Influence of Climate on the Growth of Hybrid Poplar in Michigan
Abstract
:1. Introduction
2. Experimental Methods
2.1. Study Area and Field Sampling

| County | County Abb. | Lat. (°N) | Long. (°W) | Climate Station Name | Station No. | TAVG (ºC) | PPT (mm) |
|---|---|---|---|---|---|---|---|
| Benzie | BEN | 44.63 | 86.25 | FRANKFORT 2 NE | MI2984 | 7.5 | 892 |
| Branch | BRA | 41.92 | 85.05 | COLDWATER STATE SCHOOL | MI1675 | 8.8 | 912 |
| Calhoun | CAL | 42.25 | 85.00 | BATTLE CREEK 5 NW | MI0552 | 9.1 | 899 |
| Chippewa | CHI | 46.32 | 84.52 | DE TOUR VILLAGE | MI2094 | 5.6 | 749 |
| Clare | CLA | 43.99 | 84.84 | GLADWIN | MI3170 | 7.4 | 807 |
| Gladwin | GLA | 43.99 | 84.39 | GLADWIN | MI3170 | 7.4 | 807 |
| Ingham | ING | 42.60 | 84.37 | EAST LANSING 4 S | MI2395 | 8.3 | 785 |
| Iosco | IOS | 44.28 | 83.34 | EAST TAWAS | MI2423 | 7.1 | 789 |
| Iron | IRO | 46.21 | 88.51 | STAMBAUGH 2 SSE | MI7812 | 3.8 | 775 |
| Kalkaska | KAL | 44.69 | 85.08 | TRAVERSE CITY FAA AP | MI8251 | 7.3 | 858 |
| Lake | LAK | 43.99 | 85.81 | BALDWIN | MI0446 | 7.0 | 869 |
| Marquette | MAR | 46.66 | 87.60 | MARQUETTE | MI5178 | 6.0 | 763 |
| Montcalm | MON | 43.31 | 85.15 | GREENVILLE 2 NNE | MI3429 | 8.3 | 882 |
| Oakland | OAK | 42.66 | 83.38 | PONTIAC STATE HOSPITAL | MI6658 | 9.2 | 772 |
| Ogemaw | OGE | 44.33 | 84.13 | LUPTON 1 S | MI4967 | 5.9 | 1,351 |
| Roscommon | ROS | 44.33 | 84.61 | HOUGHTON LAKE WSO AP | MI3936 | 6.4 | 726 |
| Saginaw | SAG | 43.33 | 84.05 | SAGINAW FAA AP | MI7227 | 8.3 | 804 |
| Van Buren | VAN | 42.27 | 86.31 | BLOOMINGDALE | MI0864 | 8.5 | 1,009 |
| Wexford | WEX | 44.34 | 85.58 | CADILLAC | MI1176 | 5.9 | 833 |
| Full-Sib Family | Mat. Species | Pat. Species | Genetic Acc. No. | No. Trees (No. Radii) | DBH (cm) | HT (m) | BA (m2) | VOL (m3) | SC | Chronology Start Year |
|---|---|---|---|---|---|---|---|---|---|---|
| CAL_WEX | P.GRA | P.TRE | 56-6-77 | 2 (4) | 35.2 | 22.6 | 0.0983 | 0.893 | 62.2 | 1987 |
| LAK_MAR | P.TRE | P.GRA | 27-38-27 | 2 (4) | 31.1 | 24.6 | 0.0772 | 0.812 | 79.6 | 1983 |
| BRA_CLA | P.TRE | P.GRA | 4-9-10 | 2 (4) | 30.0 | 23.4 | 0.0729 | 0.704 | 81.6 | 1983 |
| GLA_GLA | P.TRE | P.GRA | 14-21-13 | 2 (4) | 29.6 | 22.2 | 0.0689 | 0.642 | 75.0 | 1987 |
| IOS_GLA | P.TRE | P.GRA | 24-33-13 | 4 (8) | 28.5 | 21.2 | 0.0671 | 0.596 | 78.2 | 1984 |
| VAN_IRO | P.GRA | P.TRE | 78-44-34 | 6 (12) | 28.1 | 18.5 | 0.0651 | 0.514 | 68.6 | 1982 |
| SAG_CHI | P.GRA | P.TRE | 76-40-15 | 2 (4) | 28.4 | 21.2 | 0.0631 | 0.558 | 75.0 | 1987 |
| MAR_CLA1 | P.TRE | P.GRA | 32-45-10 | 2 (4) | 27.5 | 19.7 | 0.0591 | 0.488 | 71.8 | 1984 |
| CAL_IRO | P.GRA | P.TRE | 57-6-34 | 4 (8) | 27.1 | 21.0 | 0.0585 | 0.523 | 78.0 | 1984 |
| WEX_BEN | P.GRA | P.TRE | 81-46-8 | 5 (10) | 27.2 | 20.7 | 0.0585 | 0.505 | 76.6 | 1983 |
| MAR_OAK | P.TRE | P.GRA | 33-45-32 | 4 (8) | 26.4 | 17.1 | 0.0567 | 0.434 | 64.6 | 1986 |
| CHI_KAL | P.TRE | P.GRA | 8-14-21 | 2 (4) | 25.8 | 20.0 | 0.0533 | 0.432 | 80.3 | 1986 |
| ROS_OAK | P.TRE | P.GRA | 44-71-32 | 2 (4) | 25.4 | 17.8 | 0.0517 | 0.368 | 72.8 | 1984 |
| MON_VAN | P.GRA | P.TRE | 70-31-73 | 3 (6) | 22.4 | 16.8 | 0.0401 | 0.294 | 74.2 | 1987 |
| GLA_CHI | P.TRE | P.GRA | 12-21-8 | 3 (6) | 22.4 | 19.9 | 0.0393 | 0.328 | 89.1 | 1985 |
| OGE_GLA | P.GRA | P.TRE | 73-33-22 | 2 (4) | 20.8 | 15.5 | 0.0375 | 0.273 | 76.9 | 1984 |
| MAR_ING | P.TRE | P.GRA | 28-43-16 | 3 (6) | 19.3 | 16.7 | 0.0299 | 0.218 | 86.6 | 1983 |
| MAR_CLA2 | P.TRE | P.GRA | 29-43-10 | 2 (4) | 19.3 | 17.6 | 0.0294 | 0.217 | 91.5 | 1985 |
| Mean | P.TRE | P.GRA | - | 2.5 (5.1) | 25.9 | 20.0 | 0.0550 | 0.476 | 79.2 | 1985(1987)* |
