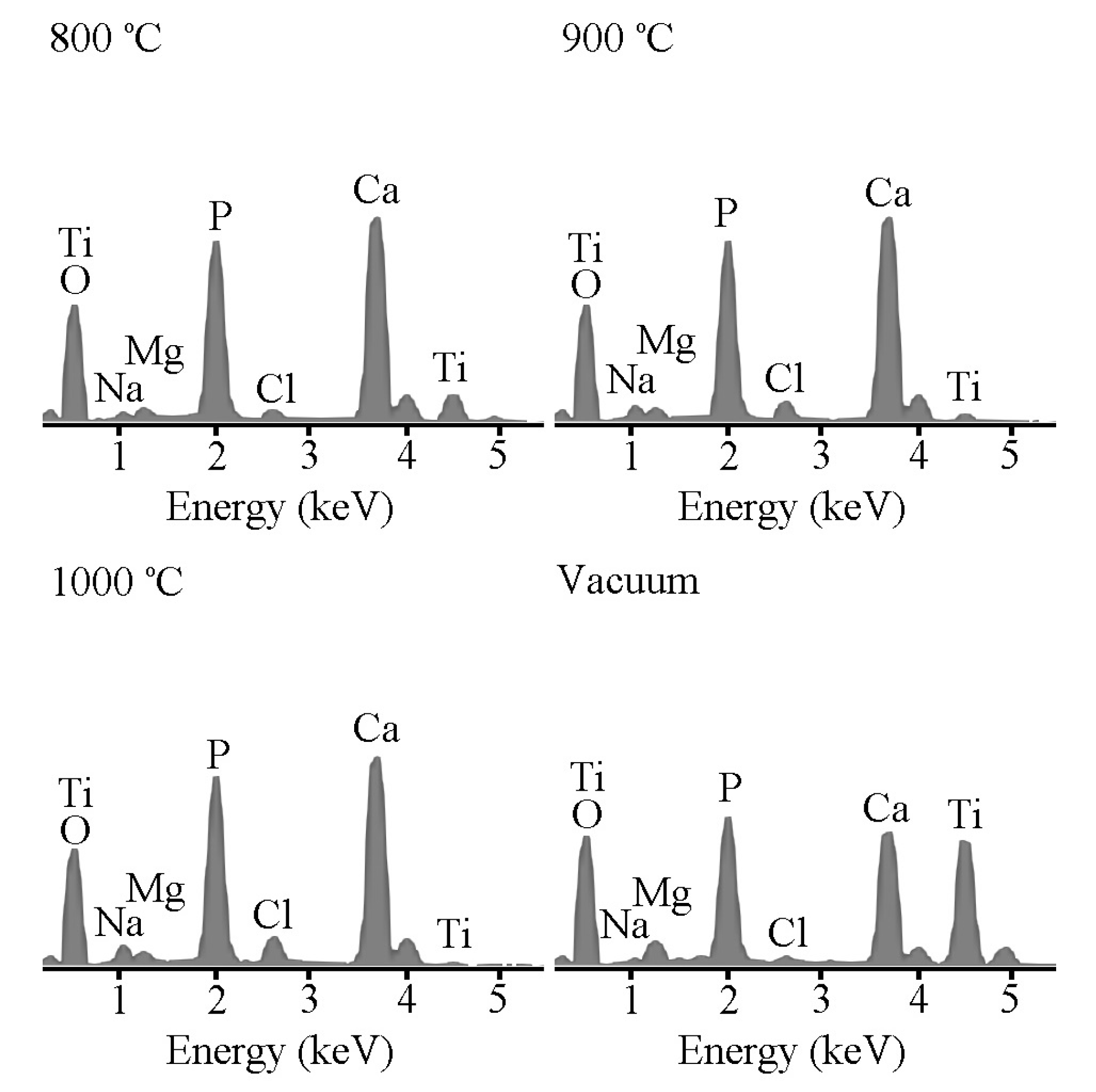
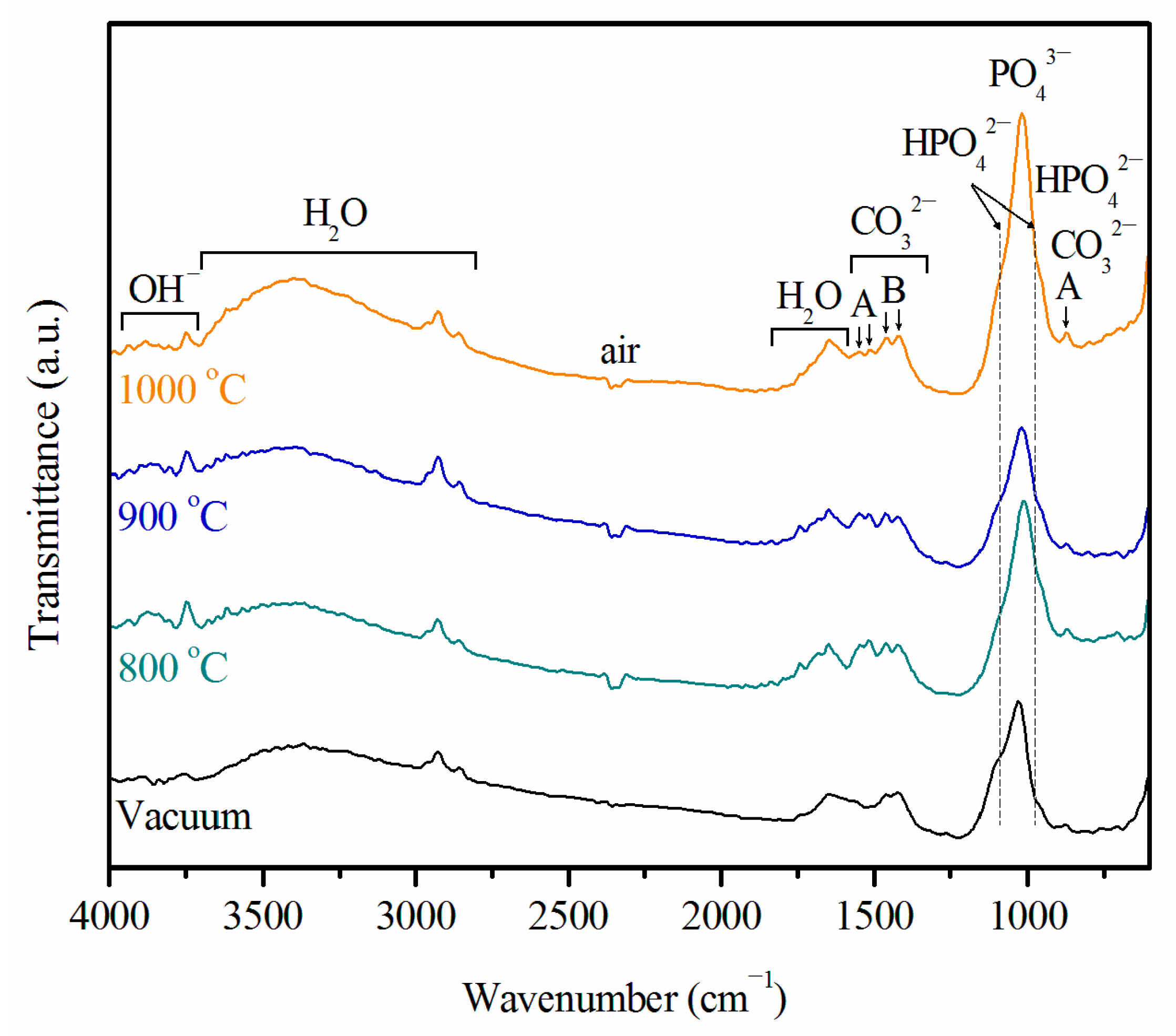
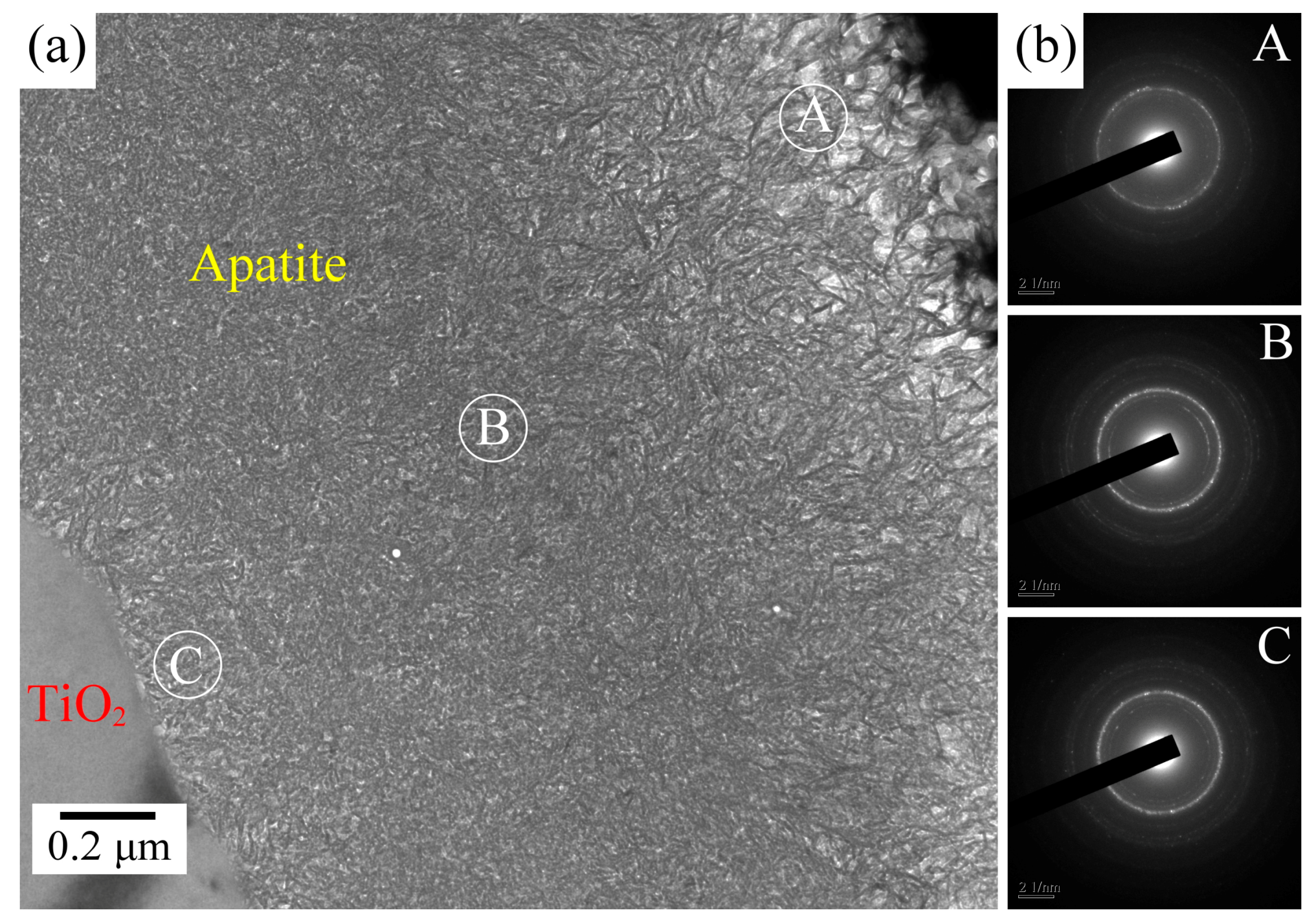
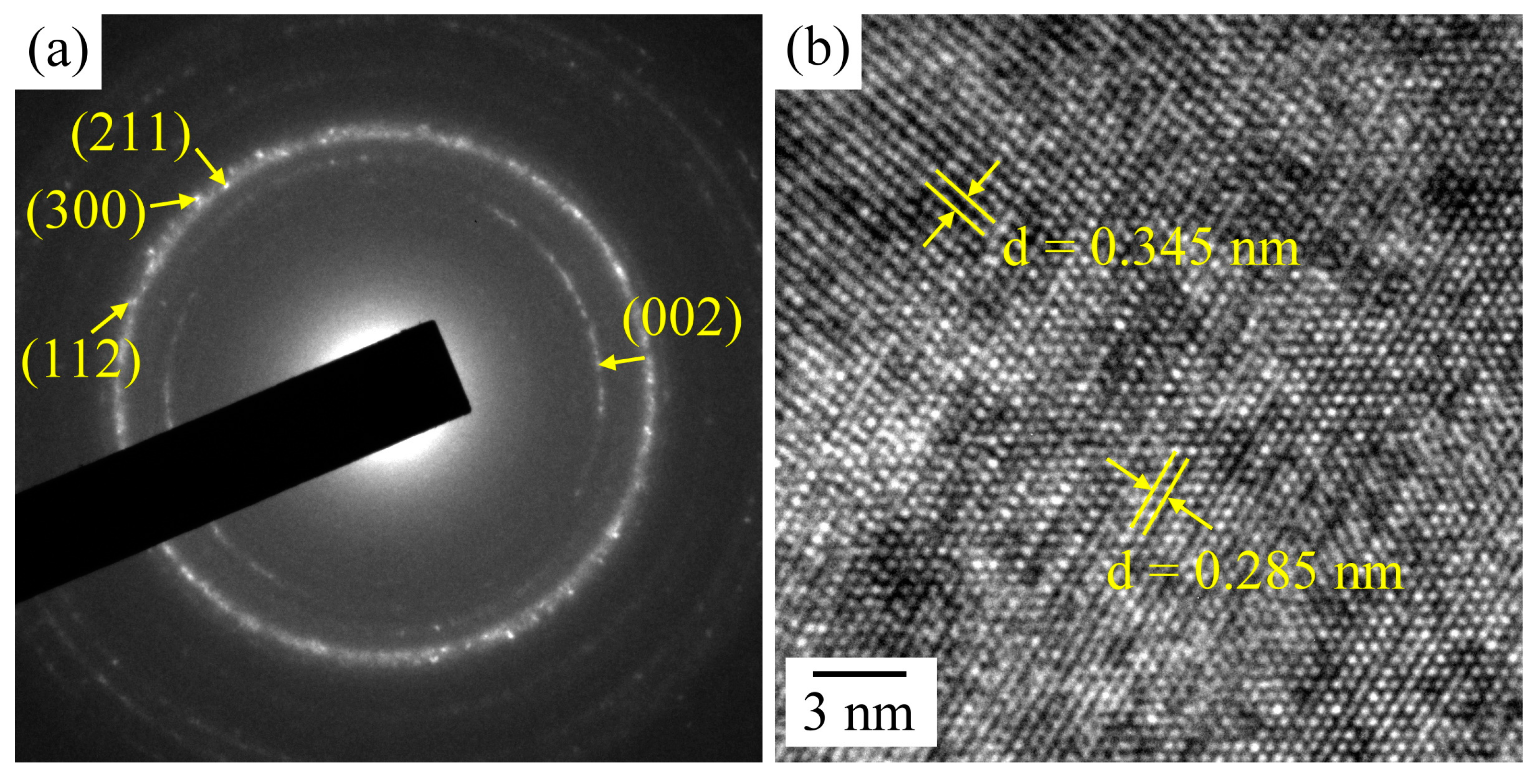
2.3. Ca–P Precipitation on the Sintered Surfaces
3. Materials and Methods
3.1. Ca–P Precipitation on the Sintered Surfaces
3.2. Examination of Apatite-Forming Ability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Syu, J.Y.; Ho, W.F. The structure and mechanical properties of as-cast Ti–25Nb–xSn alloys for biomedical applications. Mater. Sci. Eng. A 2013, 568, 1–7. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Dunand, D.C. Processing of titanium foams. Adv. Eng. Mater. 2004, 6, 369–376. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A bioactive titanium foam scaffold for bone repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Hsu, S.K.; Tsou, H.K.; Wu, S.C.; Lai, T.H.; Ho, W.F. Fabrication and characterization of porous Ti–7.5Mo alloy scaffolds for biomedical applications. J. Mater. Sci. Mater. Med. 2013, 24, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Li, S.H.; van Blitterswijk, C.A.; de Groot, K. A novel porous Ti6Al4V: Characterization and cell attachment. J. Biomed. Mater. Res. A 2005, 73, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.G.D.; Dund, C. Effect of thermal history on the superplastic expansion of argon-filled pores in titanium: Part I, kinetics and microstructure. Acta Mater. 2004, 52, 2269–2278. [Google Scholar] [CrossRef]
