Enhancement of TiO2 NPs Activity by Fe3O4 Nano-Seeds for Removal of Organic Pollutants in Water
Abstract
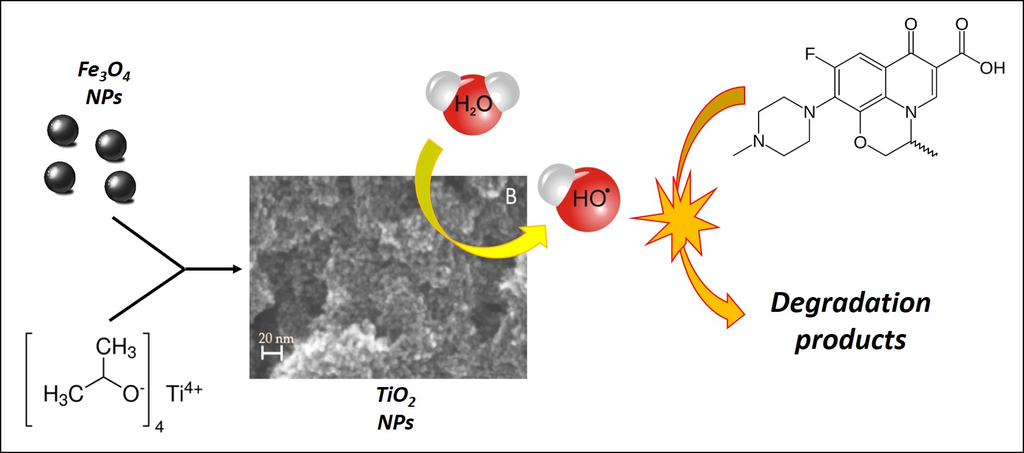
:1. Introduction
2. Results
2.1. Structural and Morphological Investigations
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Magnetite NPs
3.3. Preparation of Magnetic Photocatalysts
3.4. Characterization Techniques
3.4.1. XRD
3.4.2. FE-SEM
3.4.3. DSC
3.4.4. BET
3.5. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Kapilashrami, K.; Zhang, Y.; Liu, Y.S.; Hagfeldt, A.; Guo, J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y.; Luo, Z.; Rabenberg, L.; Heller, A. Photo-oxidative self-cleaning transparent titanium dioxide films on glass. J. Mater. Res. 1995, 10, 2842–2848. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, D.; Cho, D.L.; Lim, S.H.; Yoo, S.Y.; Kook, J.K.; Cho, Y.I.; Ohk, S.H.; Ko, Y.M. Sterilization effects of TiO2 photocatalytic film against a Streptococcus Mutans culture. Biotechnol. Bioprocess Eng. 2007, 12, 136–139. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Maraschi, F.; Dondi, D.; Fisogni, G.; Annovazzi, E.; Profumo, A.; Buttafava, A. Evaluation of UV-A and solar light photocatalytic hydrogen gas evolution from olive mill wastewater. Int. J. Hydrogen Energy 2015, 40, 4303–4310. [Google Scholar] [CrossRef]
- Liu, S.Q. Magnetic semiconductor nano-photocatalysts for the degradation of organic pollutants. Environ. Chem. Lett. 2012, 10, 209–216. [Google Scholar] [CrossRef]
- El Hajjouji, H.; Barje, F.; Pinelli, E.; Bailly, J.R.; Richard, C.; Winterton, P.; Revel, J.C.; Hafidi, M. Photochemical UV/TiO2 treatment of olive mill wastewater (OMW). Bioresour. Technol. 2008, 99, 7264–7269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.; Fasani, E.; Albini, A. Photolytic and photocatalytic degradation of fluoroquinolones in surface water under solar light. Appl. Catal. B Environ. 2012, 119–120, 32–39. [Google Scholar] [CrossRef]
- Maraschi, F.; Sturini, M.; Speltini, A.; Pretali, L.; Profumo, A.; Pastorello, A.; Kumar, V.; Ferretti, M.; Caratto, V. TiO2-modified zeolites for fluoroquinolones removal from wastewaters and reuse after solar light regeneration. J. Environ. Chem. Eng. 2014, 2, 2170–2176. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanism and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Caratto, V.; Locardi, F.; Alberti, S.; Villa, S.; Sanguineti, E.; Martinelli, A.; Balbi, T.; Canesi, L.; Ferretti, M. Different sol-gel prepartion of iron-doped TiO2 nanoparticles: Characterization, photocatalytic and cytotoxicity. J. Sol-Gel Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Locardi, F.; Sanguineti, E.; Fasoli, M.; Martini, M.; Costa, G.A.; Ferretti, M.; Caratto, V. Photocatalytic activity of TiO2 nanopowders supported on a new persistent luminescence phosphor. Catal. Commun. 2016, 74, 24–27. [Google Scholar] [CrossRef]
- Riani, P.; Napoletano, M.; Canepa, F. Synthesis, characterization and a.c. magnetic analysis of magnetite nanoparticles. J. Nanopart. Res. 2011, 13, 7013–7020. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.C.; McEvoy, S. Novel photocatalyst: Titania-coated magnetite. Activity and photodissolution. J. Phys. Chem. B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Anandan, S.; Lee, G.J.; Hsieh, S.H.; Ashokkumar, M.; Wu, J.J. Amorphous titania-coated magnetite spherical nanoparticles: Sonochemical synthesis and catalytic degradation of nonylphenol ethoxylate. Ind. Eng. Chem. Res. 2011, 50, 7874–7881. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Guo, M.; Wang, X. Preparation and properties of nano TiO2/Fe3O4 composite superparamagnetic photocatalyst. Rare Met. 2009, 28, 423–427. [Google Scholar] [CrossRef]
- Suciu, R.C.; Rosu, M.C.; Silipas, T.D.; Indrea, E.; Popescu, V.; Popescu, G.L. Fe2O3-TiO2 thin films prepared by sol-gel method. Environ. Eng. Manag. J. 2011, 10, 187–192. [Google Scholar]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 6, 533. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Wang, X.; Yang, J.L.; Jiang, X.C.; Ryan, R.; Yu, A.B. Synthesis and surface modification of magnetic nanoparticles for potential applications in sarcomas. J. Nanopart. Res. 2015, 17, 257. [Google Scholar] [CrossRef]