| Mean | P.GRA | P.TRE | - | 3.4 (6.9) | 27.0 | 19.5 | 0.0602 | 0.508 | 73.1 | 1985(1987)* |
| Mean | All Combined | - | 2.9 (5.8) | 26.3 | 19.8 | 0.0570 | 0.489 | 76.8 | 1985(1987)* | |
2.2. Sample Processing and Dendrochronological Measurements
2.3. Analyses of Full-Sib Families
2.4. Growth-Climate Analyses: Interannual Scale
3. Results
3.1. Full-Sib Family Characteristics
3.2. Relationships between growth and geographical variables and climatic normals
| Geographical Variables | DBH (cm) | Height (m) | Basal Area (m2) | Volume (m3) |
|---|---|---|---|---|
| a) Straight line distance (km) between counties: | ||||
| Maternal and paternal | −0.110 | −0.166 | −0.112 | −0.128 |
| Maternal and Ingham | −0.471* | −0.454 | −0.473* | −0.487* |
| Paternal and Ingham | 0.330 | 0.452 | 0.301 | 0.357 |
| b) Latitudinal distance (km) between counties: | ||||
| Maternal and paternal | 0.155 | 0.049 | 0.136 | 0.105 |
| Maternal and Ingham | −0.202 | −0.074 | −0.226 | −0.218 |
| Paternal and Ingham | 0.199 | 0.313 | 0.169 | 0.179 |
| Climate Normal Variables | DBH (cm) | Height (m) | Basal Area (m2) | Volume (m3) |
|---|---|---|---|---|
| a) Annual TAVG difference between counties: | ||||
| Maternal and paternal | 0.463 | 0.478* | 0.467 | 0.485* |
| Maternal and Ingham | 0.518* | 0.459 | 0.528* | 0.528* |
| Paternal and Ingham | −0.337 | −0.408 | −0.335 | −0.365 |
| b) Annual PPT difference between counties: | ||||
| Maternal and paternal | −0.021 | −0.116 | 0.034 | 0.046 |
| Maternal and Ingham | −0.074 | −0.206 | −0.014 | −0.013 |
| Paternal and Ingham | −0.118 | −0.191 | −0.117 | −0.145 |
| c) Growing season TAVG difference between counties: | ||||
| Maternal and paternal | 0.472* | 0.501* | 0.476* | 0.501* |
| Maternal and Ingham | 0.500* | 0.453 | 0.506* | 0.506* |
| Paternal and Ingham | −0.359 | −0.444 | −0.361 | −0.400 |
| d) Growing season PPT difference between counties: | ||||
| Maternal and paternal | 0.404 | 0.487* | 0.422 | 0.500* |
| Maternal and Ingham | 0.431 | 0.364 | 0.455 | 0.470* |
| Paternal and Ingham | 0.092 | −0.044 | 0.102 | 0.051 |
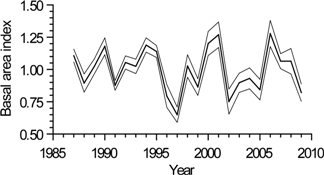
3.3. Interannual Growth Chronology Characteristics


3.4. Interannual Growth-Climate Relationships



4. Discussion
4.1. Geographical and Environmental Significance of Parental Origin
4.2. Interannual Growth-Climate Relationships
5. Conclusions
Acknowledgements
References
- Eriksson, H.M.; Hall, J.P.; Helynen, S. Rationale for forest energy production. In Bioenergy from Sustainable Forestry; Richardson, J., Bjorheden, R., Hakkila, P., Lowe, A.T., Smith, C.T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 1–17. [Google Scholar]
- Ohlrogge, J.; Allen, D.; Berguson, B.; DellaPenna, D.; Shachar-Hill, Y.; Stymne, S. Driving on biomass. Science 2009, 324, 1019–1020. [Google Scholar] [CrossRef]
- Dickmann, D.I. Silviculture and biology of short-rotation woody crops in temperature regions: then and now. Biomass Bioenergy 2006, 30, 696–705. [Google Scholar] [CrossRef]
- Demeritt, M.E. Poplar hybrids (Populus L.). Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 2. Available online: http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/populus/populus.htm (accessed on 1 August 2010).
- Riemenschneider, D.E.; Berguson, W.E.; Dickmann, D.I.; Hall, R.B.; Isebrands, J.G.; Mohn, C.A.; Stanosz, G.C.; Tuskan, G.A. Poplar breeding and testing strategies in the north-central U.S.: Demonstration of potential yield and consideration of future research needs. For. Chron. 2001, 77, 245–253. [Google Scholar] [CrossRef]