- Zhang, X.; Ayers, R.A.; Thorne, K.; Moore, J.J.; Schowengerdt, F. Combustion synthesis of porous materials for bone replacement. Biomed. Sci. Instrum. 2001, 37, 463–468. [Google Scholar] [PubMed]
- Li, J.P.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Porous Ti6Al4V scaffold directly fabricating by rapid prototyping: Preparation and in vitro experiment. Biomaterials 2006, 27, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Tsai, M.S.; Chang, T.Y.; Ho, W.F. Processing and mechanical properties of porous Ti–7.5Mo alloy. Mater. Des. 2013, 47, 21–26. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Chang, T.Y.; Ho, W.F. Effect of ball milling on properties of porous Ti–7.5Mo alloy for biomedical applications. J. Alloys Compd. 2014, 582, 793–801. [Google Scholar] [CrossRef]
- Cachinho, S.C.P.; Correia, R.N. Titanium scaffolds for osteointegration: Mechanical, in vitro and corrosion behavior. J. Mater. Sci. Mater. Med. 2008, 19, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Jiang, X.; Rowe, D.; Wei, M. Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J. Mater. Sci. Mater. Med. 2012, 23, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Geesink, R.; Klein, C.; Serekian, P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Nakamura, T.; Nishiguchi, S.; Tamura, J.; Uchida, M.; Kim, H.M.; Kokubo, T. Bioactive titanium: Effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J. Biomed. Mater. Res. 2001, 56, 562–570. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I.N. Hydroxyapatite thin films grown by pulsed laser deposition and radio-frequency magnetron sputtering: Comparative study. Appl. Surf. Sci. 2004, 228, 346–356. [Google Scholar] [CrossRef]
