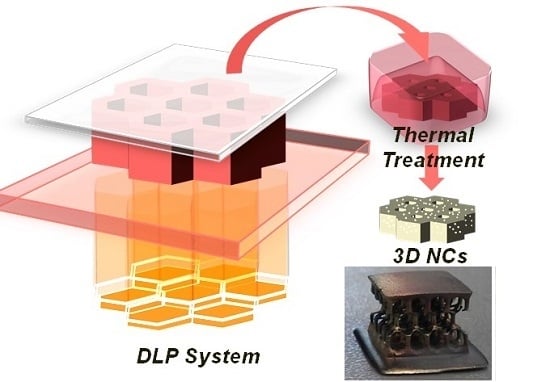
In Situ Thermal Generation of Silver Nanoparticles in 3D Printed Polymeric Structures
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the 3D Structures
2.3. Thermal Treatmeant
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| NPs | nanoparticles |
| TT | thermal treatment |
| 3D | three-dimensional |
| NCs | nanocomposites |
| SLA | stereolithography |
| DLP | digital light processing |
| DMD | digital micromirror-array device |
| DSC | differential scanning calorimetry |
| TGA | thermogravimetric analysis |
| CAD | computer-aided design |
| BAPO | bis-acyl-phosphine oxide |
| PEGDA | polyethylene glycol diacrylate |
| FESEM | field emission scanning electron microscopy |
References
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Tamborra, M.; Striccoli, M.; Comparelli, R.; Curri, M.L.; Petrella, A.; Agostiano, A. Optical properties of hybrid composites based on highly luminescent cds nanocrystals in polymer. Nanotechnology 2004, 15, S240. [Google Scholar] [CrossRef]
- Lü, C.; Gao, J.; Fu, Y.; Du, Y.; Shi, Y.; Su, Z. A ligand exchange route to highly luminescent surface-functionalized zns nanoparticles and their transparent polymer nanocomposites. Adv. Funct. Mater. 2008, 18, 3070–3079. [Google Scholar] [CrossRef]
- Lu, C.; Yang, B. High refractive index organic-inorganic nanocomposites: Design, synthesis and application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Koziej, D.; Fischer, F.; Kränzlin, N.; Caseri, W.R.; Niederberger, M. Nonaqueous tio2 nanoparticle synthesis: A versatile basis for the fabrication of self-supporting, transparent, and uv-absorbing composite films. ACS Appl. Mater. Interfaces 2009, 1, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Mutiso, R.M.; Kikkawa, J.M.; Winey, K.I. Resistive switching in silver/polystyrene/silver nano-gap devices. Appl. Phys. Lett. 2013, 103, 223302. [Google Scholar] [CrossRef]
- Ling, Q.-D.; Liaw, D.-J.; Zhu, C.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Polymer electronic memories: Materials, devices and mechanisms. Prog. Polym. Sci. 2008, 33, 917–978. [Google Scholar] [CrossRef]
- Hedayati, M.; Faupel, F.; Elbahri, M. Review of plasmonic nanocomposite metamaterial absorber. Materials 2014, 7, 1221–1248. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Schexnailder, P.; Schmidt, G. Nanocomposite polymer hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Mallouki, M.; Tran-Van, F.; Sarrazin, C.; Simon, P.; Daffos, B.; De, A.; Chevrot, C.; Fauvarque, J.F. Polypyrrole-Fe2O3 nanohybrid materials for electrochemical storage. J. Solid State Electrochem. 2007, 11, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Ingrosso, C.; Panniello, A.; Comparelli, R.; Curri, M.L.; Striccoli, M. Colloidal inorganic nanocrystal based nanocomposites: Functional materials for micro and nanofabrication. Materials 2010, 3, 1316–1352. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Leong, Y.J.; Rozen, I.; Qu, X.; Dong, R.; Wu, Z.; Gao, W.; Chung, P.H.; Wang, J.; et al. 3d-printed artificial microfish. Adv. Mater. 2015, 27, 4411–4417. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Zhu, W.; Qu, X.; Aaronson, C.; McCall, W.R.; Chen, S.; Sirbuly, D.J. 3d optical printing of piezoelectric nanoparticle–polymer composite materials. ACS Nano 2014, 8, 9799–9806. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wei, T.-S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3d printing of interdigitated li-ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.J.; Bradley, R.J.; Purssell, C.P.; Billson, D.R.; Hutchins, D.A. A simple, low-cost conductive composite material for 3d printing of electronic sensors. PLoS ONE 2012, 7, e49365. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M. 3d printing gets a boost and opportunities with polymer materials. ACS Macro Lett. 2014, 3, 382–386. [Google Scholar] [CrossRef]