- Pliura, A.; Zhang, S.Y.; MacKay, J.; Bousquet, J. Genotypic variation in wood density and growth traits of poplar hybrids at four clonal trials. For. Ecol. Manage. 2007, 238, 92–106. [Google Scholar] [CrossRef]
- White, T.L.; Addams, W.T.; Neale, D.B. Forest Genetics; CABI Publishing, CAB International: Oxfordshire, UK, 2007. [Google Scholar]
- Robison, D.; Raffa, K.F. Productivity, drought tolerance and pest status of hybrid Populus: tree improvement and silvicultural implications. Biomass Bioenergy 1998, 14, 1–20. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Hall, R.B.; Zalesny, J.A.; McMahon, B.G.; Berguson, W.E.; Stonosz, G.R. Biomass and genotype × environment interactions of Poplulus energy crops in the midwestern United States. Bioenergy Res. 2009, 2, 106–1222. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Hall, R.B.; Bauer, E.O.; Riemenschneider, D.E. Soil temperature and precipitation affect the rooting ability of dormant hardwood cuttings of Populus. Silvae Genet. 2005, 54, 47–58. [Google Scholar]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.-M.; Barbaroux, C.; Le Thiec, D.; Brechet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
- Giovannelli, A.; Deslauriers, A.; Fragnelli, G.; Scaletti, L.; Castro, G.; Rossi, S.; Crivellaro, A. Evaluation of drought response of two poplar clones through high resolution analysis of stem growth. J. Exp. Bot. 2007, 58, 2673–2683. [Google Scholar] [CrossRef]
- Monclus, R.; Villar, M.; Barbaroux, C.; Bastien, C.; Fichot, R.; Delmotte, F.M.; Delay, D.; Petit, J.-M.; Brechet, C.; Dreyer, E. Productivity, water-use efficiency and tolerance to moderate water deficit correlate in 33 poplar genotypes from a Populus deltoides × Populus trichocarpa F1 progeny. Tree Physiol. 2009, 29, 1329–1339. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Ecological Studies 183; Springer-Verlag: Berlin, Germany, 2006. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Burton, P.J.; Cumming, S.G. Potential effects of climatic change on some western Canadian forests, based on phenological enhancements to a patch model of forest succession. Water Air Soil Pollut. 1995, 82, 401–414. [Google Scholar] [CrossRef]
- Loehle, C.; LeBlanc, D. Model-based assessments of climate change effects on forests: a critical review. Ecol. Modell. 1996, 90, 1–31. [Google Scholar] [CrossRef]
- Chhin, S.; Hogg, E.H.; Lieffers, V.J.; Huang, S. Potential effects of climate change on the growth of lodgepole pine across diameter size classes and ecological regions. For. Ecol. Manage. 2008, 256, 1692–1703. [Google Scholar] [CrossRef]
- Chhin, S.; Hogg, E.H.; Lieffers, V.J.; Huang, S. 2010. Growth-climate relationships vary with height along the stem in lodgepole pine. Tree Physiol. 2010, 30, 335–345. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Michaelian, M. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Can. J. For. Res. 2008, 38, 1373–1384. [Google Scholar] [CrossRef]
- Leonelli, G.; Denneler, B.; Bergeron, Y. Climate sensitivity of trembling aspen radial growth along a productivity gradient in northeastern British Columbia, Canada. Can. J. For. Res. 2008, 38, 1211–1222. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Change Biol. 2009, 16, 711–731. [Google Scholar] [CrossRef]
- Vance, E.D.; Maguire, D.A.; Zalesny, R.S., Jr. Research strategies for increasing productivity of intensively managed forest plantations. J. For. 2010, 4, 183–192. [Google Scholar]
- Laidly, P.R. Bigtooth aspen (Populus grandidentata Michx.). Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 2. Available online: http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/populus/grandidentata.htm (accessed on 1 August 2010).
