Enhanced Photovoltaic Properties of Bulk Heterojunction Organic Photovoltaic Devices by an Addition of a Low Band Gap Conjugated Polymer
Abstract
:1. Introduction
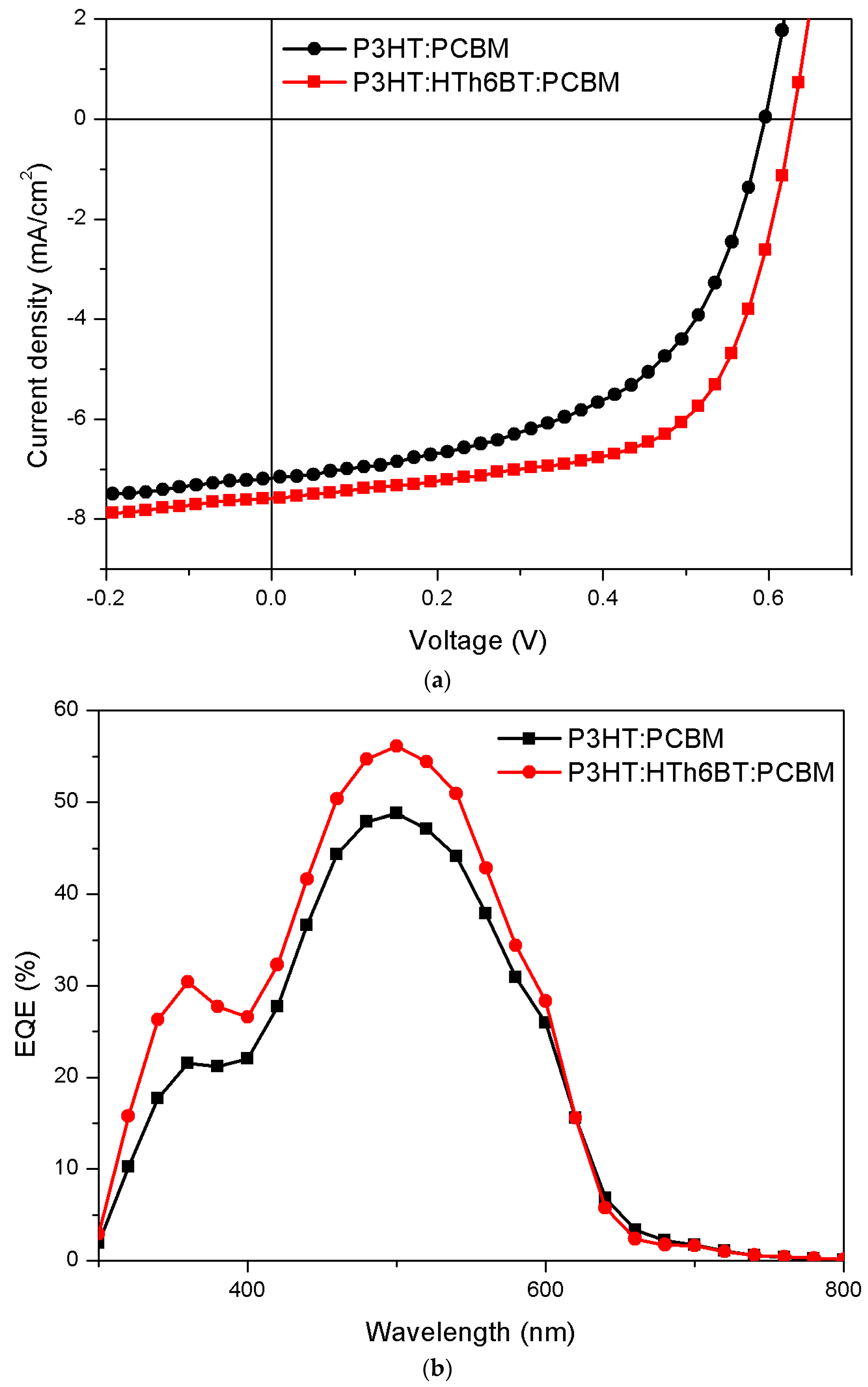
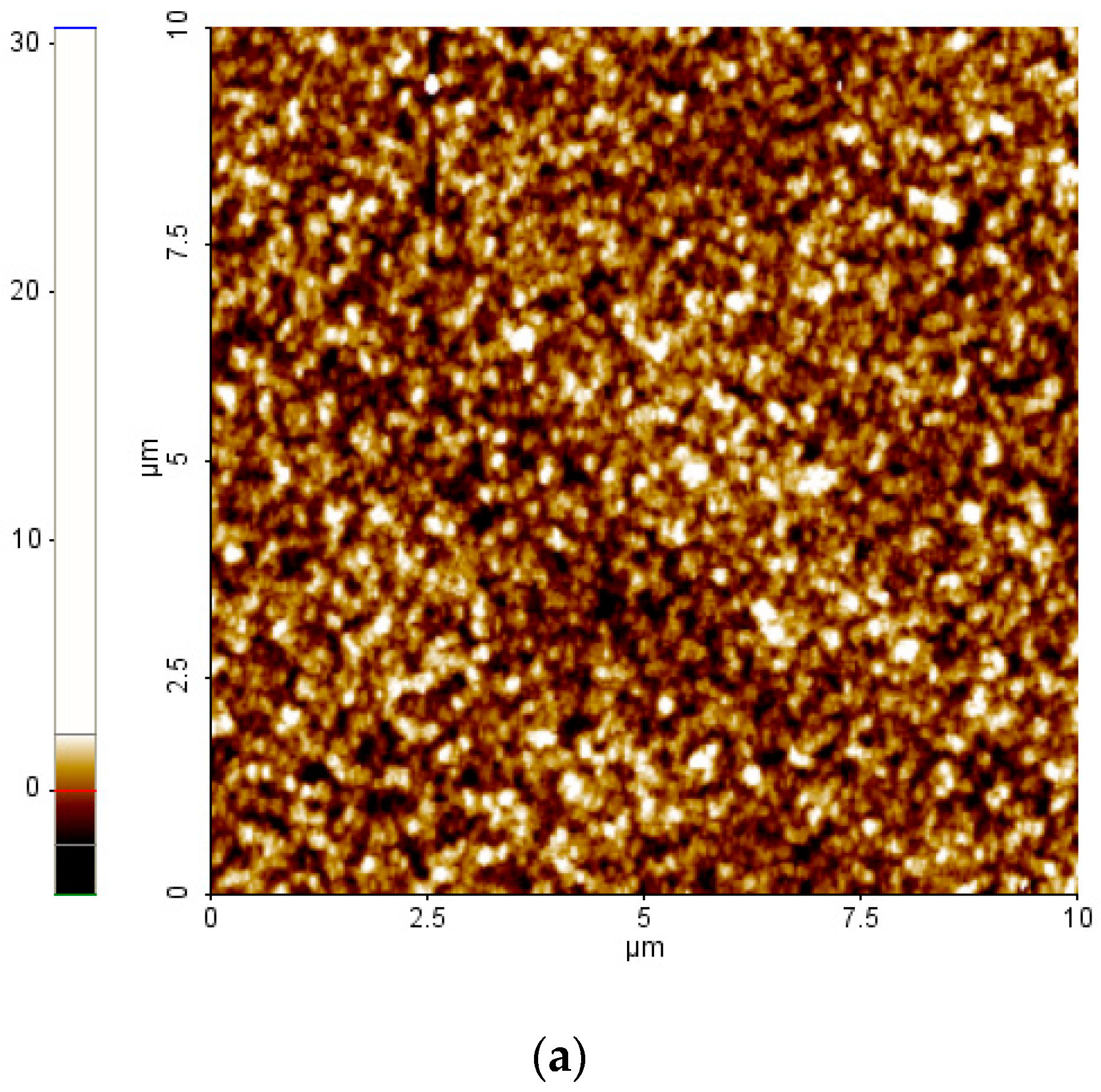
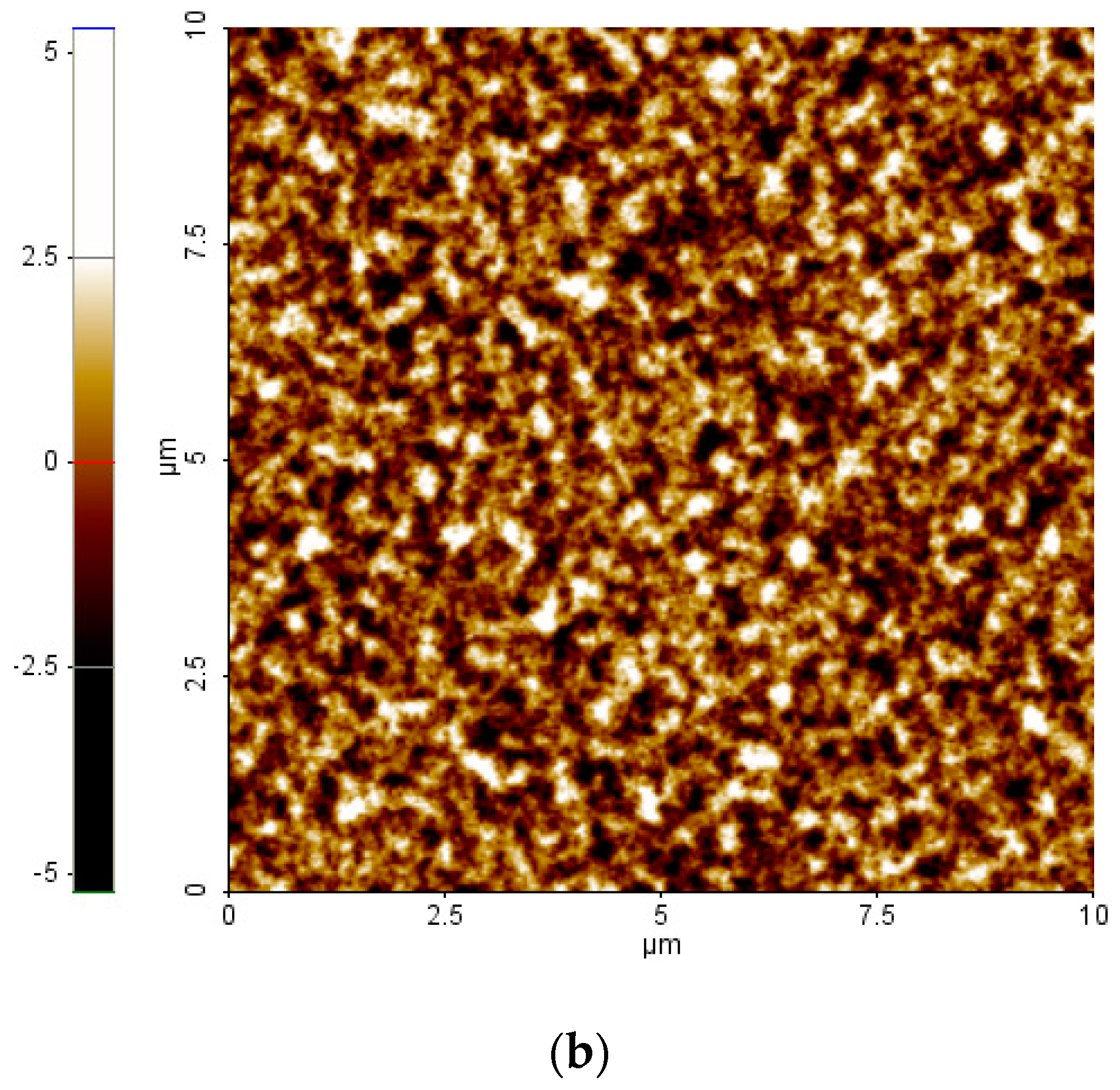
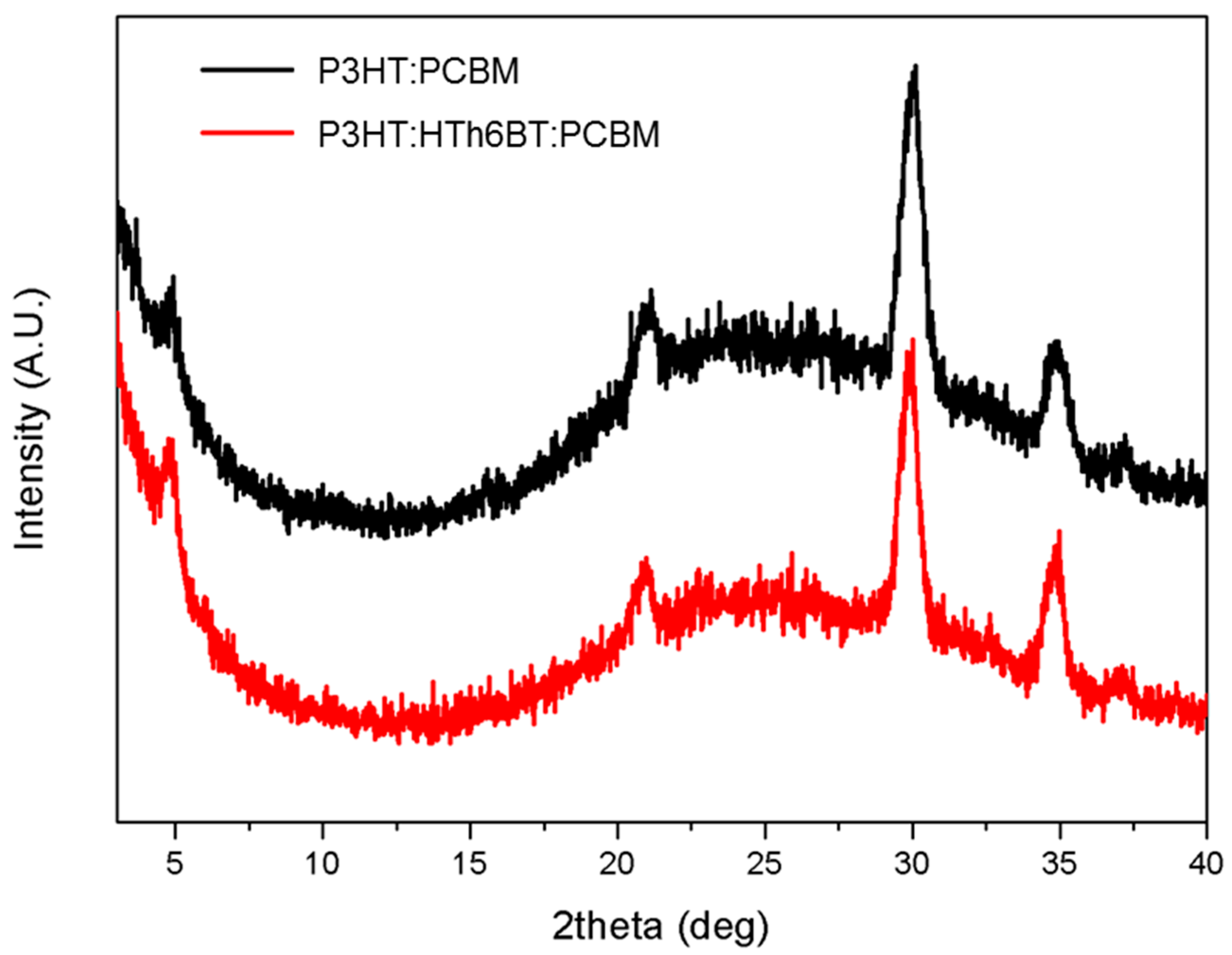
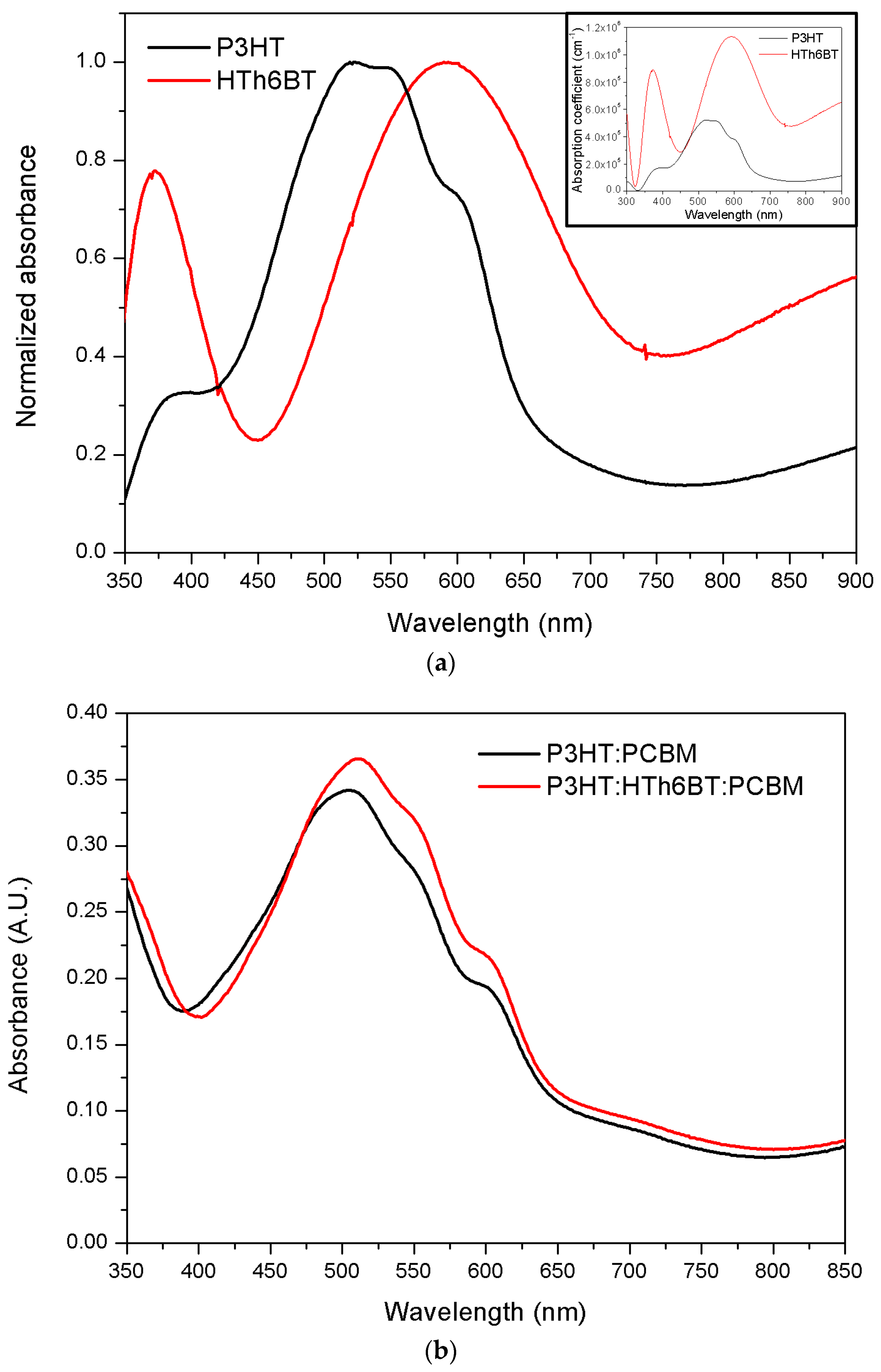
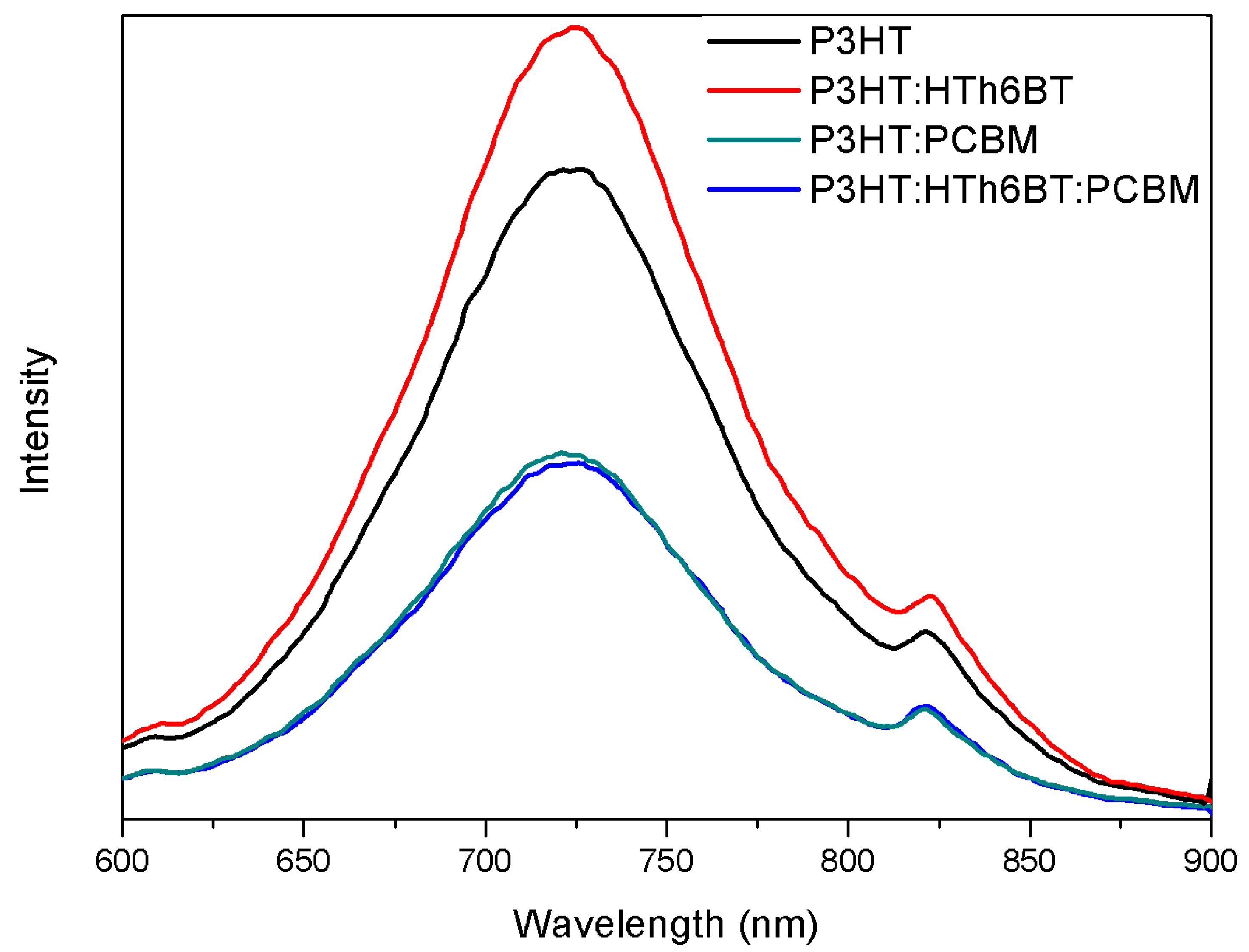
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future—Bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.W.; Lee, E.J.; Song, K.W.; Lee, J.Y.; Moon, D.K. Enhanced carrier mobility and photon-harvesting property by introducing Au nano-particles in bulk heterojunction photovoltaic cells. Org. Electron. 2013, 14, 1931–1938. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choi, M.-H.; Song, H.-J.; Moon, D.-K. Random copolymers based on 3-hexylthiophene and benzothiadiazole with induced π-conjugation length and enhanced open-circuit voltage property for organic photovoltaics. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4875–4883. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, H.; Ko, S.-J.; Uddin, M.A.; Walker, B.; Yum, S.; Jeong, J.