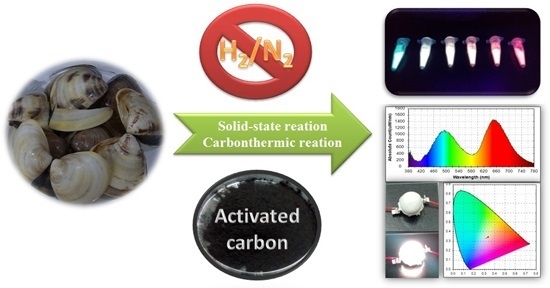
Conversion of Biowaste Asian Hard Clam (Meretrix lusoria) Shells into White-Emitting Phosphors for Use in Neutral White LEDs
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials and Synthesis
2.2. Measurements and Characterization
3. Results and Discussion
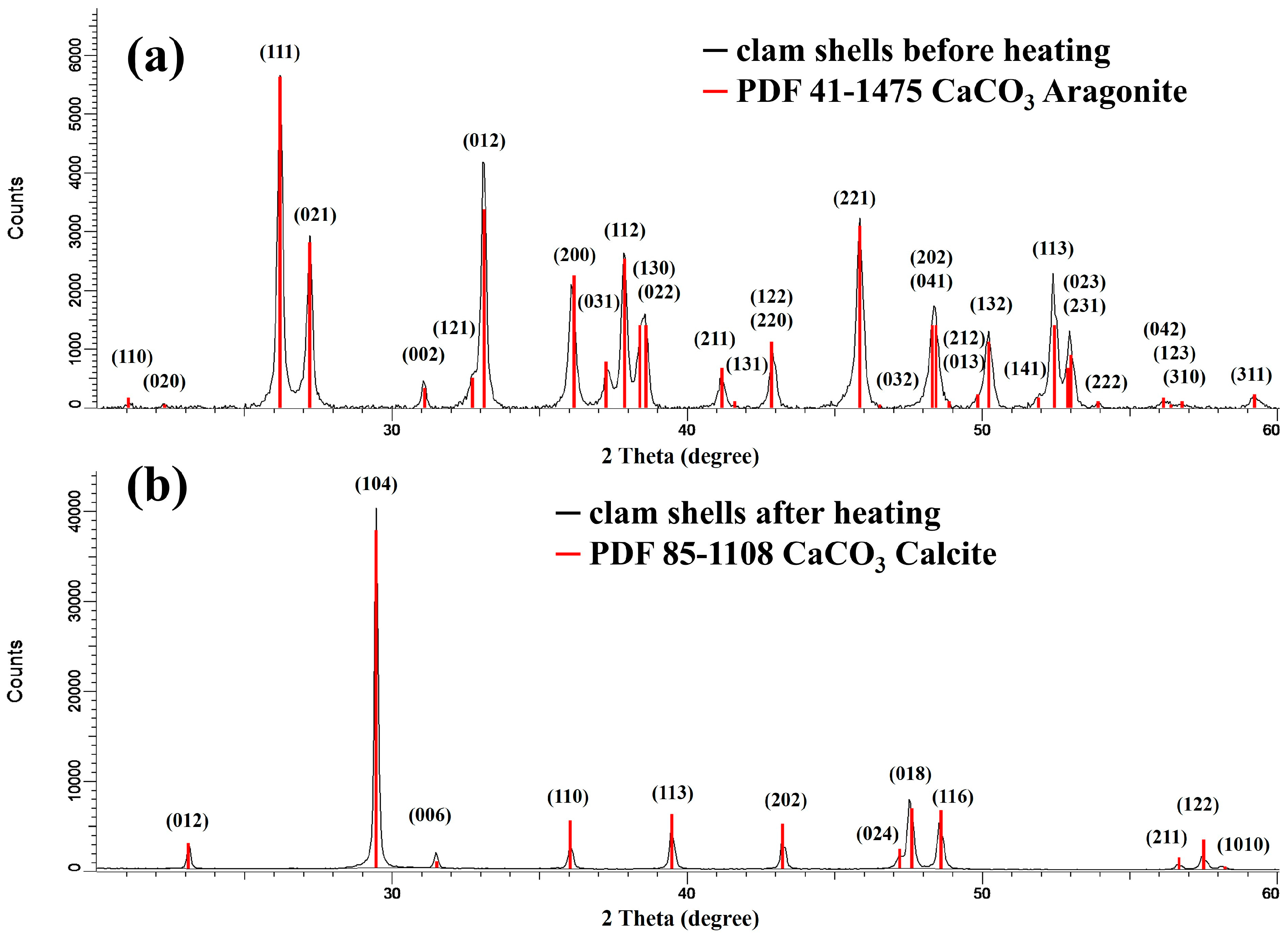
3.1. Phase Identification and Crystal Structure
3.1.1. Initial Material
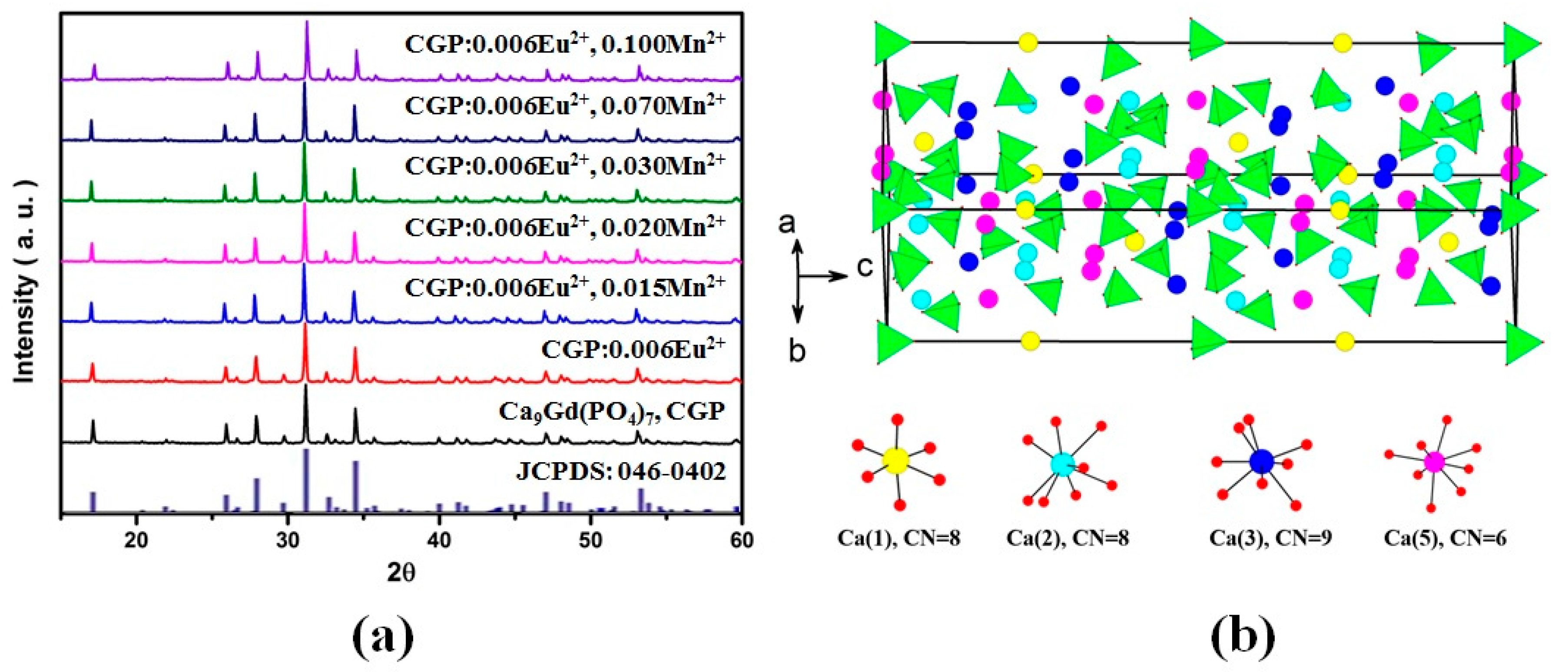
3.1.2. Crystal Structure
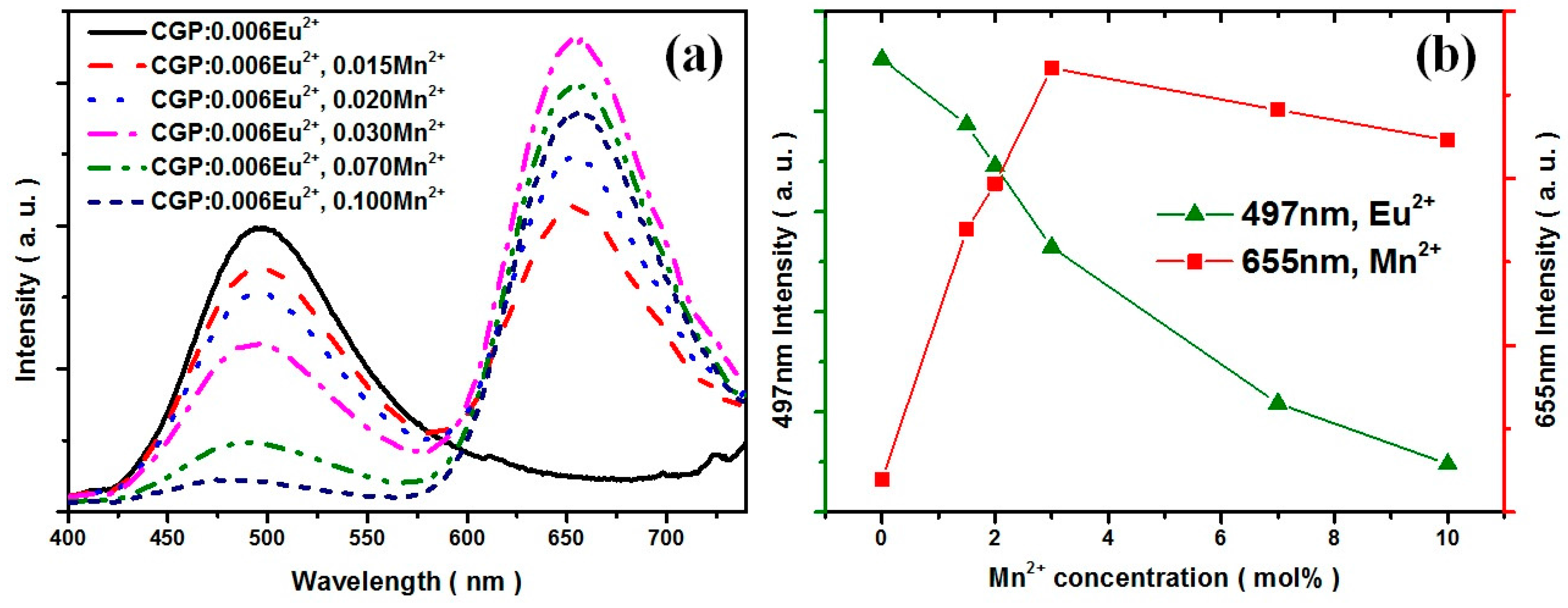
3.2. Luminescence Properties
3.2.1. Luminescent Spectra of Eu2+ and Mn2+ in the CGP
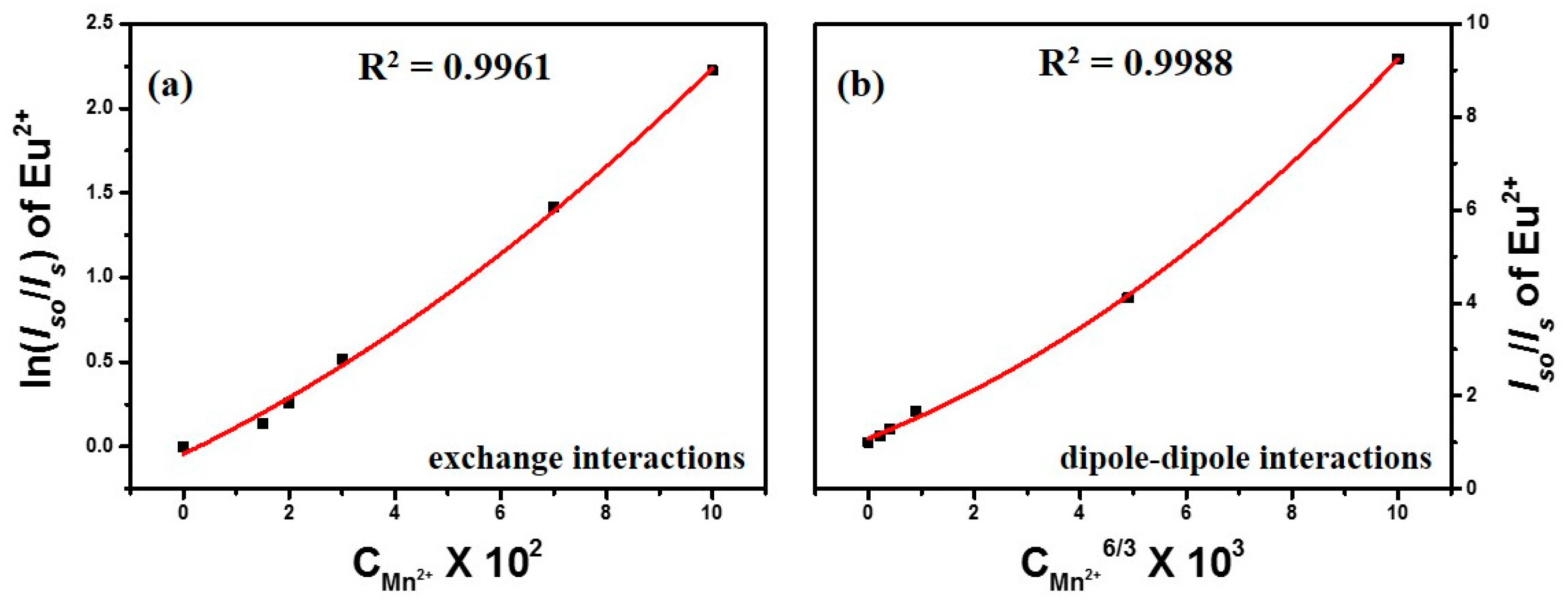
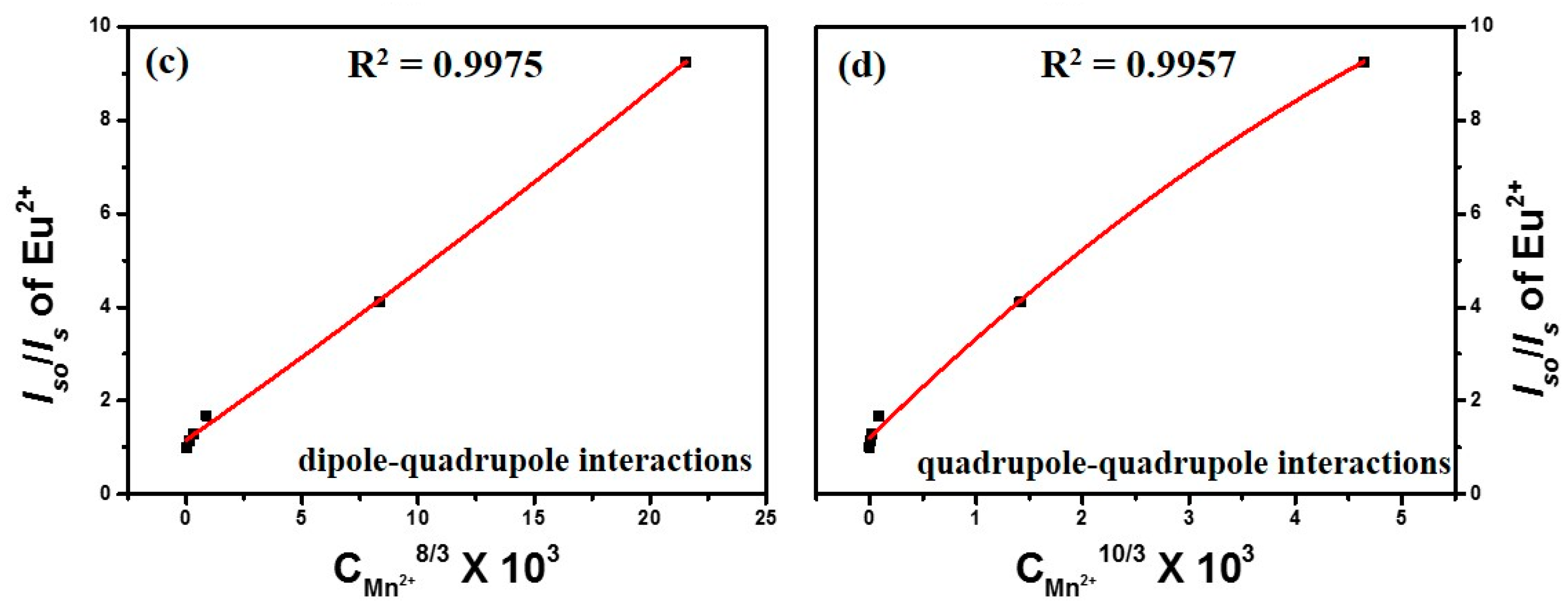
3.2.2. Energy Transfer Mechanism of CGP:Eu2+,Mn2+ Phosphors
3.2.3. CIE Coordinates of CGP:Eu2+,Mn2+
3.2.4. Quantum Efficiencies of Clam-Based and Chem-Based CGP:Eu2+,Mn2+ Phosphors
3.2.5. Thermal Stability of Clam-Based and Chem-Based CGP:Eu2+,Mn2+ Phosphors