- Quan, Z.; Wu, A.; Keefe, M.; Qin, X.; Yu, J.; Suhr, J.; Byun, J.-H.; Kim, B.-S.; Chou, T.-W. Additive manufacturing of multi-directional preforms for composites: Opportunities and challenges. Mater. Today 2015, 18, 503–512. [Google Scholar] [CrossRef]
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired detoxification using 3d-printed hydrogel nanocomposites. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3d printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Vicente Haro Gonzalez, J.; Jordá Ferrando, O.; Delgado Gordillo, J.; Ramón Blasco Puchades, J.; Portolés Griñan, L. Additive layered manufacturing: Sectors of industrial application shown through case studies. Int. J. Prod. Res. 2011, 49, 1061–1079. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Jeong, J.H.; Bajaj, P.; Collens, M.; Saif, T.; Kong, H.; Bashir, R. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip 2012, 12, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ladd, C.; So, J.-H.; Muth, J.; Dickey, M.D. 3d printing of free standing liquid metal microstructures. Adv. Mater. 2013, 25, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Chiappone, A.; Fantino, E.; Roppolo, I.; Lorusso, M.; Manfredi, D.; Fino, P.; Pirri, C.F.; Calignano, F. 3d printed peg-based hybrid nanocomposites obtained by sol–gel technique. ACS Appl. Mater. Interfaces 2016, 8, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.R.; Lambert, P.M.; Chartrain, N.A.; Ruohoniemi, D.M.; Zhang, Z.; Jangu, C.; Zhang, M.; Williams, C.B.; Long, T.E. 3d printing phosphonium ionic liquid networks with mask projection microstereolithography. ACS Macro Lett. 2014, 3, 1205–1209. [Google Scholar] [CrossRef]
- Peterson, G.I.; Larsen, M.B.; Ganter, M.A.; Storti, D.W.; Boydston, A.J. 3d-printed mechanochromic materials. ACS Appl. Mater. Interfaces 2015, 7, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Q.; Cai, X.; Zhou, S.; Kobe, B.; Yang, J. Initiator-integrated 3d printing enables the formation of complex metallic architectures. ACS Appl. Mater. Interfaces 2014, 6, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Vatani, M.; Lu, Y.; Engeberg, E.D.; Choi, J.-W. Combined 3d printing technologies and material for fabrication of tactile sensors. Int. J. Precis. Eng. Manuf. 2015, 16, 1375–1383. [Google Scholar] [CrossRef]
- Nassar, I.T.; Weller, T.M. An electrically-small, 3-d cube antenna fabricated with additive manufacturing. In Proceedings of the 2013 IEEE Topical Conference on Power Amplifiers for Wireless and Radio Applications (PAWR), Santa Clara, CA, USA, 20 January 2013; pp. 91–93.
- Lee, M.P.; Cooper, G.J.T.; Hinkley, T.; Gibson, G.M.; Padgett, M.J.; Cronin, L. Development of a 3d printer using scanning projection stereolithography. Sci. Rep. 2015, 5, 9875. [Google Scholar] [CrossRef] [PubMed]
- Corcione, C.E.; Greco, A.; Maffezzoli, A. Temperature evolution during stereolithography building with a commercial epoxy resin. Polym. Eng. Sci. 2006, 46, 493–502. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Greco, A.; Maffezzoli, A. Photopolymerization kinetics of an epoxy-based resin for stereolithography. J. Appl. Polym. Sci. 2004, 92, 3484–3491. [Google Scholar] [CrossRef]
- Corcione, C.E. Development and characterization of novel photopolymerizable formulations for stereolithography. J. Polym. Eng. 2014, 34, 85–93. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Vat photopolymerization processes. In Additive Manufacturing Technologies: 3d Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: New York, NY, USA, 2015; pp. 63–106. [Google Scholar]
- Czyżewski, J.; Burzyński, P.; Gaweł, K.; Meisner, J. Rapid prototyping of electrically conductive components using 3d printing technology. J. Mater. Process. Technol. 2009, 209, 5281–5285. [Google Scholar] [CrossRef]
- Leigh, S.J.; Purssell, C.P.; Bowen, J.; Hutchins, D.A.; Covington, J.A.; Billson, D.R. A miniature flow sensor fabricated by micro-stereolithography employing a magnetite/acrylic nanocomposite resin. Sens. Actuators A Phys. 2011, 168, 66–71. [Google Scholar] [CrossRef]
