3.1. Morphology of Carbon Nanofibers
In our previous study [
14], we showed that starch has advantages for fabricating a mesoporous carbon structure without using the template method. By electrospinning the starch solution, thin and flexible starch nanofiber webs were effectively fabricated. The morphology of the electrospun starch nanofibers included straight, smooth surfaces with no observable defects or beadings over the entire area of the web (
Figure S1). The individual starch nanofibers were cylindrical, with a diameter of 150 nm. To maintain their fibrous morphology during the carbonization process, the electrospun starch nanofibers were stabilized at 250 °C in air for 1 h. During the stabilization process, the fibers absorb oxygen and consequently rearrange into a more stable ladder configuration. After the nanofibers were stabilized, they were carbonized by heating in vacuum (10
−6 Torr) at temperatures from 700 to 1400 °C. Over 90% carbon content was obtained in the carbonized starch nanofibers heat-treated at >700 °C, and the carbon/oxygen ratio was increased almost linearly by the carbonization temperatures (
Figure S2). This process is a demonstration of successful conversion of starch to carbon nanofibers.
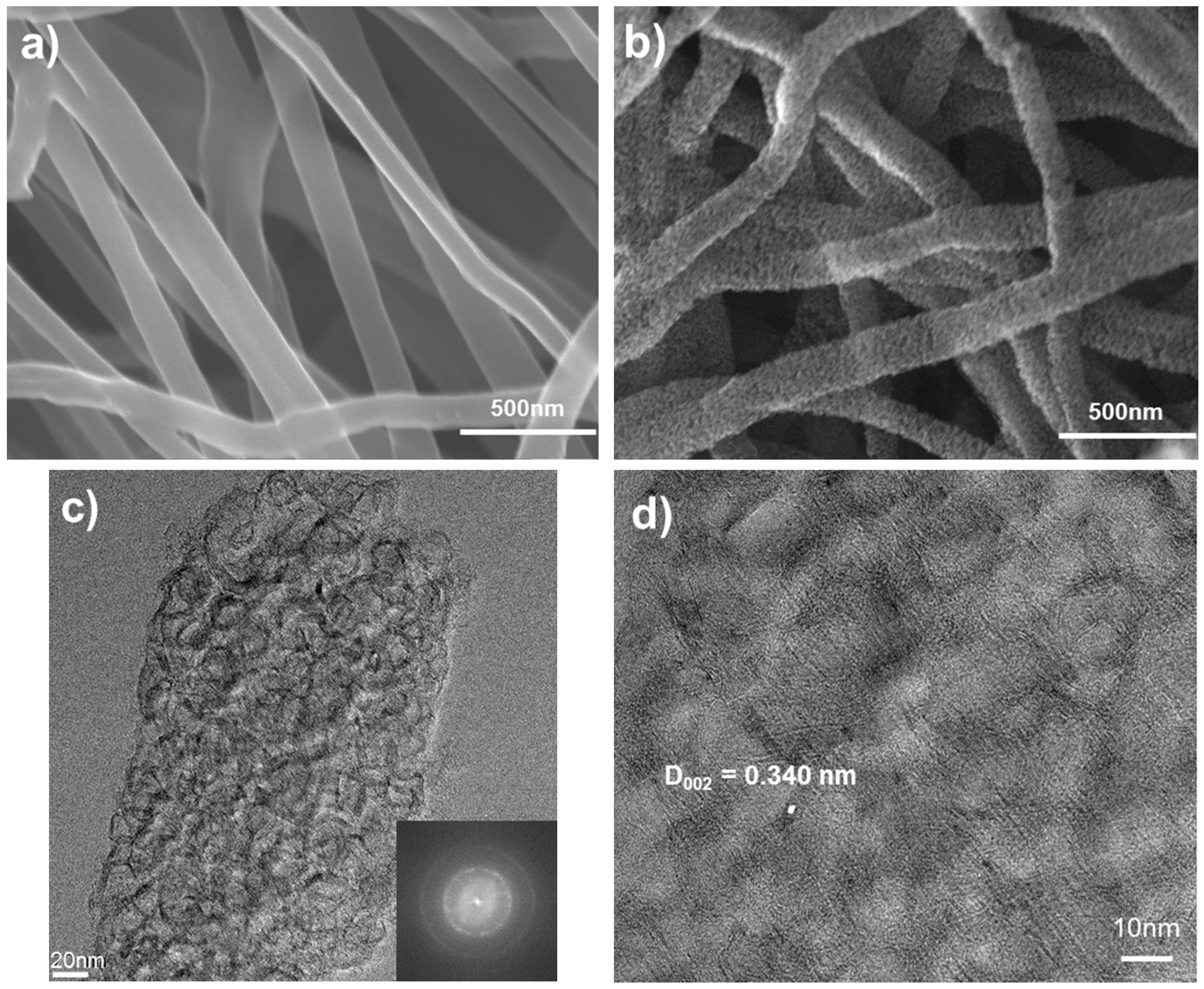
The surface of the carbon nanofibers that had been heat-treated at 700 °C was very smooth, as indicated by the micrographs in
Figure 1a. However, the carbon nanofibers carbonized at 1400 °C exhibited a highly porous structure, as shown in
Figure 1b. The transmission electron micrographs of the highly porous carbon nanofibers (
Figure 1c,d) represent a turbostratic structure consisting of carbon shells of 10–15 graphitic layers around a central core, and covered by pyrolytic carbon. In general, the d
002 spacing of graphite is 0.3354 nm, whereas the d
002 spacing of turbostratic carbon is about 0.338–0.341 nm [
15]. In a high-resolution transmission electron micrograph image (
Figure 1d) the d