3.2.6. White-Emitting LED Packages by Near-UV Chip
3.3. The Production Cost of Clam-Based Phosphors
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yao, Z.T.; Chen, T.; Li, H.Y.; Xia, M.S.; Ye, Y.; Zheng, H. Preparation, characterization, and antibacterial activity of shell waste loaded with silver. J. Mater. Sci. 2013, 48, 8580–8587. [Google Scholar] [CrossRef]
- Mahshuri, Y.; Amalina, M.A. Hardness and compressive properties of calcium carbonate derived from clam shell filled unsaturated polyester composites. Mater. Res. Innov. 2014, 18, 291–294. [Google Scholar] [CrossRef]
- Xia, M.S.; Yao, Z.T.; Ge, L.Q.; Chen, T.; Li, H.Y. A potential bio-filler: The substitution effect of furfural modified clam shell for carbonate calcium in polypropylene. J. Compos. Mater. 2015, 49, 807–816. [Google Scholar] [CrossRef]
- Yao, Z.T.; Chen, T.; Li, H.Y.; Xia, M.S.; Ye, Y.; Zheng, H. Mechanical and thermal properties of polypropylene (PP) composites filled with modified shell waste. J. Hazard. Mater. 2013, 262, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.T.; Xia, M.S.; Ge, L.Q.; Chen, T.; Li, H.Y.; Ye, Y.; Zheng, H. Mechanical and thermal properties of polypropylene (PP) composites filled with CaCO3 and shell waste derived bio-fillers. Fiber. Polym. 2014, 15, 1278–1287. [Google Scholar] [CrossRef]
- Nurfitri, I.; Maniam, G.P.; Hindryawati, N.; Yusoff, M.M.; Ganesan, S. Potential of feedstock and catalysts from waste in biodiesel preparation: A review. Energy Convers. Manag. 2013, 74, 395–402. [Google Scholar] [CrossRef]
- Suryaputra, W.; Winata, I.; Indraswati, N.; Ismadji, S. Waste capiz (Amusium cristatum) shell as a new heterogeneous catalyst for biodiesel production. Renew. Energy 2013, 50, 795–799. [Google Scholar] [CrossRef]
