The Preparation of Modified Industrial Waste Polyacrylonitrile for the Adsorptive Recovery of Pt(IV) from Acidic Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of Waste Textile
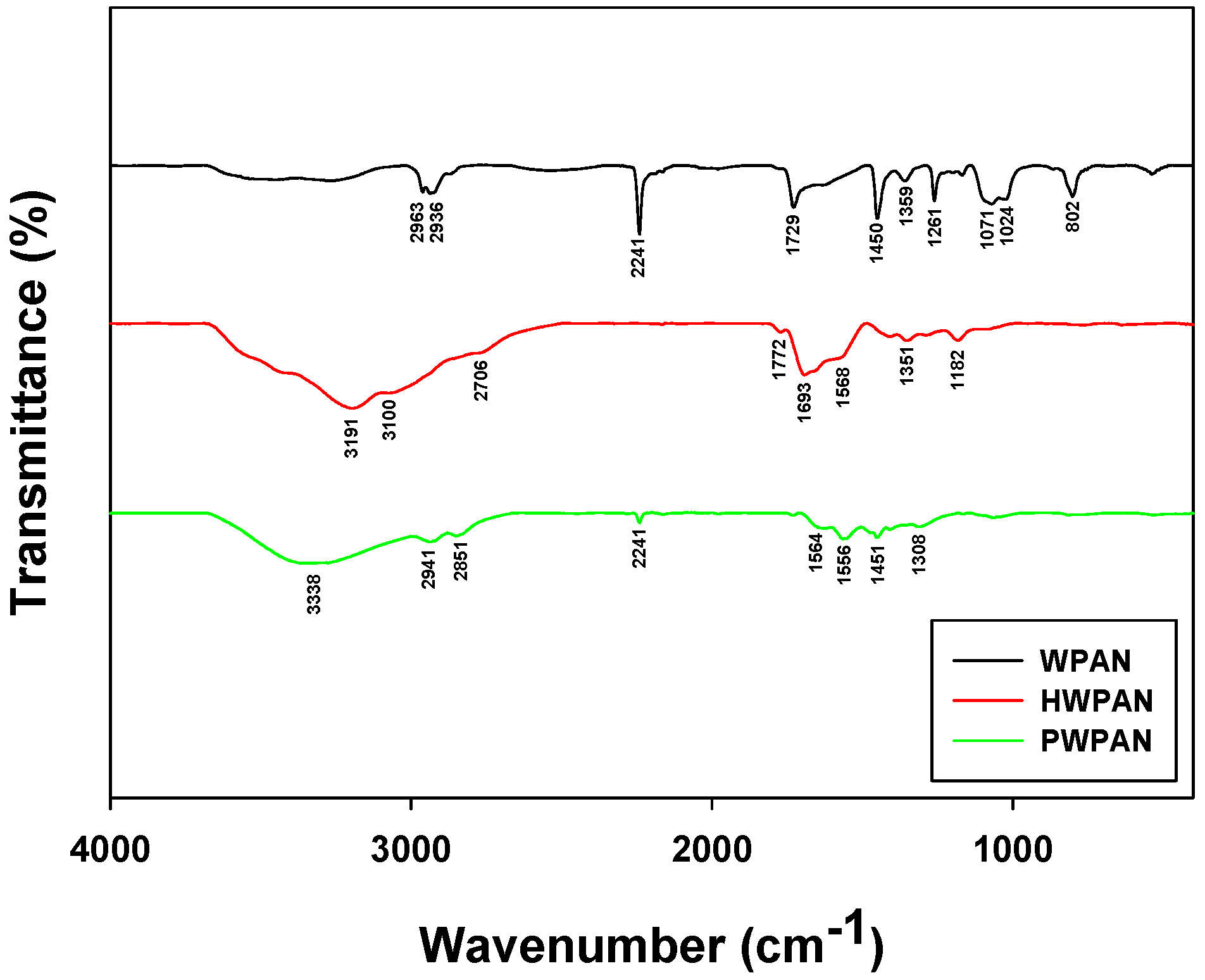
2.3. FTIR Analyses
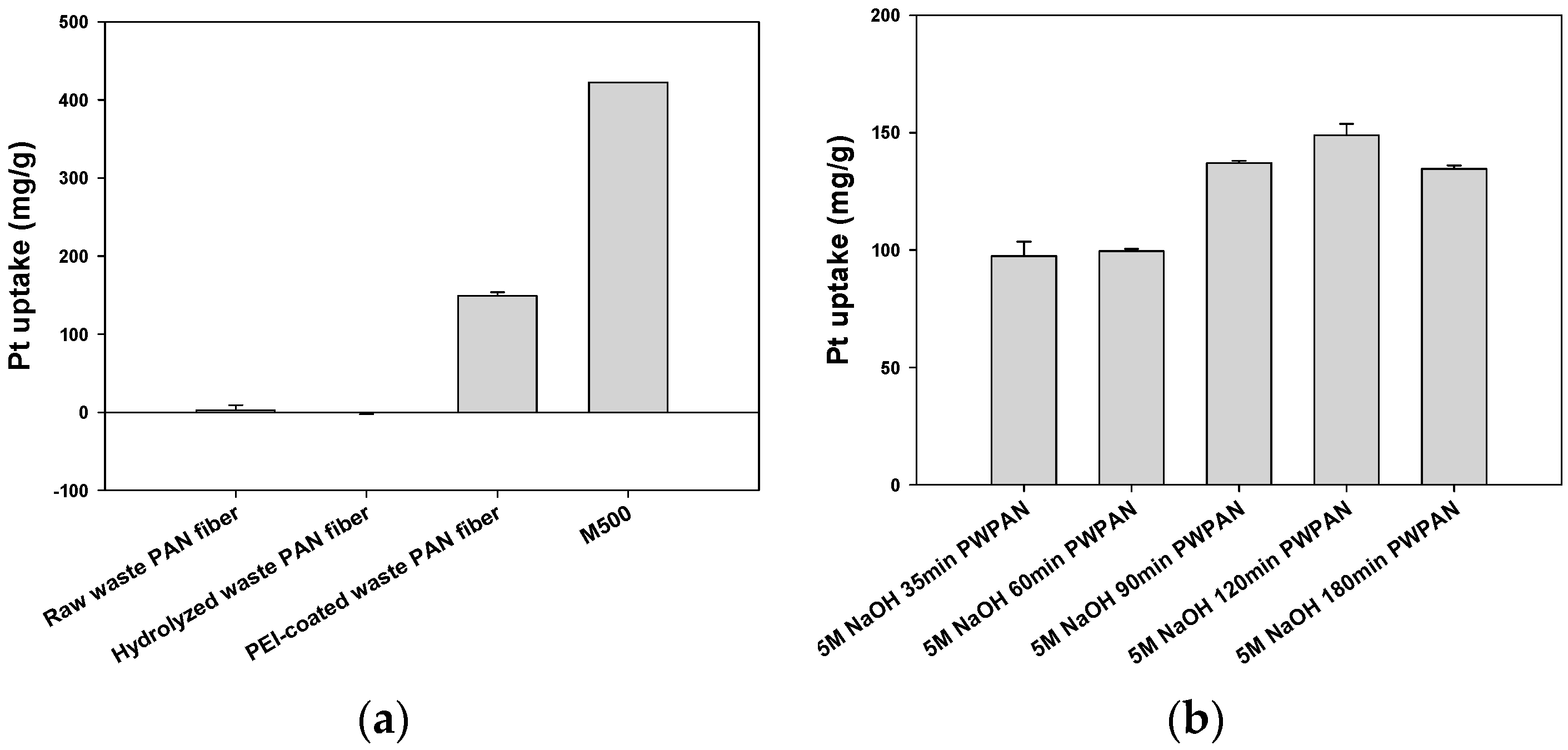
2.4. Batch Sorption Experiments
2.5. Column Experiments
3. Results and Discussion
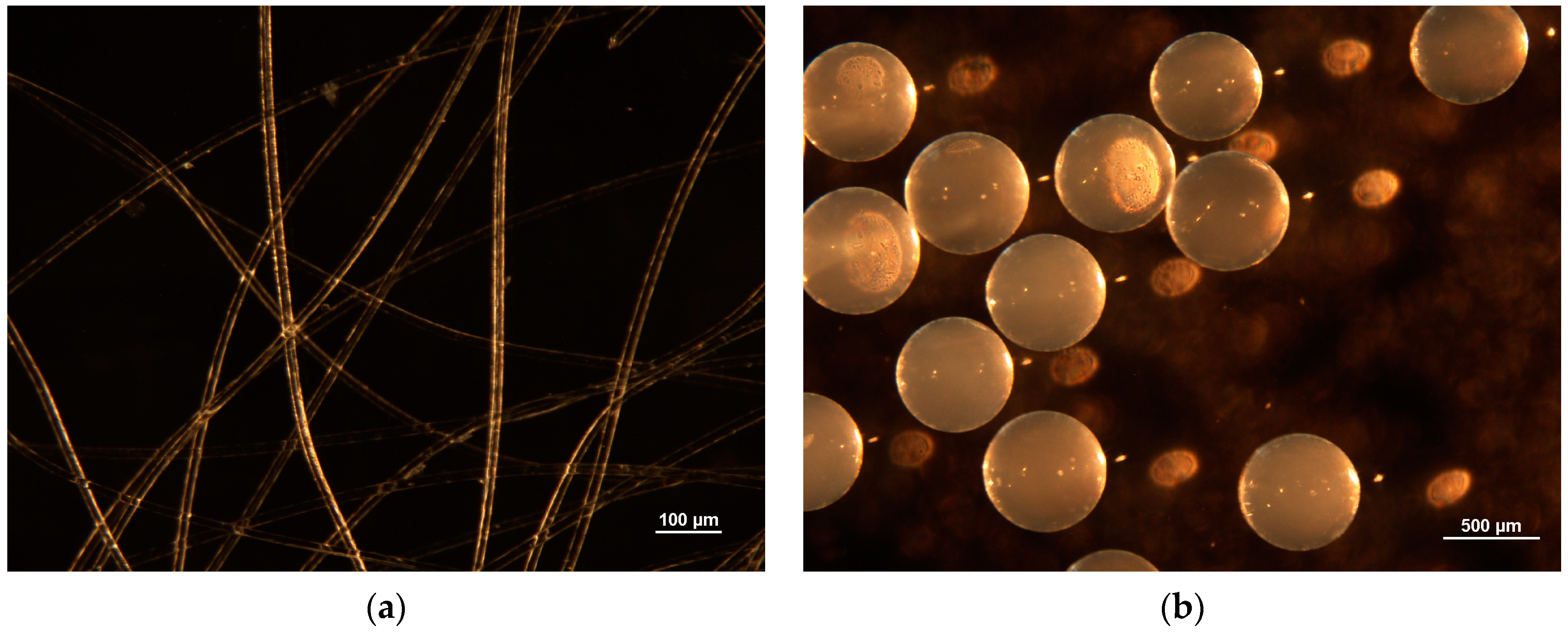
3.1. Characterization of the PEI-Coated Waste PAN Textile
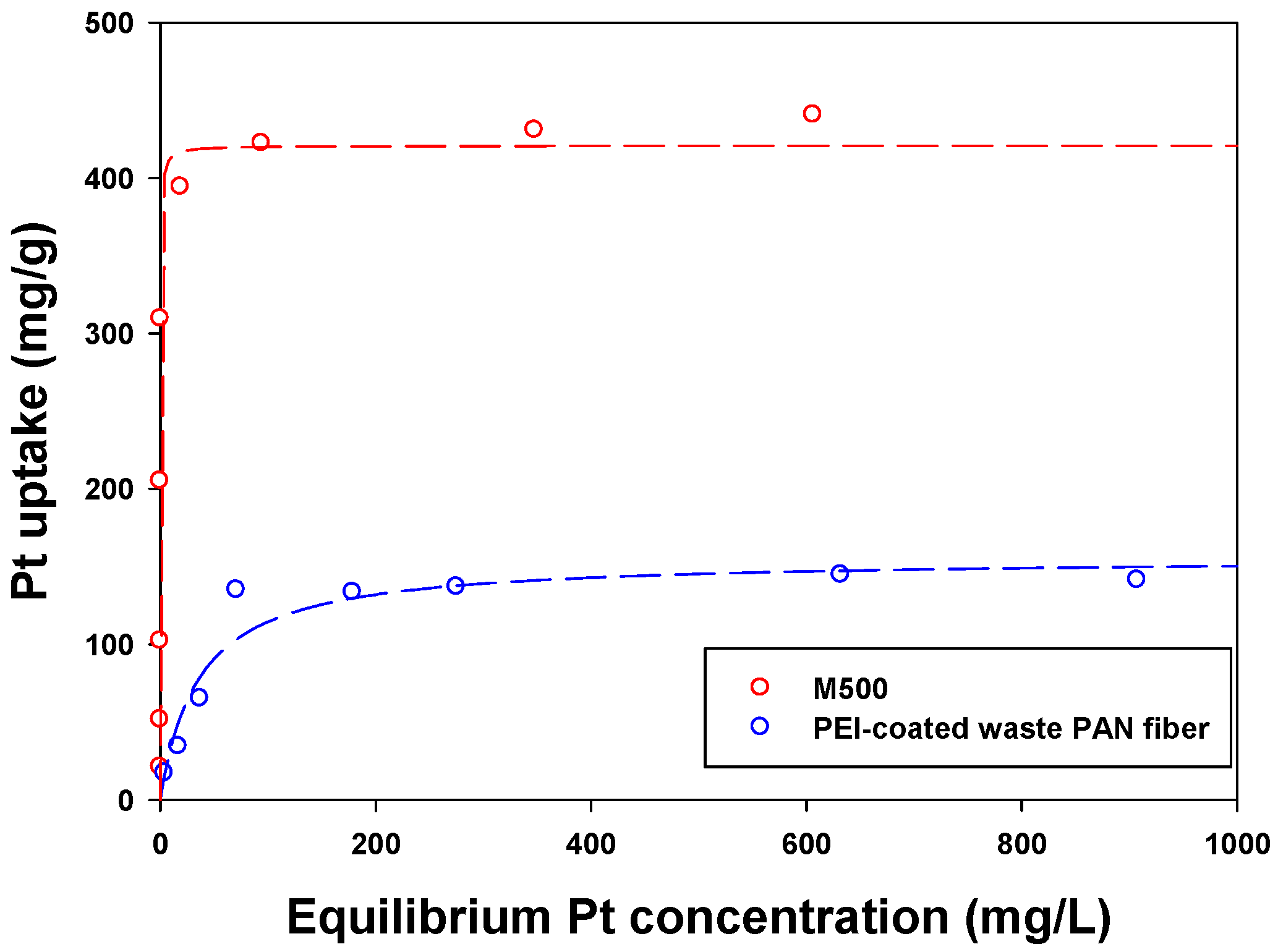
3.2. Sorption Isotherm
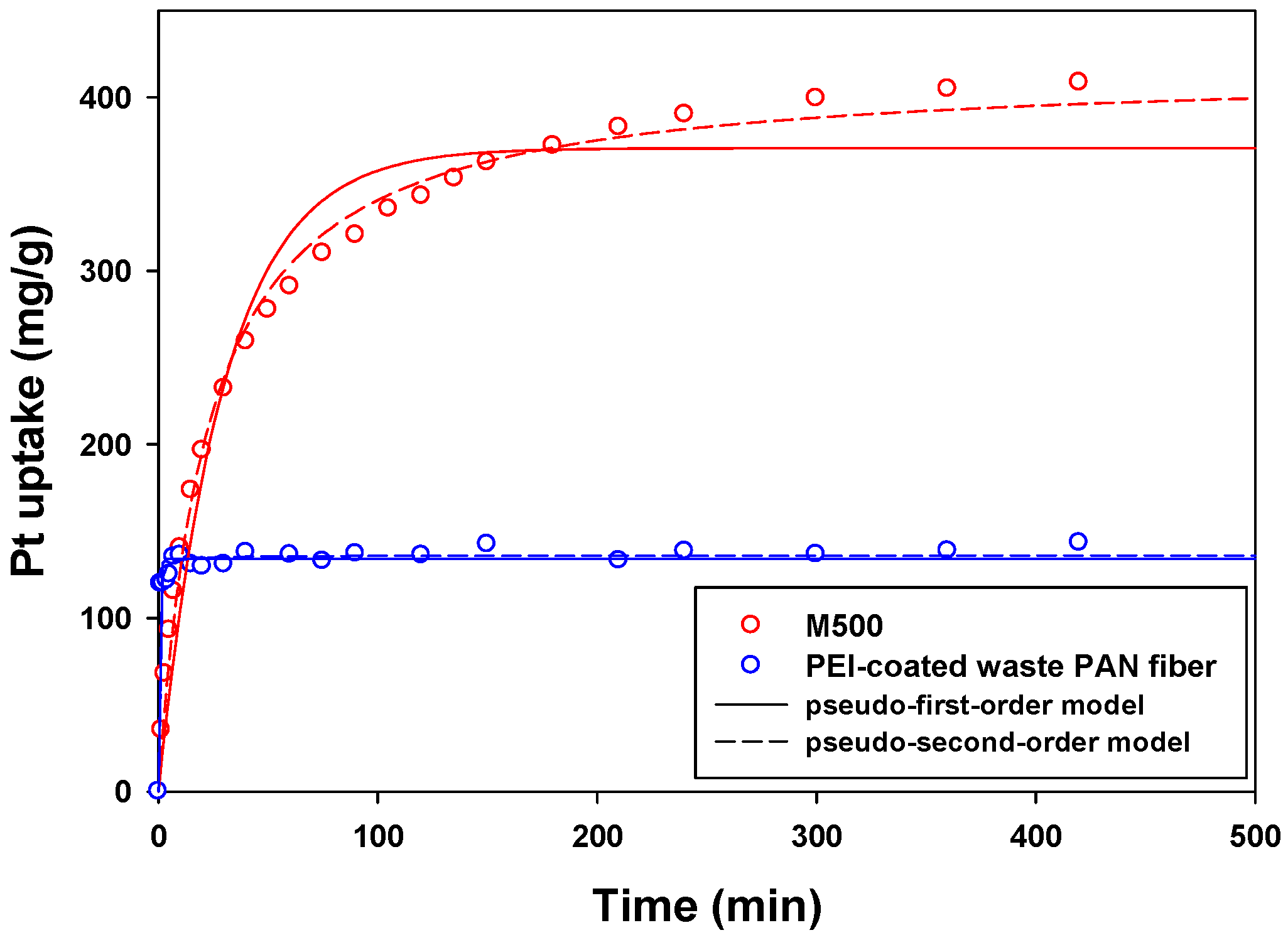
3.3. Sorption Kinetics
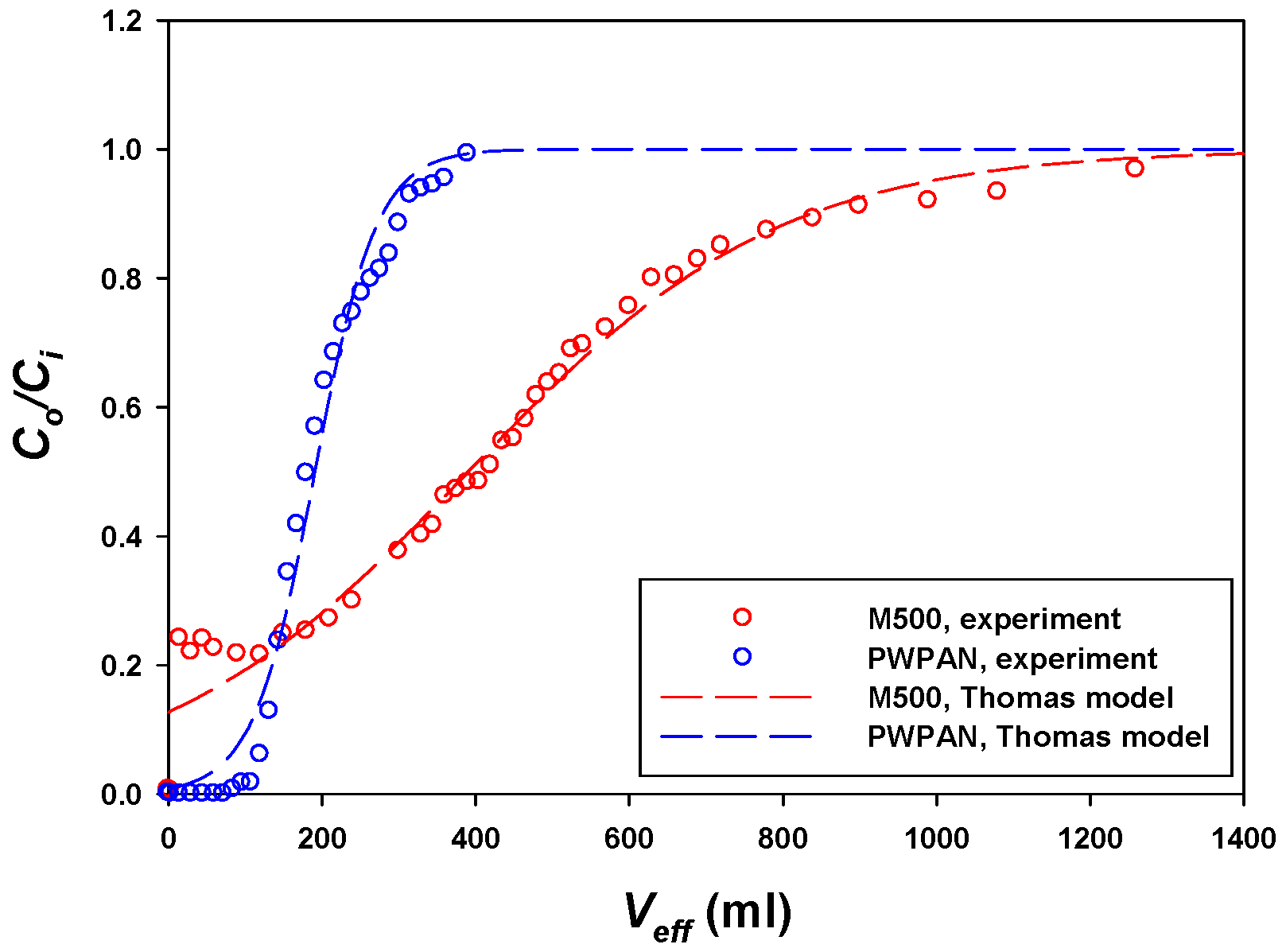
3.4. Column Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Won, S.W.; Kotte, P.; Wei, W.; Lim, A.; Yun, Y.-S. Biosorbents for recovery of precious metals. Bioresour. Technol. 2014, 160, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Vincent, T.; Parodi, A.; Guibal, E. Pt recovery using Cyphos IL-101 immobilized in biopolymer capsules. Sep. Purif. Technol. 2008, 62, 470–479. [Google Scholar] [CrossRef]