-E.; Yun, M.H.; Shin, T.J.; Hwang, S.; et al. Semi-crystalline photovoltaic polymers with efficiency exceeding 9% in a ~300 nm thick conventional single-cell device. Energy Environ. Sci. 2014, 7, 3040–3051. [Google Scholar] [CrossRef]
- Marrocchi, A.; Facchetti, A.; Lanari, D.; Petrucci, C.; Vaccaro, L. Current methodologies for a sustainable approach to π-conjugated organic semiconductors. Energy Environ. Sci. 2016, 9, 763–786. [Google Scholar] [CrossRef]
- Bura, T.; Blaskovits, J.T.; Leclerc, M. Direct (Hetero)arylation Polymerization: Trends and Perspectives. J. Am. Chem. Soc. 2016, 138, 10056–10071. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.Y.; Dong, Q.; Yin, H.; Leung, W.W.K.; Yang, Q.; Lee, H.K.H.; Tsang, S.W.; So, S.K. Impact of Solvent Additive on Carrier Transport in Polymer:Fullerene Bulk Heterojunction Photovoltaic Cells. Adv. Mater. Interfaces 2015, 2, 1500166. [Google Scholar] [CrossRef]
- Heo, S.W.; Kim, S.H.; Lee, E.J.; Moon, D.K. Enhanced performance in bulk heterojunction solar cells by introducing naphthalene derivatives as processing additives. Sol. Energy Mater. Sol. Cells 2013, 111, 16–22. [Google Scholar] [CrossRef]