- Zuo, S.; Du, Y.; Liu, F.; Han, D.; Qi, C. Influence of ceria promoter on shell-powder-supported Pd catalyst for the complete oxidation of benzene. Appl. Catal. A Gen. 2013, 451, 65–70. [Google Scholar] [CrossRef]
- Bramhe, S.; Kim, T.N.; Balakrishnan, A.; Chu, M.C. Conversion from biowaste Venerupis clam shells to hydroxyapatite nanowires. Mater. Lett. 2014, 135, 195–198. [Google Scholar] [CrossRef]
- Chiang, C.H.; Liu, T.H.; Lin, H.Y.; Kuo, H.Y.; Chu, S.Y. Effects of flux additives on characteristics of Y2.95Al5O12:0.05Ce3+ phosphor: Thermal stability and application to WLEDs. J. Disp. Technol. 2015, 11, 466–470. [Google Scholar] [CrossRef]
- Kang, F.W.; Zhang, Y.; Peng, M.Y. Controlling the energy transfer via multi luminescent centers to achieve white light/tunable emissions in a single-phased X2-type Y2SiO5:Eu3+,Bi3+ phosphor for ultraviolet converted LEDs. Inorg. Chem. 2015, 54, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; You, H.; Song, Y.; Yang, M.; Liu, K.; Zheng, Y.; Huang, Y.; Zhang, H. White-light