002 spacing of the carbon nanofiber produced was about 0.340 nm, and this indicates that the structure of the starch nanofibers carbonized at 1400 °C was turbostratic carbon.
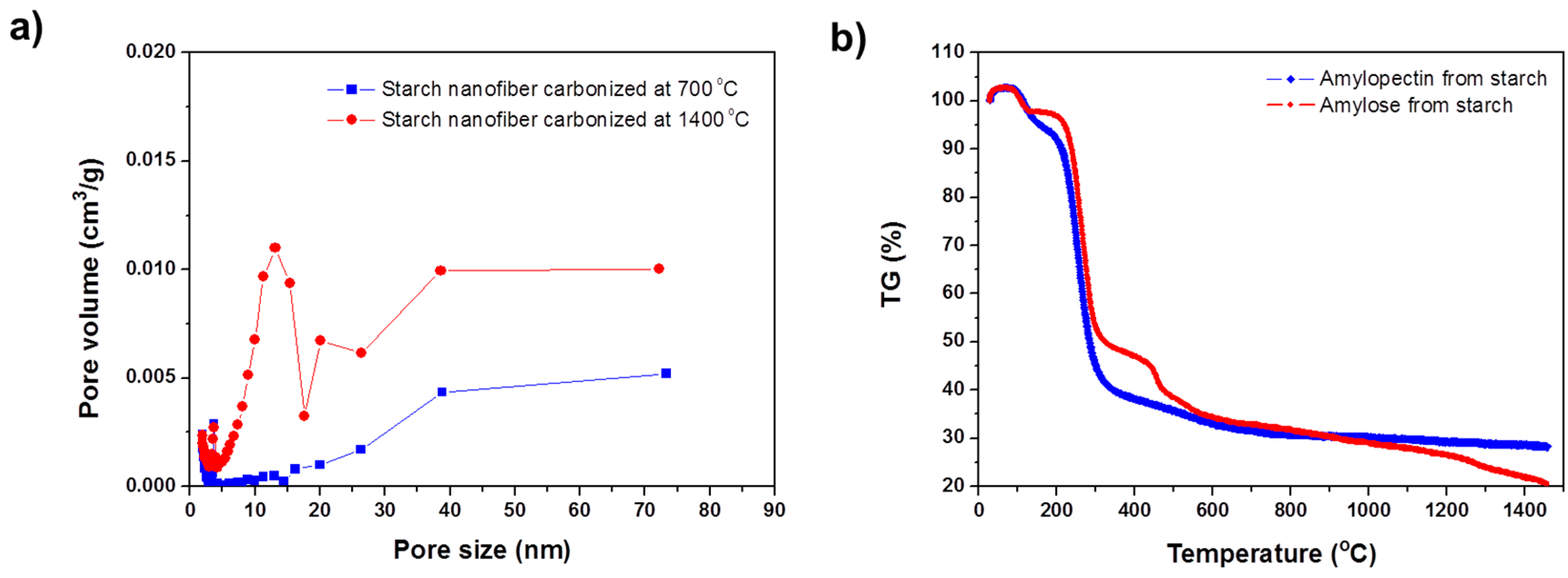
Figure 2a shows the pore size distributions obtained by applying density functional theory (DFT) to nitrogen adsorption isotherms, measured at 77 K. The starch nanofibers carbonized at 700 °C mostly contained micropores with an average diameter of 2 nm. In contrast, the pore diameters of the nanofiber carbonized at 1400 °C were distributed in the mesoporous region (5–10 nm). This novel mesoporous structure originated from the characteristic structure of starch. The starch consists of linear structures of amylose and branched structures of amylopectin. The starch microstructure shows alternating domains of amorphous amylose and crystalline amylopectin in a nanoscale lamellar structure [
16,
17]. Due to the difference in their crystallinity, these two polysaccharides show contrasting thermal behavior during the carbonization process.
Figure 2b shows thermograms of amylose and amylopectin from the starch. A small weight loss prior to 200 °C is related to desorption of water and the drastic weight losses that occurred between 250 and 700 °C corresponded to dehydration and decarboxylation. As seen in the thermograms, amorphous amylose is almost completely decomposed at around 1400 °C and crystalline amylopectin shows more stable behavior to >1000 °C. In a native starch, the amorphous regions are located between the amylopectin crystalline layers, which are approximately 6 nm apart. Therefore, due to decomposition of the amorphous amylose regions at high temperature, the starch nanofibers carbonized at 1400 °C exhibited abundant mesopores, with an average pore diameter of 4.5 nm and the starch nanofibers carbonized at 700 °C exhibited microporous surfaces with an average pore diameter of 2.0 nm due to the pyrolysis of functional groups as seen in