- Oh, I.H.; Nomura, N.; Chiba, A.; Murayama, Y.; Masahashi, N.; Lee, B.T.; Hanada, S. Microstructures and bond strengths of plasma-sprayed hydroxyapatite coatings on porous titanium substrates. J. Mater. Sci. Mater. Med. 2005, 16, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J. Biomed. Mater. Res. 1996, 32, 409–417. [Google Scholar] [CrossRef]
- Spriano, S.; Bronzoni, M.; Vernè, E.; Maina, G.; Bergo, V.; Windler, M. Characterization of surface modified Ti–6Al–7Nb alloy. J. Mater. Sci. Mater. Med. 2005, 16, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Hayakawa, S.; Tsuru, K.; Osaka, A. A comparative study of in vitro apatite deposition on heated-, H2O2-, and NaOH-treated titanium substrates. J. Biomed. Mater. Res. 2001, 54, 172–178. [Google Scholar] [CrossRef]
- Wang, X.X.; Yan, W.; Hayakawa, S.; Tsuru, K.; Osaka, A. Apatite deposition on thermally and anodically oxided titanium surfaces in simulated body fluid. Biomaterials 2003, 24, 4631–4637. [Google Scholar] [CrossRef]
- Feng, B.; Chen, J.Y.; Qi, S.K.; Zhao, J.Z.; He, L.; Zhang, X.D. Characterization of surface oxide films on titanium and bioactivity. J. Mater. Sci. Mater. Med. 2002, 13, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Siva Rama Krishna, D.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Int. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 layers on cp–Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 59, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Sunny, M.C.; Sharma, C.P. Titanium-protein interaction: Changes with oxide layer thickness. J. Biomater. Appl. 1991, 6, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cui, D.Z.; Jeon, H.R.; Chung, H.J.; Park, Y.J.; Kim, O.S.; Kim, Y.J. Surface characteristics of thermally treated titanium surfaces. J. Periodontal Implant Sci. 2012, 42, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Hsu, S.K.; Wu, S.C.; Wang, P.H.; Ho, W.F. Design and characterization of highly porous titanium foams with bioactive surface sintering in air. J. Alloys Compd. 2013, 575, 326–332. [Google Scholar] [CrossRef]
- Kokubo, T.; Miyaji, F.; Kim, H.M.; Nakamura, T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, T.S.N.S.; Raman, S.G.S.; Seshadri, S.K. Thermal oxidation of CP Ti—An electrochemical and structural characterization. Mater. Charact. 2010, 61, 589–597. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Balas, F.; Izquierdo-Barba, I.; Vallet-Regí, M. In vitro structural changes in porous HA/β-TCP scaffolds in simulated body fluid. Acta Biomater. 2009, 5, 2738–2751. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, H.K. Preparation and characterization of hydrophilic TiO2 film. Bull. Korean Chem. Soc. 2002, 23, 745–748. [Google Scholar]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mat. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J.H.; Lee, C.S.; Hanawa, T. Osteoconductivity of hydrophilic microstructured titanium implants with phosphate ion chemistry. Acta Biomater. 