- Heo, S.W.; Song, K.W.; Choi, M.H.; Oh, H.S.; Moon, D.K. Influence of alkanediol series as processing additives in photo-active layer on the power conversion efficiency of polymer solar cells. Sol. Energy Mater. Sol. Cells 2013, 114, 82–88. [Google Scholar] [CrossRef]
- Heo, S.W.; Song, K.W.; Moon, D.K. Enhanced performance in bulk heterojunction solar cells with alkylidene fluorene donor by introducing modified PFN-OH/Al bilayer cathode. RSC Adv. 2014, 4, 6776. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.-Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous Enhancement of Open-Circuit Voltage, Short-Circuit Current Density, and Fill Factor in Polymer Solar Cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.W.; Lee, E.J.; Seong, K.W.; Moon, D.K. Enhanced stability in polymer solar cells by controlling the electrode work function via modification of indium tin oxide. Sol. Energy Mater. Sol. Cells 2013, 115, 123–128. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, E.J.; Kim, D.H.; Moon, D.K.; Lee, S. Solution-processed interlayer of n-type small molecules for organic photovoltaic devices: Enhancement of the fill factor due to ordered orientation. Sol. Energy Mater. Sol. Cells 2015, 141, 232–239. [Google Scholar] [CrossRef]
- Lu, L.; Xu, T.; Chen, W.; Landry, E.S.; Yu, L. Ternary blend polymer solar cells with enhanced power conversion efficiency. Nat. Photonics 2014, 8, 716–722. [Google Scholar] [CrossRef]
- Savoie, B.M.; Dunaisky, S.; Marks, T.J.; Ratner, M.A. The Scope and Limitations of Ternary Blend Organic Photovoltaics. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Mai, C.-K.; Collins, S.D.; Bazan, G.C.; Nguyen, T.-Q.; Heeger, A.J. Polymer Homo-Tandem Solar Cells with Best Efficiency of 11.3%. Adv. Mater. 2015, 27, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Sharma, G.D.; Mikroyannidis, J.A. Improved power conversion efficiency of bulk heterojunction poly(3-hexylthiophene):PCBM photovoltaic devices using small molecule additive. Sol. Energy Mater. Sol. Cells 2011, 95, 1219–1223. [Google Scholar] [CrossRef]
- Lim, E.; Lee, S.; Lee, K.K. Improved photovoltaic performance of P3HT:PCBM cells by addition of a low band-gap oligomer. Chem. Commun. 2011, 47, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.D.; Singh, S.P.; Roy, M.S.; Mikroyannidis, J.A. Solution processed bulk heterojunction polymer solar cells with low band gap DPP-CN small molecule sensitizer. Org. Electron. 2012, 13, 1756–1762. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Noh, S.; Howard, J.B.; Thompson, B.C. Influence of Surface Energy on Organic Alloy Formation in Ternary Blend Solar Cells Based on Two Donor Polymers. ACS Appl. Mater. Interfaces 2016, 8, 27931–27941. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Fan, Q.; Guo, X.; Guo, B.; Li, W.; Zhang, Y.; Zhang, M.; Li, Y. Efficient ternary blend all-polymer solar cells with a polythiophene derivative as a hole-cascade material. J. Mater. Chem. A 2016, 4, 14752–14760. [Google Scholar] [CrossRef]
- Flamini, R.; Marrocchi, A.; Spalletti, A. Quantitative cascade energy transfer in semiconductor thin films. Photochem. Photobiol. Sci. 2014, 13, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; George, Z.; Ma, Z.; Bergqvist, J.; Tvingstedt, K.; Vandewal, K.; Wang, E.; Andersson, L.M.; Andersson, M.R.; Zhang, F.; et al. Semi-Transparent Tandem Organic Solar Cells with 90% Internal Quantum Efficiency. Adv. Energy Mater. 2012, 2, 1467–1476. [Google Scholar] [CrossRef]
- Khlyabich, P.P.; Burkhart, B.; Thompson, B.C. Compositional Dependence of the Open-Circuit Voltage in Ternary Blend Bulk Heterojunction Solar Cells Based on Two Donor Polymers. J. Am. Chem. Soc. 