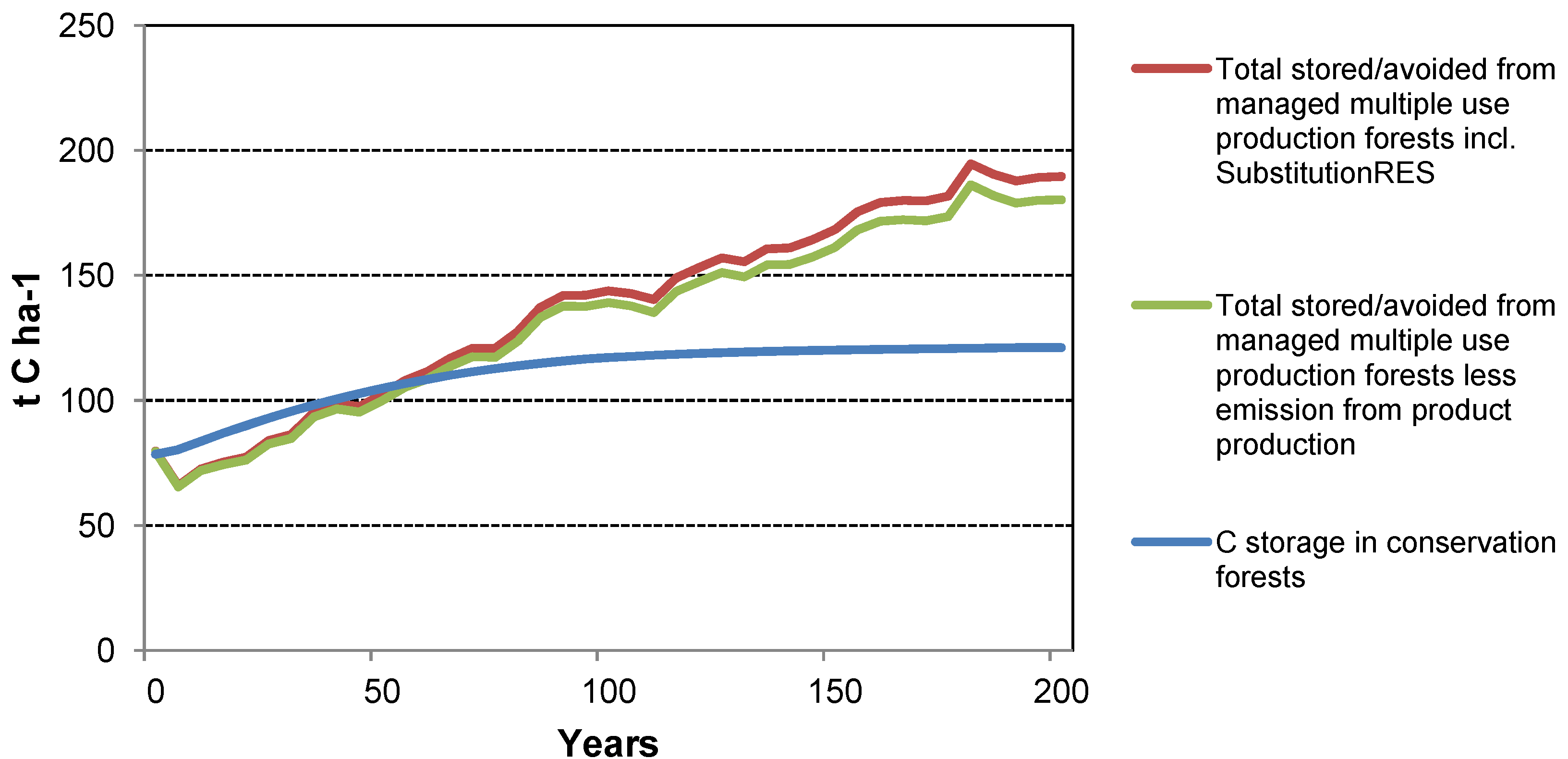1. Introduction
Australia has approximately 4% of the world’s forests, comprising 147.4 million hectares (Mha) of native forest and 2.0 Mha of forestry plantations, covering about 19% of the continent [
1]. Approximately 9.4 Mha of publicly owned native forests are managed for multiple use (including timber production) in Australia [
1]. The National Forest Policy Statement set a course for major change in Australia’s native forest industry, with the objectives of: establishing a comprehensive, adequate and representative forest reserve system; implementing ecologically sustainable forest management practices; and establishing an internationally competitive, value-added industry [
2]. As a consequence in New South Wales (NSW), the area of public native forests managed for multiple use was reduced from 2.6 Mha in 1990 [
3] to 1.3 Mha by 2008 [
4] through conversion to conservation reserves.