- Won, S.W.; Kim, S.; Kotte, P.; Lim, A.; Yun, Y.-S. Cationic polymer-immobilized polysulfone-based fibers as high performance sorbents for Pt(IV) recovery from acidic solutions. J. Hazard. Mater. 2013, 263, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Han, M.H.; Choi, S.B.; Yun, Y.-S. Biosorption of reactive black 5 by Corynebacterium glutamicum biomass immobilized in alginate and polysulfone matrices. Chemosphere 2007, 68, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Fiber and Textile Waste Utilization. Waste Biomass Valori. 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Koduru, J.R.; Lee, K.D. Separation studies of Pd(II) from acidic chloride solutions of Pt(IV), Ni(II) and Rh(III) by using 4-aroyl-3-phenyl-5-isoxazolones. J. Chem. 2012, 9, 756–765. [Google Scholar]
- Poochai, C.; Pongprayoon, T. Enhancing dispersion of carbon nanotube in polyacrylonitrile matrix using admicellar polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 67–74. [Google Scholar] [CrossRef]
- Lee, K.I.; Li, J.; Fei, B.; Xin, J.H. Mechanism study of heat stabilization of polyacrylonitrile nanofibers against alkaline hydrolysis. Polym. Degrad. Stab. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Ljubomirova, V.; Djingova, R. Speciation of inorganic platinum-chloride complexes in spiked environmental samples by SPE and ICP-AES. Anal. Chim. Acta 2008, 614, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Methylene blue adsorption by algal biomass based materials: Biosorbents characterization and process behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan(chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Wu, J.; Cai, G.; Liu, J.; Ge, H.; Wang, J. Eco-friendly surface modification on polyester fabrics by esterase treatment. Appl. Surf. Sci. 2014, 295, 150–157. [Google Scholar] [CrossRef]