Table 1. In addition, though the surface area of starch nanofiber carbonized at 1400 °C was relatively lower than that of the starch nanofiber carbonized at 700 °C, the mesopore volume (
Vme) of it was 4 times higher than that of the starch nanofiber carbonized at 700 °C. This result suggests a new approach for tailoring both the size and the distribution of pores on the carbon nanofibers by adjusting the composition of amylose and amylopectin in the native starch.
3.2. Activation Process for the CNT-Carbon Nanofibers
While the carbon nanofibers show adequate pore distribution of mesopores, the surface area of the carbon nanofiber was relatively lower than that of activated carbon nanofiber. However, it is well known that the surface structure of carbon materials can be controlled by the activation process.
Moreover, hybridization with the CNT in the carbon nanofibers could be expected to result in better mechanical and functional properties. Therefore, the CNT-carbon nanofibers were fabricated using the same experimental procedure and then activated to obtain increased surface area and extensively developed internal pore structure, which make the activated carbon nanofibers highly adsorptive.
There are two main techniques for activation (physical and chemical), depending on whether gaseous or solid activating agents are used. Physically, activated carbons generally exhibit a fine pore structure, which is ideal for the adsorption of compounds from both liquid and vapor phase. The CNT-carbon nanofibers were activated physically in CO
2. During the activation process, a reaction is triggered between carbon atoms of the carbon nanofibers and the oxidizing gas, as in the following reaction:
This activation process etches the carbon backbones and gives rise to the creation and development of mesopores on the smooth surface of the CNT-carbon nanofibers. This is because the amorphous structure reacted faster than the crystalline structure.