- Perala, D.A. Quaking aspen (Populus tremuloides Michx.). Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 2. Available online: http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/populus/tremuloides.htm (accessed on 1 August 2010).
- Reighard, G.L. Physiological Genetics Studies of Populus grandidentata, Populus tremuloides, and their hybrid, Populus × smithii. Ph.D. Dissertation, Michigan State University, East Lansing, MI, USA, 1984. [Google Scholar]
- Reighard, G.L.; Hanover, J.W. Progeny testing of native aspens and their hybrids for biomass production in Michigan. In Proceedings of the 29th Northeastern Forest Tree Improvement Conference, Morgantown, WV, 1985; Demeritt, M.D., Jr., Ed.; West Virginia University: Morgantown, WV, 1985; pp. 5–22. [Google Scholar]
- Monthly Surface Data; National Climatic Data Center: Asheville, NC, USA, 2010. Available online: http://cdo.ncdc.noaa.gov/pls/plclimprod/somdmain.somdwrapper?datasetabbv=DS3220&countryabbv=&georegionabbv=&forceoutside=.
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; The University of Arizona Press: Tuscon, AZ, USA, 1996. [Google Scholar]
- Yamaguchi, D.K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 21, 414–416. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Lieffers, V.J. Using dendrochronology to obtain annual data for modelling stand development: a supplement to permanent sample plots. Forestry 2009, 82, 163–173. [Google Scholar] [CrossRef]
- Gevorkiantz, S.R.; Olsen, L.P. Composite Volume Tables for Timber and Their Application in the Lake States; USDA For. Serv. Tech. Bull. 1104; USDA Forest Service: Washington, DC, USA, 1955. [Google Scholar]
- Wang, Y.; Titus, S.J.; LeMay, V.M. Relationships between tree slenderness coefficients and tree or stand characteristics for major species in boreal mixedwood forests. Can. J. For. Res. 1998, 28, 1171–1183. [Google Scholar] [CrossRef]
- Hogg, E.H. Temporal scaling of moisture and the forest-grassland boundary in western Canada. Agr. Forest Meteorol. 1997, 84, 115–122. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Frontier, S. Étude de la décroissance des valeurs propres dans une analyse en composantes principales: comparaison avec le modèle du baton brisé. J. Exp. Mar. Biol. Ecol. 1976, 25, 67–75. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Developments in Environmental Modeling 20; Elsevier Science B.V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Roach, D.A.; Wulff, R.D. Maternal effects in plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Rossiter, M.C. Incidence and consequences of inherited environmental effects. Annu. Rev. Ecol. Syst. 1996, 27, 451–476. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Fox, C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef]
- Andersson, B. Aftereffects of maternal environment on autumn frost hardiness in Pinus sylvestris seedlings in relation to cultivation techniques. Tree Physiol. 1994, 14, 313–322. [Google Scholar] [CrossRef]
- Wei, R.-P.; Lindgren, K.; Lindgren, D. Parental environmental effects on cold acclimation and height growth in lodgepole pine seedlings. Silvae Genet. 2001, 50, 252–257. [Google Scholar]
- Chmura, D.J. Phenology differs among Norway spruce populations in relation to local variation in altitude of maternal stands in the Beskidy Mountains. New For. 2006, 32, 21–31. [Google Scholar] [CrossRef]
- Reighard, G.L.; Hanover, J.W. Shoot and root development and dry matter partitioning in Populus grandidentata, Populus tremuloides, and P. × smithii. Can. J. For. Res. 1990, 20, 849–852. [Google Scholar] [CrossRef]