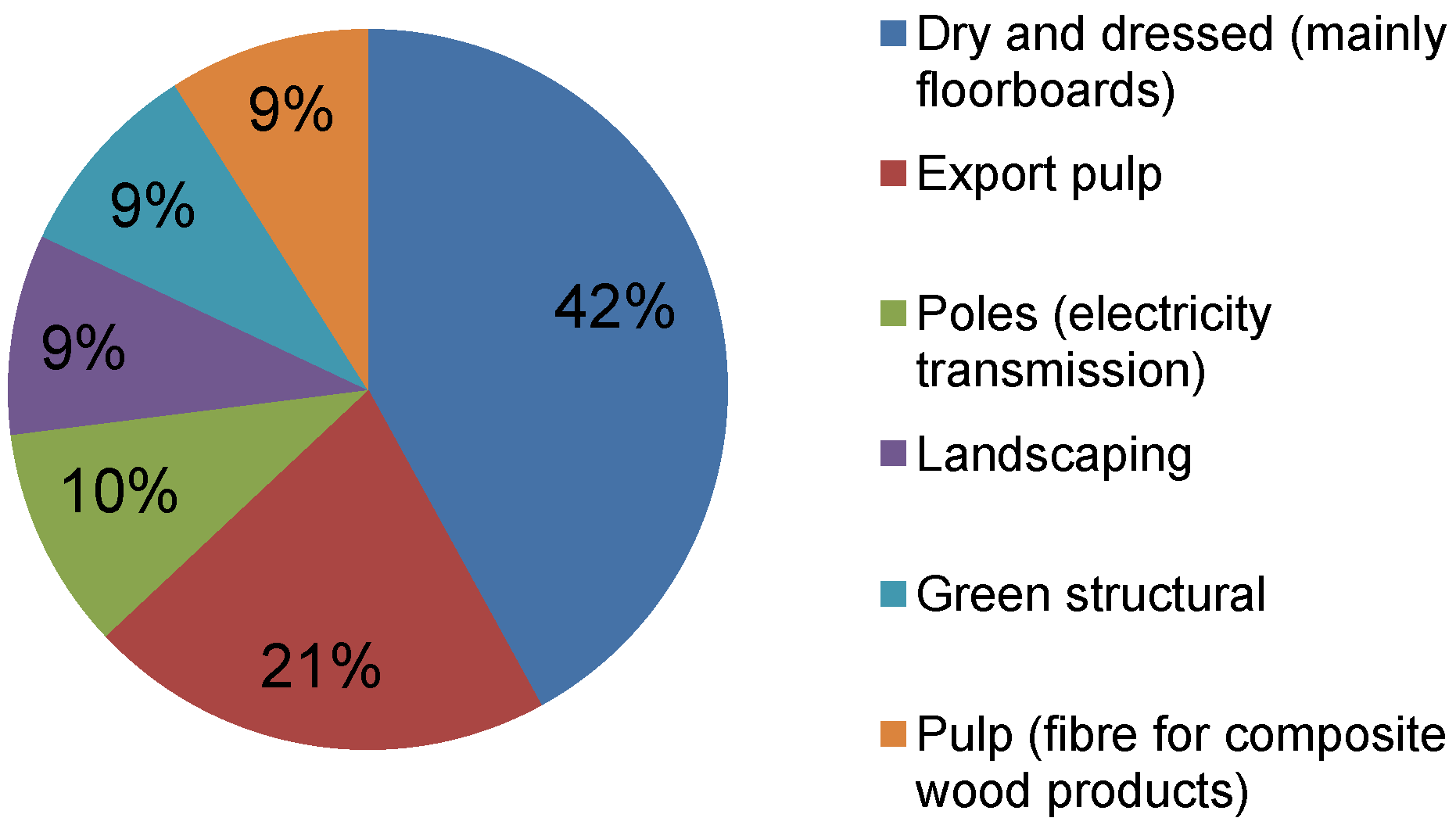
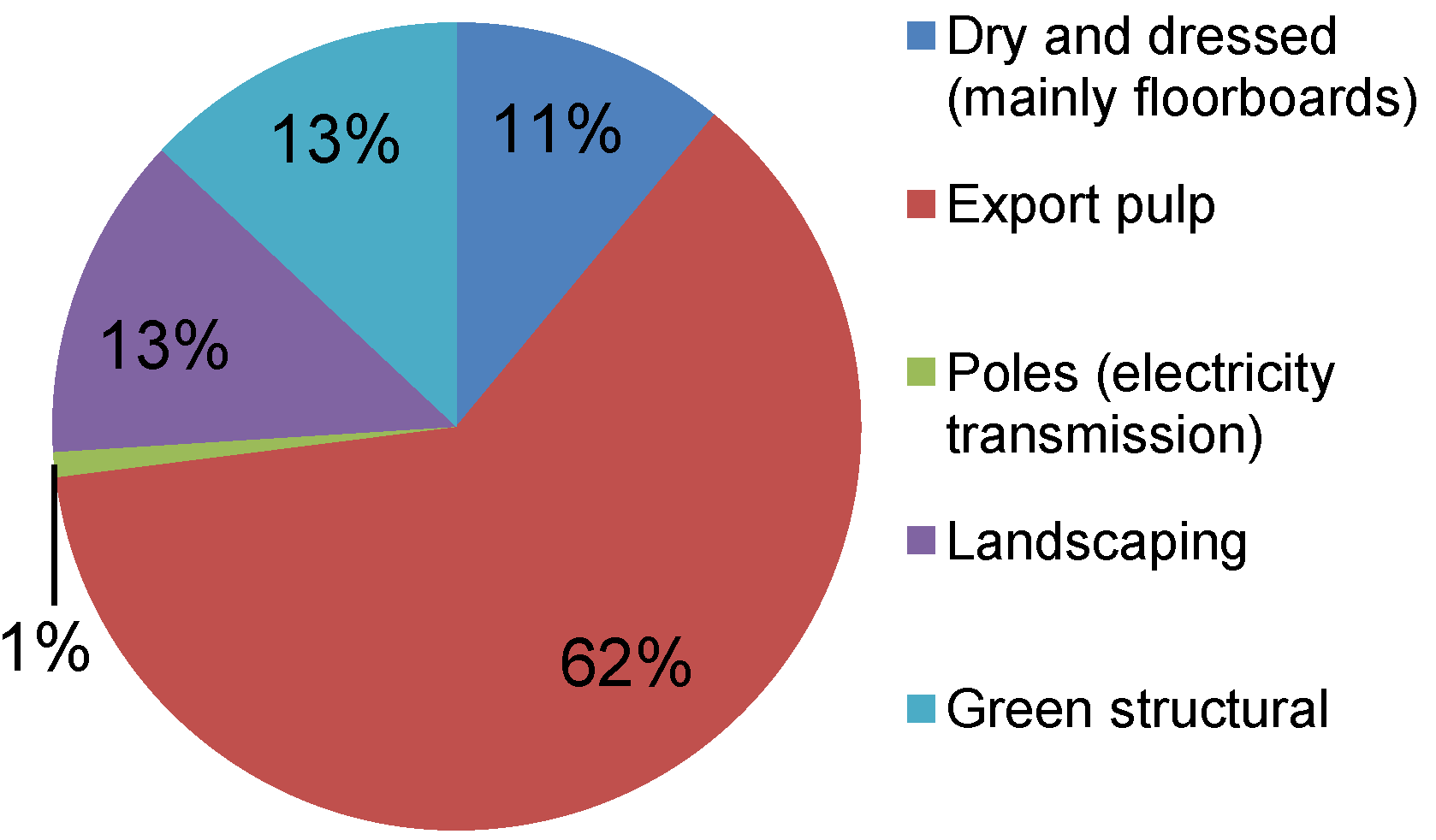
When forests are harvested in Australia the amount of biomass removed for processing into wood products varies between 45% and 65% for different forest types, ages and locations [
5]. The proportion of extracted logs in different product classes varies substantially between tree species. The proportion of highly value-added hardwood products such as floorboards, decking and furniture has increased from 29% in 1995/1996 to 62% in 2008/2009 [
4].