2012, 134, 9074–9077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, D.; Lu, K.; Zhang, J.; Xia, B.; Zhao, Y.; Fang, J.; Wei, Z. Synergistic effect of polymer and small molecules for high-performance ternary organic solar cells. Adv. Mater. 2015, 27, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Fang, J.; Lu, K.; Wang, Z.; Ma, W.; Wei, Z. Conjugated Polymer-Small Molecule Alloy Leads to High Efficient Ternary Organic Solar Cells. J. Am. Chem. Soc. 2015, 137, 8176–8183. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, W.; Xu, T.; Yu, L. High-performance ternary blend polymer solar cells involving both energy transfer and hole relay processes. Nat. Commun. 2015, 6, 7327. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Guerrero, A.; Chambon, S.; Hirsch, L.; Garcia-Belmonte, G. Light-modulated TiOx Interlayer Dipole and Contact Activation in Organic Solar Cell Cathodes. Adv. Funct. Mater. 2014, 24, 6234–6240. [Google Scholar] [CrossRef]
- Wang, W.; Guo, S.; Herzig, E.M.; Sarkar, K.; Schindler, M.; Magerl, D.; Philipp, M.; Perlich, J.; Müller-Buschbaum, P. Investigation of Morphological Degradation of P3HT:PCBM Bulk Heterojunction Films Exposed to Long-Term Host Solvent Vapor. J. Mater. Chem. A 2016, 4, 3743–3753. [Google Scholar] [CrossRef]