- Nguyen, P.U.; Dickmann, D.I.; Pregitzer, K.S.; Hendrick, R. Late-season changes in allocation of starch and sugar to shoots, coarse roots, and fine roots in two hybrid poplar clones. Tree Physiol. 1990, 7, 95–105. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Dickmann, D.I.; Isebrands, J.G.; Blake, T.J.; Kosola, K.; Kort, J. Physiological ecology of poplars. In Poplar Culture in North America; Dickmann, D.I., Isebrands, J.G., Eckenwalder, J.E., Richardson, J., Eds.; NRC Research Press: Ottawa, Canada, 2001; Part A, Chapter 3; pp. 77–118. [Google Scholar]
- Landhausser, S.M.; Lieffers, V.J. Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees 2003, 17, 471–476. [Google Scholar] [CrossRef]
- Telewski, F.W.; Aloni, R.; Sauter, J.J. Physiology of secondary tissues of Populus. In Biology of Populus and Its Implications for Management and Conservation; Stettler, R.F., Bradshaw, H.D., Jr., Heilman, P.E., Hinckley, T.M., Eds.; NRC Research Press, National Research Council of Canada: Ottawa, Canada, 1996; Part II, Chapter 13; pp. 459–489. [Google Scholar]
- Van Vokenburgh, E.; Taylor, G. 1996. Leaf growth physiology. In Biology of Populus and Its Implications for Management and Conservation; Stettler, R.F., Bradshaw, H.D., Jr., Heilman, P.E., Hinckley, T.M., Eds.; NRC Research Press, National Research Council of Canada: Ottawa, Canada, 1996; Part II, Chapter 18; pp. 459–489. [Google Scholar]
- Blake, T.J.; Sperry, J.; Tschaplinski, T.J.; Wang, S.S. Water relations. In Biology of Populus and Its Implications for Management and Conservation; Stettler, R.F., Bradshaw, H.D., Jr., Heilman, P.E., Hinckley, T.M., Eds.; NRC Research Press, National Research Council of Canada: Ottawa, Canada, 1996; Part II, Chapter 16; pp. 401–422. [Google Scholar]
- DeBell, D.S.; Harrington, C.A.; Clendenen, G.W.; Zasada, J.C. Tree growth and stand development of four Populus clones in large monoclonal plots. New For. 1997, 14, 1–18. [Google Scholar] [CrossRef]
- Devine, W.D.; Harrington, C.A.; DeBell, D.S. Intra-annual growth and mortality of four Populus clones in pure and mixed plantings. New For. 2010, 39, 287–299. [Google Scholar] [CrossRef]
- Yu, Q.; Tigerstedt, P.M.A.; Haapanen, M. Growth and phenology of hybrid aspen clones (Populus tremula L. × Populus tremuloides Michx.). Silva Fenn. 2001, 35, 15–25. [Google Scholar]
- Deslauriers, A.; Giovannelli, A.; Rossi, S.; Castro, G.; Fragnelli, G.; Traversi, L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol. 2009, 29, 1223–1235. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: the Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- McCabe, G.J.; Betancourt, J.L.; Gray, S.T.; Palecki, M.A.; Hidalgo, H.G. Associations of multi-decadal sea-surface temperature variability with US drought. Quat. Int. 2008, 188, 31–40. [Google Scholar] [CrossRef]
- Grier, C.C. Foliage loss due to snow, wind, and winter drying damage; its effects on leaf biomass of some western conifer forests. Can. J. For. Res. 1988, 18, 1097–1102. [Google Scholar] [CrossRef]
- Havranek, W.M.; Tranquillini, W. Physiological processes during winter dormancy and their ecological significance. In Ecophysiology of Coniferous Forests; Smith, W.K., Hinckley, T.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 95–124. [Google Scholar]
- Begum, S.; Nakaba, S.; Bayramzadeh, V.; Oribe, Y.; Kubo, T.; Funada, R. Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiol. 2008, 28, 1813–1819. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chhin, S. Influence of Climate on the Growth of Hybrid Poplar in Michigan. Forests 2010, 1, 209-229. https://doi.org/10.3390/f1040209
Chhin S. Influence of Climate on the Growth of Hybrid Poplar in Michigan. Forests. 2010; 1(4):209-229. https://doi.org/10.3390/f1040209
Chicago/Turabian StyleChhin, Sophan. 2010. "Influence of Climate on the Growth of Hybrid Poplar in Michigan" Forests 1, no. 4: 209-229. https://doi.org/10.3390/f1040209