- Stefan, M.; Pana, O.; Leostean, C.; Bele, C.; Silipas, D.; Senila, M.; Gautron, E. Synthesis and characterization of Fe3O4-TiO2 core-shell nanoparticles. J. Appl. Phys. 2014, 116, 114312. [Google Scholar] [CrossRef]
- Alvarez, P.M.; Jaramillo, J.; Lopez-Pinero, F.; Plucinski, P.K. Preparation and characterization of magnetic TiO2 nanoparticles and their utilization for the degradation of emerging pollutants in water. Appl. Catal. B Environ. 2010, 100, 338–345. [Google Scholar] [CrossRef]
- Ma, J.Q.; Guo, S.B.; Guo, X.H.; Ge, H.G. Liquid-phase deposition of TiO2 nanoparticles on core-shell Fe3O4@SiO2 spheres: Preparation, characterization, and photocatalytic activity. J. Nanopart. Res. 2015, 17, 307. [Google Scholar] [CrossRef]
- Pang, S.C.; Kho, S.Y.; Chin, S.F. Fabrication of magnetite/silica/titania core-shell nanoparticles. J. Nanomater. 2012, 2012, 125. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Kang, L.; Wang, X.; Gao, R. Magnetic (γ-Fe2O3@SiO2)n@TiO2 functional hybrid nanoparticles with actived photocatalytic ability. J. Phys. Chem. C 2009, 113, 4008–4011. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic titanium dioxide based nanomaterials: Synthesis, characteristics, and photocatalytic applicaton in pollutant degradation. J. Mater. Chem. A 2015, 3, 17511–17524. [Google Scholar] [CrossRef]
- ISO 10678:2010(en). Available online: https://www.iso.org/obp/ui/#iso:std:iso:10678:ed-1:v1:en (accessed on 29 August 2016).
- Caratto, V.; Setti, L.; Campodonico, S.; Carnasciali, M.M.; Botter, R.; Ferretti, M. Synthesis and characterization of nitrogen-doped TiO2 nanoparticles prepared by sol-gel method. J. Sol-Gel Sci. Technol. 2012, 63, 16–22. [Google Scholar] [CrossRef]
- Tian, G.; Fu, H.; Jing, L.; Xin, B.; Pan, K. Preparation and characterization of stable biophase TiO2 photocatalyst with high crystallinity, large surface area, and enhanced photoactivity. J. Phys. Chem. C 2008, 112, 3083–3089. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of enviromental micorpollutants. Sci. Total Environ. 2014, 500–501, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, J.; Tobin, C.M.; White, L.O.; MacGowan, A.P.; Hedges, A.J. Ofloxacin photodegradation products possess antimicrobial activity. Drugs 1999, 58, 171–172. [Google Scholar] [CrossRef]
- Caratto, V.; Aliakbarian, B.; Casazza, A.A.; Setti, L.; Bernini, C.; Perego, P.; Ferretti, M. Inactivation of Escherichia coli on anatase and rutile nanoparticles using UV and fluorescent light. Mater. Res. Bull. 2013, 48, 2095–2101. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, M.F.; Cerrato, G.; Morandi, S.; Gatto, S.; Oldani, V.; Di Michele, A.; Bianchi, C.L. Photoactive TiO2-montmorillonite composite for degradation of organic dyes in water. J. Photochem. Photobiol. A 2014, 295, 57–63. [Google Scholar] [CrossRef]





| Sample | BET Surface Area (m2/g) |
|---|---|
| A | 174.83 |
| B | 286.73 |
| C | 302.39 |
| D | 341.86 |
| Sample | Fe3O4 NPs (mL) | TISOP (mL) | 2-Propanol (mL) | H2O (mL) |
|---|---|---|---|---|
| A | 0.71 | 6 | 200 | 30 |
| B | 0.35 | 6 | 200 | 30 |
| C | 0.18 | 6 | 200 | 30 |
| D | 0 | 6 | 200 | 30 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, S.; Caratto, V.; Locardi, F.; Alberti, S.; Sturini, M.; Speltini, A.; Maraschi, F.; Canepa, F.; Ferretti, M. Enhancement of TiO2 NPs Activity by Fe3O4 Nano-Seeds for Removal of Organic Pollutants in Water. Materials 2016, 9, 771. https://doi.org/10.3390/ma9090771
Villa S, Caratto V, Locardi F, Alberti S, Sturini M, Speltini A, Maraschi F, Canepa F, Ferretti M. Enhancement of TiO2 NPs Activity by Fe3O4 Nano-Seeds for Removal of Organic Pollutants in Water. Materials. 2016; 9(9):771. https://doi.org/10.3390/ma9090771
Chicago/Turabian StyleVilla, Silvia, Valentina Caratto, Federico Locardi, Stefano Alberti, Michela Sturini, Andrea Speltini, Federica Maraschi, Fabio Canepa, and Maurizio Ferretti. 2016. "Enhancement of TiO2 NPs Activity by Fe3O4 Nano-Seeds for Removal of Organic Pollutants in Water" Materials 9, no. 9: 771. https://doi.org/10.3390/ma9090771