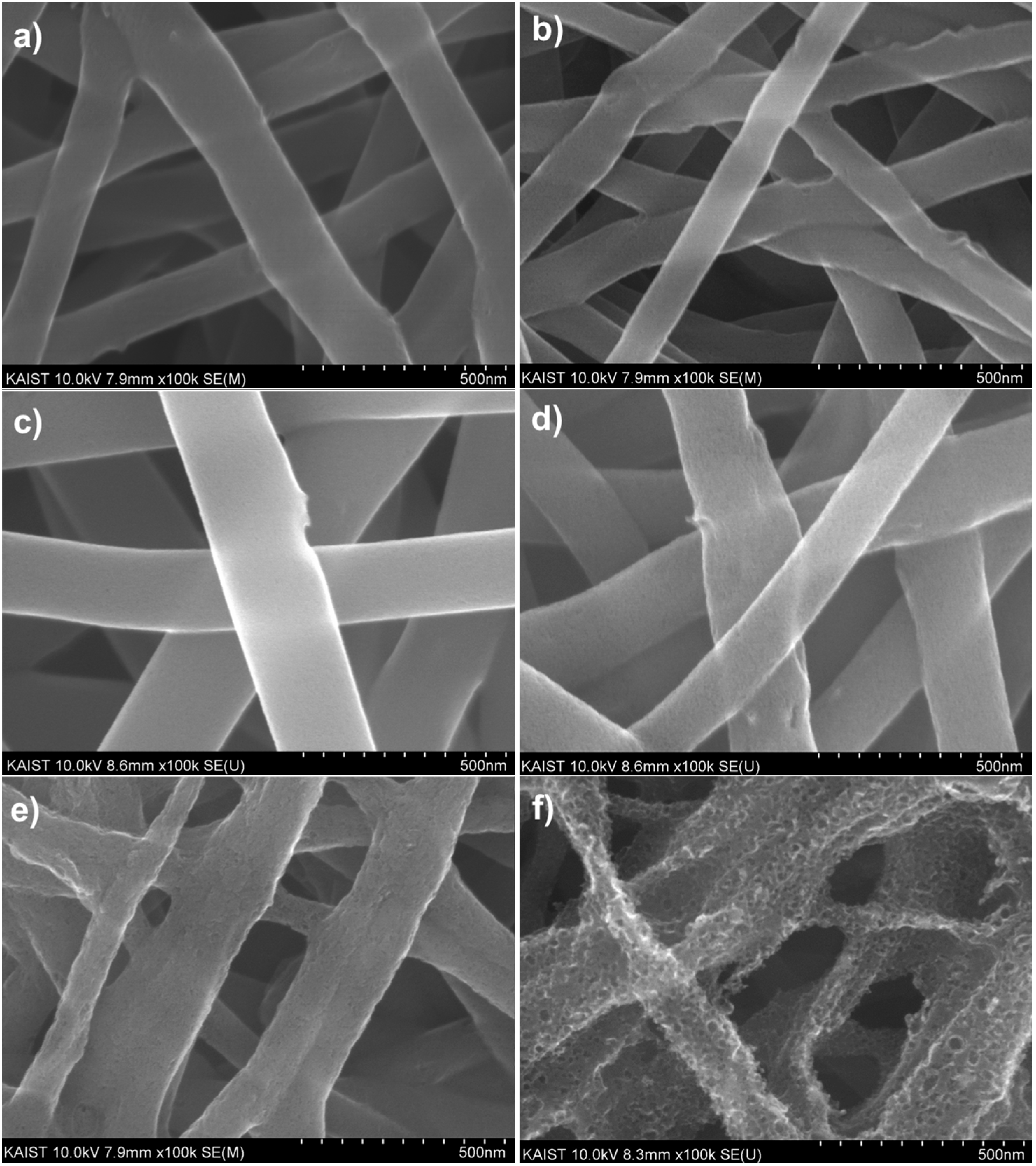
The morphological changes of the CNT-carbon nanofibers were observed under the various activation conditions. Before activation, the surface morphology of CNT-carbon nanofibers carbonized at 700 °C was very smooth, as indicated in
Figure 3a, and showed a mostly microporous structure. As shown in
Figure 3b,c, there were no morphological changes in the CNT-carbon nanofibers under the condition of 700 °C for 1 h, and 800 °C for 15 min. However, note that the surface of CNT-carbon nanofibers becomes porous after activation at 800 °C for 30 min (
Figure 3d). After this condition is achieved, the surface of the CNT-carbon nanofibers is greatly etched by the CO
2 gas and the pore size becomes larger (
Figure 3e,f).