- Kwak, I.S.; Won, S.W.; Chung, Y.S.; Yun, Y.-S. Ruthenium recovery from acetic acid waste water through sorption with bacterial biosorbent fibers. Bioresour. Technol. 2013, 128, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wołowicz, A.; Hubicki, Z. The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Jermakowicz-Bartkowiak, D.; Kolarz, B.N. Poly(4-vinylpyridine) resins towards perrhenate sorption and desorption. React. Funct. Polym. 2011, 71, 95–103. [Google Scholar] [CrossRef]
- Marinho, R.S.; da Silva, C.N.; Afonso, J.C.; da Cunha, J.W. Recovery of platinum, tin and indium from spent catalysts in chloride medium using strong basic anion exchange resins. J. Hazard. Mater. 2011, 192, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Malkoc, E.; Nuhoglu, Y.; Abali, Y. Cr(VI) adsorption by waste acorn of Quercus ithaburensis in fixed beds: Prediction of breakthrough curves. Chem. Eng. J. 2006, 119, 61–68. [Google Scholar] [CrossRef]
- Sharififard, H.; Soleimani, M.; Ashtiani, F.Z. Evaluation of activated carbon and bio-polymer modified activated carbon performance for palladium and platinum removal. J. Taiwan Inst. Chem. Eng. 2012, 43, 696–703. [Google Scholar] [CrossRef]