At the end of their service life, the vast majority of harvested wood products (HWPs) in Australia are deposited in landfill. Although some HWPs may be recycled at least once, eventually a high proportion of recycled HWPs will also end up in landfills. The majority of the C in HWPs deposited in landfill remains undecomposed [
6]. Carbon in HWPs in landfill is quantified from estimates of waste composition and volume, and assumed decay rates [
7]. Decomposition of organic materials in landfills results in the generation of greenhouse gas (GHG), mainly carbon dioxide and methane in approximately equal proportions. Emissions typically occur over a period of approximately 30 years after the waste has been deposited. The decomposition factors used are critical to the calculation of GHG emissions from landfills, as methane is a GHG 21–25 times more powerful than carbon dioxide. In the IPCC Guidelines [
7] it is currently assumed that 50% of the C in HWPs in landfill is released as a result of decomposition. However, recent field-based research (e.g., [
6]), and a recently published experimental study in the USA [
8], have demonstrated that HWPs in landfill represent a long term C store, with minimal or no decomposition taking place.
Besides storing C sequestered during forest growth, HWPs can provide additional GHG mitigation benefits through the substitution for other more energy and GHG-intensive materials such as steel, aluminium, plastic and concrete [
9]. Research from around the world has shown that the life-cycle GHG impact of HWPs is significantly lower than that of competing, non-renewable products (e.g., Australia and New Zealand [
10,
11,
12,
13]; Europe [
14,
15]; US [
16,
17]). A meta-analysis of twenty European and North-American studies found an average reduction of two tonnes of C for each tonne of C in HWPs substituted for non-wood products [
14].
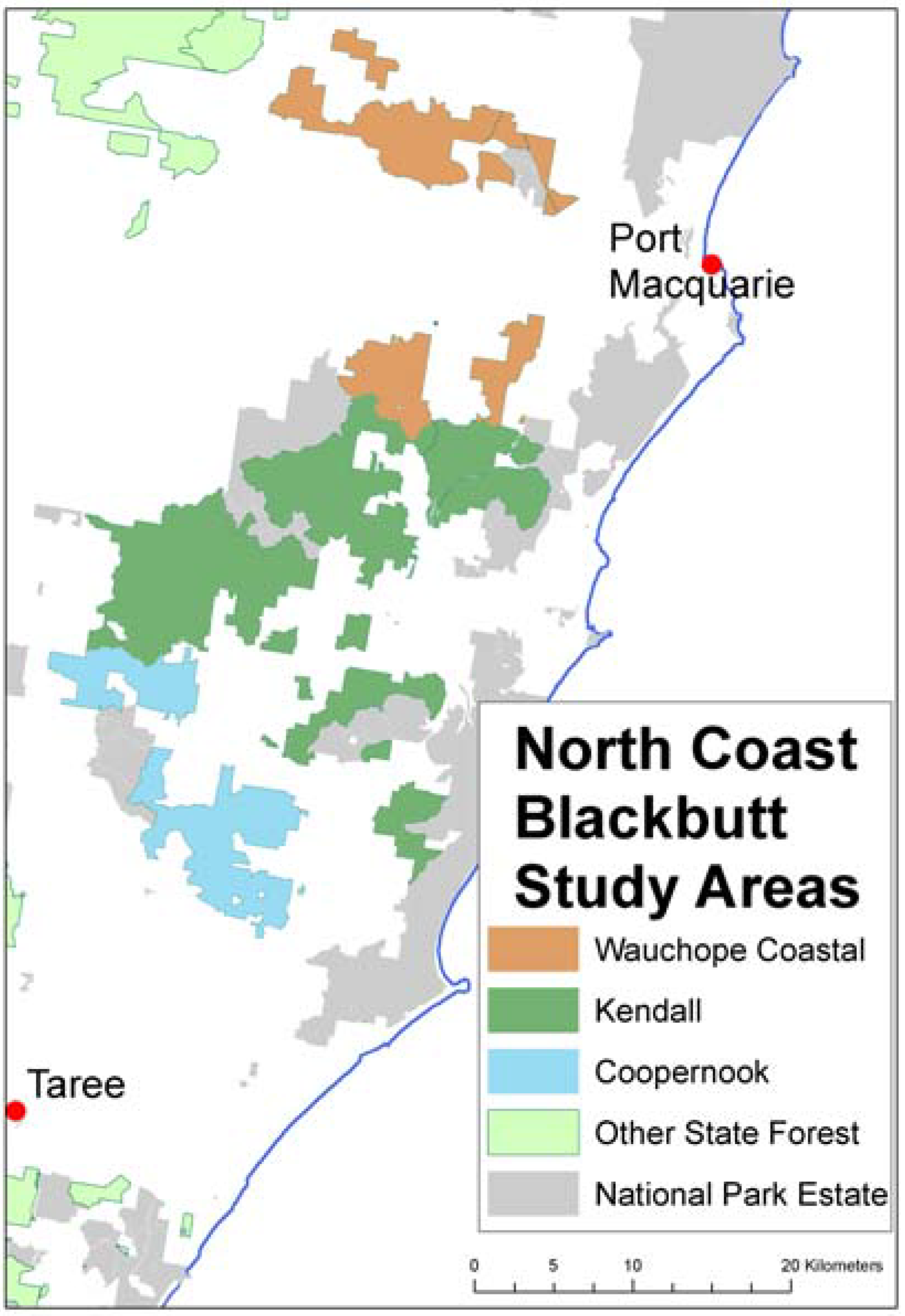
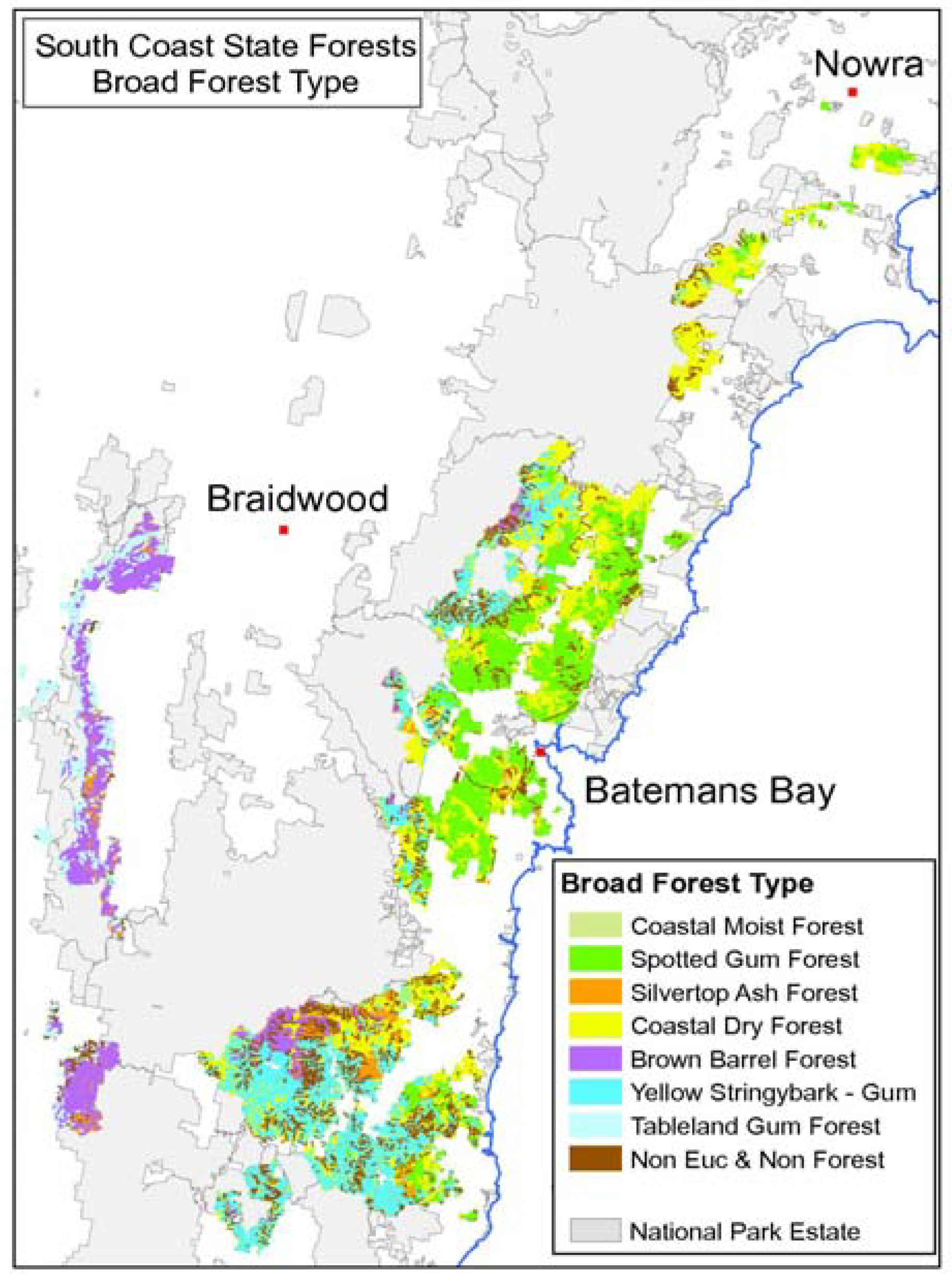
Similar benefits through fossil fuel displacement may be achieved by the use of harvest residues for bioenergy production. Forest residues comprise the bole of the tree remaining after the commercial logs are removed, the crown, bark, limbs, and entire trees that are not marketable due to size, bends and twists or internal defect such as rot. On the north coast of NSW over 600,000 tonnes per annum of sawlogs and other products are sold each year, with the remaining 300,000+ tonnes of forest residue left on the ground. In practice, harvest residues often create a fire risk and usual practice is to burn them post-harvest. This adds operational cost and risk and results in GHG emissions. If not burnt, these residues are assumed to decay over a 10–20 year time frame releasing their C back into the atmosphere [
18]. In Australia it is a requirement by 2020 that 20% of the electricity generation is produced from renewable sources [
19]. This will represent a substantial increase from current levels (8% of the electricity is currently generated from renewable sources [
20]), representing a significant opportunity for increased biomass use.
Greenhouse emissions due to fire events are an important component of the global carbon cycle [
21]. Fire is an intrinsic aspect of the ecology and management of SE Australian forests and woodlands [
21]. Prescribed burning is the principal means of managing fuel levels in Australian forests, with the aim to reduce wildfire risk [
22]. The frequency and severity of wildfire events vary significantly according to the forest type. There is circumstantial evidence that fuel reduction programs have reduced the impact of wildfires on forest land: in NSW wildfires have been low in State Forests, but widespread on other land tenures [
23].