To investigate changes in the pore structure, the specific surface area and pore size distribution of the CNT-carbon nanofibers were analyzed under the various activation conditions shown in
Table 2. During the activation process, sufficient numbers of mesopores and micropores were created. As the activation time increased at 800 °C, the pore width increased from 3.1 to 6.8 nm, and the Brunauer-Emmett-Teller (BET) specific surface area decreased from 982.4 to 350.1 m
2·g
−1. As a result, we can conclude that the optimum conditions for activation of the CNT-carbon nanofibers should be 800 °C for 30 min. This is particularly true for the application of this material as electrochemical electrodes, because of the abundance of mesopores that provide easy access to ions and provide a higher BET specific surface area.
3.3. Electrochemical Characterizations of the Porous Carbon Nanofibers
The electrochemical properties of the porous carbon and CNT-carbon nanofibers with various pore structures were characterized using the galvanostatic charge/discharge test. The porous nanofibers were applied for the anode as they were, because they are free-standing due to their good mechanical properties and exhibit superior electrical conductivity. Porous nanofibers with three different pore structures were used as anodes and tested at a rate of 0.1 C in the voltage range 0.01–3 V.
Figure 4a shows the initial charge/discharge curves of the porous carbon nanofibers heat-treated under different conditions.
For all the types of porous carbon nanofibers, there was no significant plateau shown in the charge/discharge profiles, which is similar to that of a typical non-graphitic carbon material. Porous carbon nanofibers heat-treated at 700 and 1400 °C exhibit a reversible capacity of 380 and 202 mAh·g−1, respectively. The specific capacity of the porous carbon nanofiber heat-treated at 700 °C and activated at 800 °C for 30 min was enhanced to 540 mAh·g−1.
In the first charge and discharge cycle, an irreversible capacity loss (a common phenomenon in carbonaceous electrodes) is observed. This irreversible capacity loss arises from reduction of the electrolyte, which results in the formation of a solid electrolyte interphase (SEI) over the large surface area of the sample; or from irreversible insertion of lithium into certain positions (such as in the vicinity of residual hydrogen atoms) in the carbon material [
18,
19]. In
Figure 4a, a gradual slope near 0.8 V appeared in the charge/discharge profiles; this represents the SEI formation. The 700 °C heat-treated nanofiber and the activated nanofiber show irreversible capacity. After these porous carbon nanofibers were charged (lithium ion inserted), a certain amount of lithium ions could not be extracted from the carbon nanofiber electrode during discharge, due to interruption by the SEI layer formed at the interface between electrode and electrolyte. From this phenomenon, we can conclude that these irreversible capacities originated from formation of an SEI layer caused by decomposition of the electrolyte, owing to the porous carbon nanofibers having relatively high specific surface area. However, the carbon nanofibers heat-treated at 1400 °C showed good reversible capacity in spite of a little lower specific capacity, which is mainly due to their turbostratic structure. The structure has partially graphitized regions and a slightly high d
002 space of 0.34–0.35 nm (as seen in
Figure 1c,d). This allows lithium ions to be easily inserted and extracted without collapsing its structure, in contrast with carbons with disordered structure, such as the carbon nanofiber heat-treated at 700 °C or activated carbon nanofiber.
The cycle performances of three kinds of carbon nanofibers are shown in
Figure 4b. The charge capacity of the carbon nanofiber heat-treated at 700 °C showed some decrease over the initial few cycles, but then stabilized after six cycles and showed specific capacity of 254 mAh·g
−1 after 30 cycles. For the activated carbon nanofiber, the specific capacity stabilized after ten cycles and exhibited a specific capacity of 338 mAh·g
−1 after 30 cycles. The 1400 °C heat-treated carbon nanofiber showed good cycle performance, with about 90% retention after 30 cycles, due to its turbostratic structure.