- Yugang, D.; Yuan, Z.; Yiping, T.; Dichen, L. Nano-tio 2 -modified photosensitive resin for rp. Rap. Prototyp. J. 2011, 17, 247–252. [Google Scholar] [CrossRef]
- Kalsoom, U.; Peristyy, A.; Nesterenko, P.N.; Paull, B. A 3d printable diamond polymer composite: A novel material for fabrication of low cost thermally conducting devices. RSC Adv. 2016, 6, 38140–38147. [Google Scholar] [CrossRef]
- Licciulli, A.; Corcione, C.E.; Greco, A.; Amicarelli, V.; Maffezzoli, A. Laser stereolithography of ZrO2 toughened Al2O3. J. Eur. Ceram. Soc. 2004, 24, 3769–3777. [Google Scholar] [CrossRef]
- Scalera, F.; Esposito Corcione, C.; Montagna, F.; Sannino, A.; Maffezzoli, A. Development and characterization of uv curable epoxy/hydroxyapatite suspensions for stereolithography applied to bone tissue engineering. Ceram. Int. 2014, 40, 15455–15462. [Google Scholar] [CrossRef]
- Gmeiner, R.; Mitteramskogler, G.; Stampfl, J.; Boccaccini, A.R. Stereolithographic ceramic manufacturing of high strength bioactive glass. Int. J. Appl. Ceram. Technol. 2015, 12, 38–45. [Google Scholar] [CrossRef]
- Fantino, E.; Chiappone, A.; Roppolo, I.; Manfredi, D.; Bongiovanni, R.; Pirri, C.F.; Calignano, F. 3d printing of conductive complex structures with in situ generation of silver nanoparticles. Adv. Mater. 2016, 28, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Roppolo, I.; Chiappone, A.; Bocchini, S.; Perrone, D.; Chiolerio, A. Silver nanoparticle ink technology: State of the art. Nanotechnol. Sci. Appl. 2016, 9, 1–13. [Google Scholar] [PubMed]
- Roppolo, I.; Doriguzzi Bozzo, A.; Castellino, M.; Chiappone, A.; Perrone, D.; Bejtka, K.; Bocchini, S.; Sangermano, M.; Chiolerio, A. Dual step irradiation process for in situ generation and patterning of silver nanoparticles in a photocured film. RSC Adv. 2016, 6, 14832–14843. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C. Silver as an example of the applications of photochemistry to the synthesis and uses of nanomaterials. Photochem. Photobiol. 2012, 88, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.-I.; Bingham, J.M.; Ringe, E.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Correlated structure and optical property studies of plasmonic nanoparticles. J. Phys. Chem. C 2011, 115, 9291–9305. [Google Scholar] [CrossRef]
- Krishnan, K.; Tsuruoka, T.; Mannequin, C.; Aono, M. Mechanism for conducting filament growth in self-assembled polymer thin films for redox-based atomic switches. Adv. Mater. 2016, 28, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Chiolerio, A.; Roppolo, I.; Sangermano, M. Radical diffusion engineering: Tailored nanocomposite materials for piezoresistive inkjet printed strain measurement. RSC Adv. 2013, 3, 3446–3452. [Google Scholar] [CrossRef]








| Treatment | Tg VALUE (°C) | ||
|---|---|---|---|
| No TT | −28 | ||
| TT_Air | @100 °C | @150 °C | @200 °C |
| 10′ | −25 | −25 | −25 |
| 30′ | −25 | −26 | −38 |
| 60′ | −26 | −30 | −37 |
| TT_Vacuum | - | @150 °C | @200 °C |
| 30′ | - | −26 | −26 |
| 60′ | −27 | −28 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantino, E.; Chiappone, A.; Calignano, F.; Fontana, M.; Pirri, F.; Roppolo, I. In Situ Thermal Generation of Silver Nanoparticles in 3D Printed Polymeric Structures. Materials 2016, 9, 589. https://doi.org/10.3390/ma9070589
Fantino E, Chiappone A, Calignano F, Fontana M, Pirri F, Roppolo I. In Situ Thermal Generation of Silver Nanoparticles in 3D Printed Polymeric Structures. Materials. 2016; 9(7):589. https://doi.org/10.3390/ma9070589
Chicago/Turabian StyleFantino, Erika, Annalisa Chiappone, Flaviana Calignano, Marco Fontana, Fabrizio Pirri, and Ignazio Roppolo. 2016. "In Situ Thermal Generation of Silver Nanoparticles in 3D Printed Polymeric Structures" Materials 9, no. 7: 589. https://doi.org/10.3390/ma9070589