2009, 5, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Tang, S.; Li, Y.; Long, T.; Qu, X.H.; Yu, D.G.; Guo, Y.J.; Guo, Y.P.; Zhu, Z.A. Fabrication, characterization, and biocompatibility of ethyl cellulose/carbonated hydroxyapatite composite coatings on Ti6Al4V. J. Mater. Sci. Mater. Med. 2014, 25, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Wen, C.; Guan, S.; Peng, L.; Ren, C.; Wang, X.; Hu, Z. Characterization and degradation behavior of AZ31 alloy surface modified by bone-like hydroxyapatite for implant applications. Appl. Surf. Sci. 2009, 255, 6433–6438. [Google Scholar] [CrossRef]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J. Biomed. Mater. Res. 2001, 57, 441–448. [Google Scholar] [CrossRef]
- Kaneko, H.; Uchida, M.; Kim, H.M.; Kokubo, T.; Nakamura, T. Process of apatite formation induced by anatase on titanium metal in simulated body fluid. Key Eng. Mater. 2002, 218–220, 649–652. [Google Scholar] [CrossRef]
- Ma, J.; Wong, H.; Kong, L.B.; Peng, K.W. Biomimetic processings of nanocrystallite bioactive apatite coating on titanium. Nanotechnology 2003, 14, 619–623. [Google Scholar] [CrossRef]
- Lu, X.; Leng, Y. TEM study of calcium phosphate precipitation on bioactive titanium surfaces. Biomaterials 2004, 25, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Zhang, Q.; Zhang, X.; Gu, Z.; Chen, J. BCP coatings on pure titanium plates by CD method. Mater. Sci. Eng. C 2007, 27, 781–786. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Caillard, D.; Conforto, E. Preferred growth orientation of biomimetic apatite crystals. J. Crystal Growth 2007, 304, 464–471. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]


| Ion concentration (mM) | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HPO42− | SO42− | HCO3− |
|---|---|---|---|---|---|---|---|---|
| Blood plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 1.0 | 0.5 | 27.0 |
| SBF | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 1.0 | 0.5 | 4.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-B.; Hsu, H.-C.; Wu, S.-C.; Hsu, S.-K.; Wang, P.-H.; Ho, W.-F. Microstructure and Characteristics of Calcium Phosphate Layers on Bioactive Oxide Surfaces of Air-Sintered Titanium Foams after Immersion in Simulated Body Fluid. Materials 2016, 9, 956. https://doi.org/10.3390/ma9120956
Lee H-B, Hsu H-C, Wu S-C, Hsu S-K, Wang P-H, Ho W-F. Microstructure and Characteristics of Calcium Phosphate Layers on Bioactive Oxide Surfaces of Air-Sintered Titanium Foams after Immersion in Simulated Body Fluid. Materials. 2016; 9(12):956. https://doi.org/10.3390/ma9120956
Chicago/Turabian StyleLee, Hung-Bin, Hsueh-Chuan Hsu, Shih-Ching Wu, Shih-Kuang Hsu, Peng-Hsiang Wang, and Wen-Fu Ho. 2016. "Microstructure and Characteristics of Calcium Phosphate Layers on Bioactive Oxide Surfaces of Air-Sintered Titanium Foams after Immersion in Simulated Body Fluid" Materials 9, no. 12: 956. https://doi.org/10.3390/ma9120956