The high specific capacity of the activated carbon nanofiber is derived from the enlarged specific surface area and increased adsorption of lithium ions on the internal surfaces of the nanopores formed by small graphene sheets. When graphite is fully intercalated with lithium, there is one layer of lithium per carbon layer. However, if a carbon is made up of small single layers, lithium can be adsorbed on each side of the single layers, resulting in up to two lithium atoms per six carbons, without requiring lithium atoms to occupy nearest-neighbor sites. Therefore, the carbon materials with lots of carbon sheets arranged as single layers can have high specific capacity [
20]. To analyze the number of carbon sheets arranged as single layers, the empirical parameter,
R, is used. This parameter is defined as the ratio of the height of the (002) Bragg peak to the background [
21].
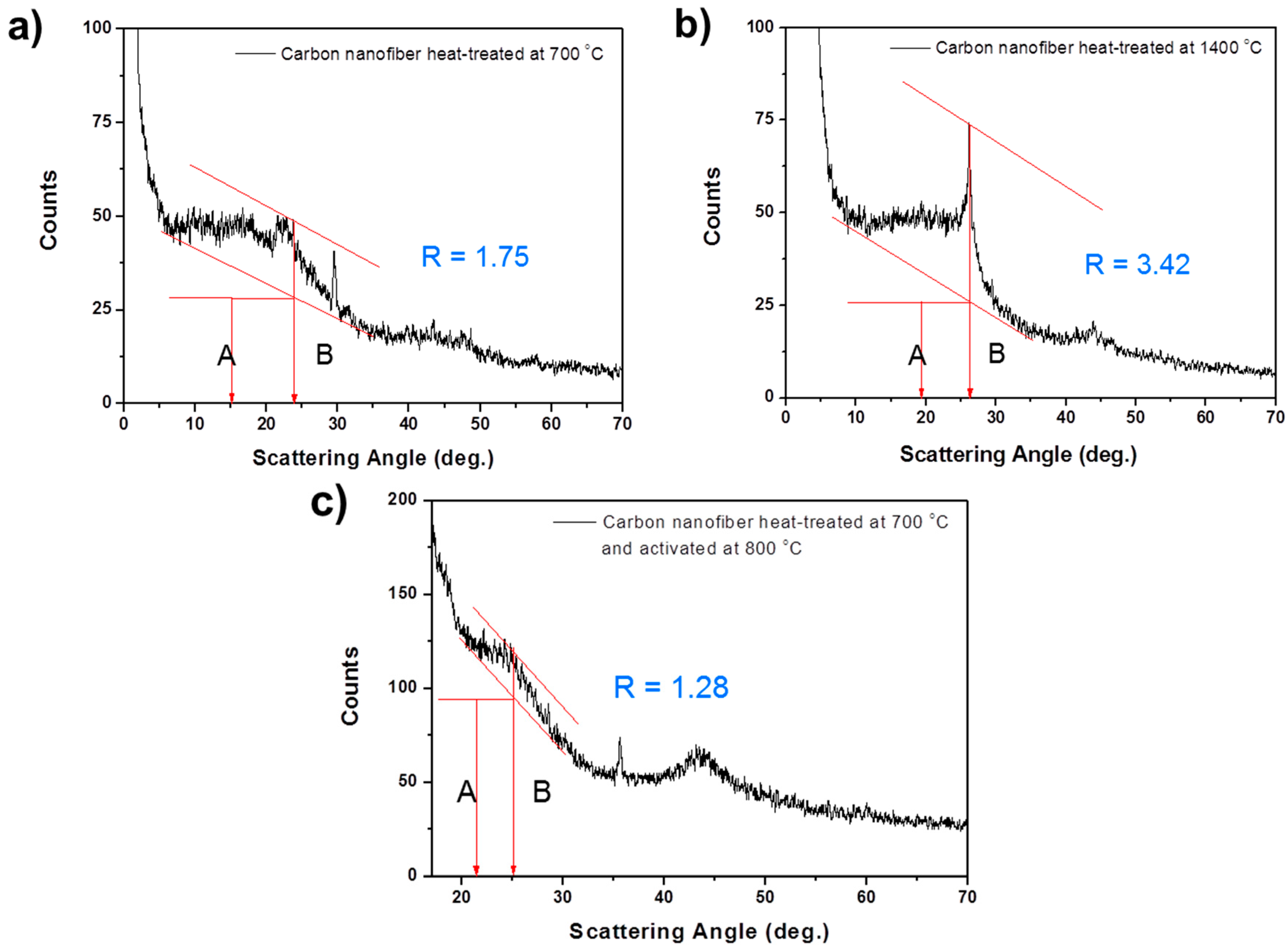
In the XRD result, the background (
A) is established by drawing a straight line connecting the data on either side of the peak. The peak height (
B) is determined by drawing the line tangent to the estimated linear background, which intersects the (002) peak at a single point. To compare a rate of the single layer in the carbon nanofibers heat-treated at different conditions, XRD patterns of the carbon nanofibers heat-treated at 700 and 1400 °C, and of the activated carbon nanofibers, were obtained (see
Figure 5). By calculating the R value of each kind of carbon nanofiber, we determined that carbon heat-treated at 700 °C and activated at 800 °C has the lowest
R value (1.28). This means that it has the most single layers, when compared with carbon heat-treated at 700 °C (
R = 1.75) and 1400 °C (
R = 3.42). From this analysis, we can conclude that activated carbon nanofiber has a high specific capacity of 540 mAh·g
−1 because it contains many single layers in its structure.
3.4. Effect of CNT on the Electrochemical Properties of the Porous CNT-Carbon Nanofibers
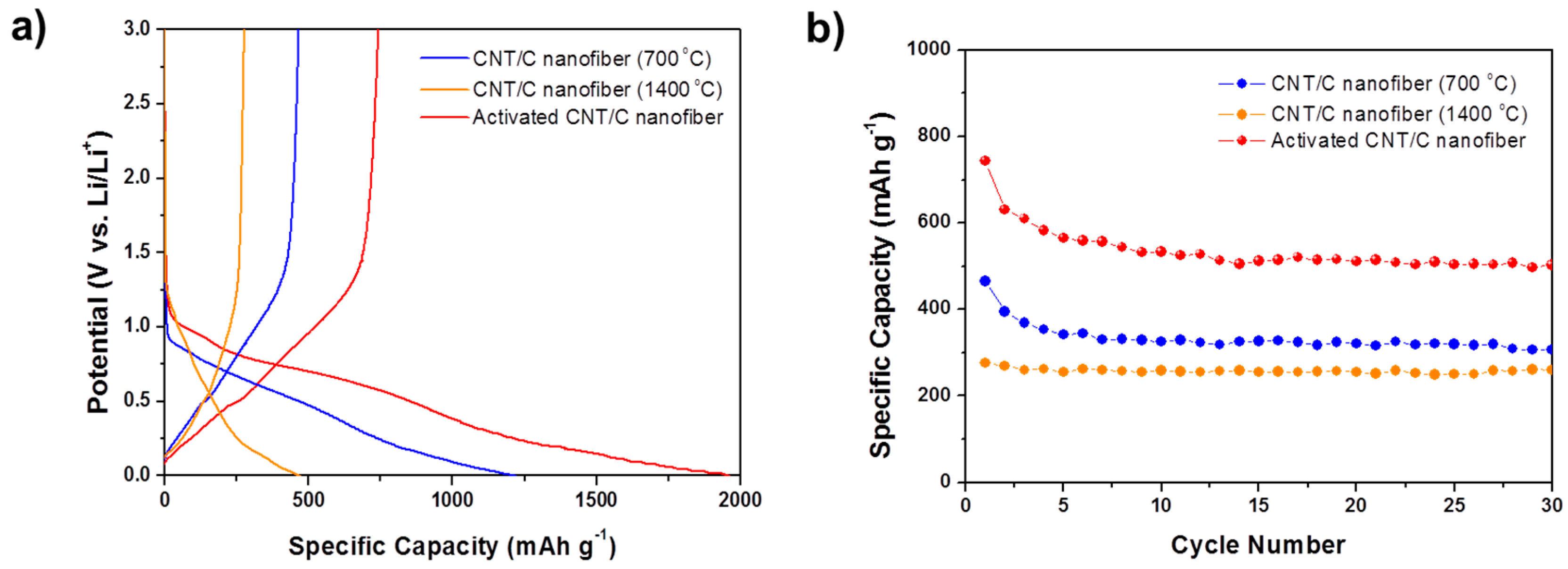