- Bouthinon, B.; Clerc, R.; Vaillant, J.; Verilhac, J.-M.; Faure-Vincent, J.; Djurado, D.; Ionica, I.; Man, G.; Gras, A.; Pananakakis, G.; et al. Impact of Blend Morphology on Interface State Recombination in Bulk Heterojunction Organic Solar Cells. Adv. Funct. Mater. 2015, 25, 1090–1101. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhou, H.; Price, S.C.; You, W. Parallel-like bulk heterojunction polymer solar cells. J. Am. Chem. Soc. 2012, 134, 5432–5435. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-H.; Jhuo, H.-J.; Cheng, Y.-S.; Chen, S.-A. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kwon, Y.; Choi, B.-D.; Ade, H.; Han, Y.S. Improved efficiency of bulk heterojunction poly(3-hexylthiophene):[6,6]-phenyl-C[sub 61]-butyric acid methyl ester photovoltaic devices using discotic liquid crystal additives. Appl. Phys. Lett. 2010, 96, 183305. [Google Scholar] [CrossRef]
- Choi, M.-H.; Ko, E.J.; Han, Y.W.; Lee, E.J.; Moon, D.K. Control of polymer-packing orientation in thin films through chemical structure of D-A type polymers and its application in efficient photovoltaic devices. Polymer 2015, 74, 205–215. [Google Scholar] [CrossRef]