- Shen, S.; Guishen, L.; Pan, T.; He, J.; Guo, Z. Selective adsorption of Pt ions from chloride solutions obtained by leaching chlorinated spent automotive catalysts on ion exchange resin Diaion WA21J. J. Colloid Interface Sci. 2011, 364, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Zhang, Q.; Li, C.; Bao, C.; Liu, X.; Xiao, P. Adsorption of Au(III), Pd(II), and Pt(IV) from Aqueous Solution onto Graphene Oxide. J. Chem. Eng. Data 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J. Hazard. Mater. 2009, 172, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Hasegawa, H.; Sugimoto, W.; Maki, T.; Ueda, K. Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Bioresour. Technol. 2008, 99, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Won, S.W.; Mao, J.; Kwak, I.-S.; Sathishkumar, M.; Yun, Y.-S. Platinum recovery from ICP wastewater by a combined method of biosorption and incineration. Bioresour. Technol. 2010, 101, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, J.; Liang, X.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J. Hazard. Mater. 2010, 182, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lee, S.Y.; Won, S.W.; Yun, Y.S. Surface modified bacterial biosorbent with poly(allylamine hydrochloride): Development using response surface methodology and use for recovery of hexachloroplatinate (IV) from aqueous solution. Water Res. 2010, 44, 5919–5928. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Kim, S.; Wu, X.H.; Kwak, I.-S.; Zhou, T.; Yun, Y.-S. A sustainable cationic chitosan/E. coli fiber biosorbent for Pt(IV) removal and recovery in batch and column systems. Sep. Purif. Technol. 2015, 143, 32–39. [Google Scholar] [CrossRef]
| Functional Group | Matrix | Structure | Mean Bead Size | Total Capacity |
|---|---|---|---|---|
| quaternary amine | crosslinked polystyrene | gel type | 0.64 ± 0.05 | 1.3 eq/L |