Figure 6a shows the charge/discharge profiles of 1 wt % CNT-carbon nanofibers. At a rate of 0.1 C, the reversible capacity of the CNT-carbon nanofibers heat-treated at 700 and 1400 °C shows 465 mAh·g
−1 and 276 mAh·g
−1, respectively. The reversible capacity of the CNT-activated carbon nanofiber was shown to be very high (743 mAh·g
−1). Irreversible capacity was also observed in the first charge/discharge profiles of the CNT-carbon nanofibers, as seen in those of the carbon nanofibers. The first discharge capacity of the CNT-carbon nanofiber heat-treated at 700 °C and activated at 800 °C, showed a very high value (1960 mAh·g
−1), but this decreased to 743 mAh·g
−1, less than half of the initial discharge capacity. This shows a larger irreversible capacity compared with carbon nanofiber heat-treated under the same conditions, but without CNTs. From this comparison, we know that when lithium is extracted from the CNT-carbon nanocomposite fibers (charge), it is trapped and cannot be extracted by CNTs. The plateau near 0.8 V, which is attributed to SEI formation, is also clearly visible in this sample. Therefore, we can conclude that large irreversible capacity comes from decomposition of an electrolyte resulting in the formation of the SEI layer, due to its very high specific surface area of 973 m
2·g
−1.
The cycle performances of the CNT-carbon nanofibers are shown in
Figure 6b. For the activated carbon nanofibers, the specific capacity decreases over the initial few cycles but then stabilizes and remains at about 510 mAh·g
−1 after 30 cycles, which is significantly higher than the theoretical specific capacity of graphite (372 mAh·g
−1).
Compared with the carbon nanofibers, all three types of the CNT-carbon nanofibers showed enhanced specific capacity. In
Table 3, specific capacities of all three types of the CNT-carbon nanofibers are summarized as a function of heat-treated conditions and their physical properties such as electrical conductivity and BET specific surface area. This high specific capacity is mainly due to its larger specific surface area, which provides