| Photoactive Layer | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) | Rs (Ω·cm2) | Rsh (Ω·cm2) |
|---|---|---|---|---|---|---|
| P3HT:PCBM | 7.1 | 0.596 | 52.9 | 2.2 | 9.02 | 2300 |
| (6.9) | (0.596) | (49.9) | (2.02) | |||
| HTh6BT:PCBM [5] | 5.5 | 0.820 | 34.6 | 1.6 | - | - |
| P3HT:HTh6BT:PCBM | 7.6 | 0.636 | 62.3 | 3.0 | 7.50 | 3340 |
| (7.5) | (0.636) | (59.8) | (2.85) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.J.; Choi, M.H.; Moon, D.K. Enhanced Photovoltaic Properties of Bulk Heterojunction Organic Photovoltaic Devices by an Addition of a Low Band Gap Conjugated Polymer. Materials 2016, 9, 996. https://doi.org/10.3390/ma9120996
Lee EJ, Choi MH, Moon DK. Enhanced Photovoltaic Properties of Bulk Heterojunction Organic Photovoltaic Devices by an Addition of a Low Band Gap Conjugated Polymer. Materials. 2016; 9(12):996. https://doi.org/10.3390/ma9120996
Chicago/Turabian StyleLee, Eui Jin, Min Hee Choi, and Doo Kyung Moon. 2016. "Enhanced Photovoltaic Properties of Bulk Heterojunction Organic Photovoltaic Devices by an Addition of a Low Band Gap Conjugated Polymer" Materials 9, no. 12: 996. https://doi.org/10.3390/ma9120996