| Sorbent | Langmuir Isotherm | Pseudo-First-Order | Pseudo-Second-Order | Thomas Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/g) | (L/mg) | R2 | (mg/g) | (L/min) | R2 | (mg/g) | (g/mg/min) | R2 | (mL·min/mg) | (mg/g) | R2 | |
| M500 | 420.76 | 5.4522 | 0.81 | 370.62 | 0.0335 | 0.98 | 417.25 | 0.0001 | 0.99 | 0.0573 | 354.99 | 0.97 |
| PWPAN | 155.75 | 0.0278 | 0.92 | 133.97 | 2.0301 | 0.98 | 135.95 | 0.0370 | 0.99 | 0.2925 | 119.70 | 0.98 |
| Sorbent | qmax (mg/g) | Reference |
|---|---|---|
| Activated carbon | 45.5 | [20] |
| Bio-polymer modified activated carbon | 52.6 | [20] |
| Polysulfone-based fibers | 45.1 | [5] |
| Polymer-immobilized polysulfone-based fibers | 296.2 | [5] |
| Poly(allylamine hydrochloride)-modified E. coli | 348.8 | [27] |
| Glycine-modified crosslinked chitosan resin | 122.5 | [24] |
| Thiourea-modified chitosan | 129.9 | [23] |
| Ethylenediamine-modified magnetic chitosan nanoparticles | 171 | [26] |
| Graphene Oxide | 71.4 | [22] |
| Diaion WA21J | 5.69 | [21] |
| Poly(allylamine hydrochloride)-modified E. coli/chitosan fiber | 263.8 | [28] |
| PEI-modified E. coli | 108.8 | [25] |
| PWPAN | 155.75 | Present study |
| M500 | 420.76 | Present study |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.I.; Kim, S.; Cho, C.-W.; Yun, Y.-S. The Preparation of Modified Industrial Waste Polyacrylonitrile for the Adsorptive Recovery of Pt(IV) from Acidic Solutions. Materials 2016, 9, 988. https://doi.org/10.3390/ma9120988
Yoon SI, Kim S, Cho C-W, Yun Y-S. The Preparation of Modified Industrial Waste Polyacrylonitrile for the Adsorptive Recovery of Pt(IV) from Acidic Solutions. Materials. 2016; 9(12):988. https://doi.org/10.3390/ma9120988
Chicago/Turabian StyleYoon, Sung Il, Sok Kim, Chul-Woong Cho, and Yeoung-Sang Yun. 2016. "The Preparation of Modified Industrial Waste Polyacrylonitrile for the Adsorptive Recovery of Pt(IV) from Acidic Solutions" Materials 9, no. 12: 988. https://doi.org/10.3390/ma9120988