emission from a single-emitting-component Ca9Gd(PO4)7:Eu2+,Mn2+ phosphor with tunable luminescent properties for near-UV light-emitting diodes. J. Mater. Chem. 2010, 20, 9061–9067. [Google Scholar] [CrossRef]
- Huang, C.-H.; Liu, W.-R.; Chen, T.-M. Single-Phased white-light phosphors Ca9Gd(PO4)7:Eu2+,Mn2+ under near-ultraviolet excitation. J. Phys. Chem. C 2010, 114, 18698–18701. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coordin. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Shi, J.; Gong, M. Luminescent properties and energy transfer of orange-emitting phosphor Ca10Na(PO4)7:Eu2+,Mn2+ for NUV LEDs. Mater. Res. Bull. 2014, 57, 1–5. [Google Scholar] [CrossRef]
- Guo, N.; Huang, Y.; You, H.; Yang, M.; Song, Y.; Liu, K.; Zheng, Y. Ca9Lu(PO4)7:Eu2+,Mn2+: A potential single-phased white-light-emitting phosphor suitable for white-light-emitting diodes. Inorg. Chem. 2010, 49, 10907–10913. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.L. A Theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Philips Res. Rep. 1969, 24, 131–144. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-Y.; Wang, C.-M.; Lin, T.-Y.; Lin, H.-M. Conversion of Biowaste Asian Hard Clam (Meretrix lusoria) Shells into White-Emitting Phosphors for Use in Neutral White LEDs. Materials 2016, 9, 979. https://doi.org/10.3390/ma9120979
Chang T-Y, Wang C-M, Lin T-Y, Lin H-M. Conversion of Biowaste Asian Hard Clam (Meretrix lusoria) Shells into White-Emitting Phosphors for Use in Neutral White LEDs. Materials. 2016; 9(12):979. https://doi.org/10.3390/ma9120979
Chicago/Turabian StyleChang, Tsung-Yuan, Chih-Min Wang, Tai-Yuan Lin, and Hsiu-Mei Lin. 2016. "Conversion of Biowaste Asian Hard Clam (Meretrix lusoria) Shells into White-Emitting Phosphors for Use in Neutral White LEDs" Materials 9, no. 12: 979. https://doi.org/10.3390/ma9120979