many adsorption sites for lithium ions and higher electrical conductivity, compared with carbon nanofibers without CNT. Also, the CNT could effectively enhance the electrical conductivity of the carbon nanofibers due to its superior electrical properties. The electrical resistivity of the carbon nanofibers and the CNT-carbon nanofibers were measured using the 2-probe-point technique and the electrical conductivities were calculated using the following equation:
where
R is electrical resistance in Ω,
A is the cross-sectional area in cm
2, and
L is the distance between electrodes in cm. As seen in
Table 3, the CNT-carbon nanofibers showed higher electrical conductivity compared to the carbon nanofibers with the same heat-treatment and activation condition. From these results, we can see that CNT is an effective material for enhancing the electrical conductivity of the carbon nanofibers, and that it induces the increased specific capacity of the carbon nanofibers.
The enhancement of the specific capacity with increase of the fraction of small graphene sheets can be predicted using a simple line plot, the capacity (0 to the theoretical value of Li
2C
6, 744 mAh·g
−1) versus the single-layer fraction as shown in
Figure 7. For the carbon nanofibers without CNT, the specific capacity increases with increase in the fraction of single layers in their carbon structure. These results fit well with the estimated values. However, the specific capacity of the CNT-carbon nanocomposite fibers is much greater than the value predicted value from the single-layer fraction by XRD analysis. From the result of the increase in specific capacity with the addition of CNT, we can see that CNT plays a key role in providing a large surface area (adsorbed sites) and in creating a conductive path for ions.
Therefore, the CNT-carbon nanofibers can be applied as electrodes of Li-ion batteries. Their electrochemical properties are adjustable by controlling the surface morphology and pore structure by manipulating the conditions for heat-treatment and the activation process.