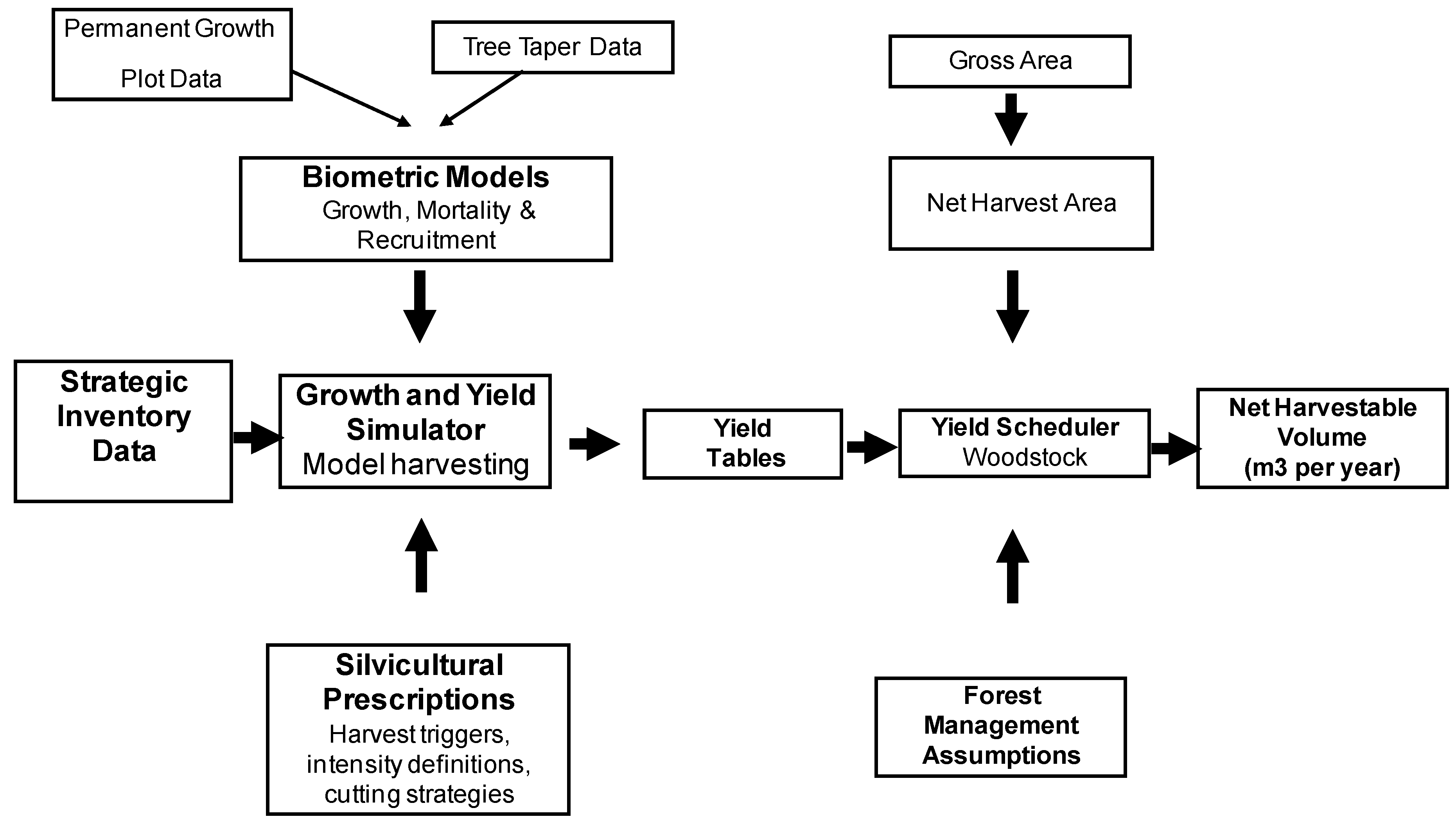
The key objective of this paper is to estimate the impact on net GHG emissions to the atmosphere of converting multiple use production forests into forests managed for conservation purposes only in NSW. In order to understand the full contribution that forests managed for wood products can deliver in reducing GHG emissions, we estimate the impact of different harvest scenarios on carbon levels in forest and wood products over time, for the two case study regions. In addition we assess the potential emissions offset due to product substitution and displacement of fossil energy emissions.
4. Discussion
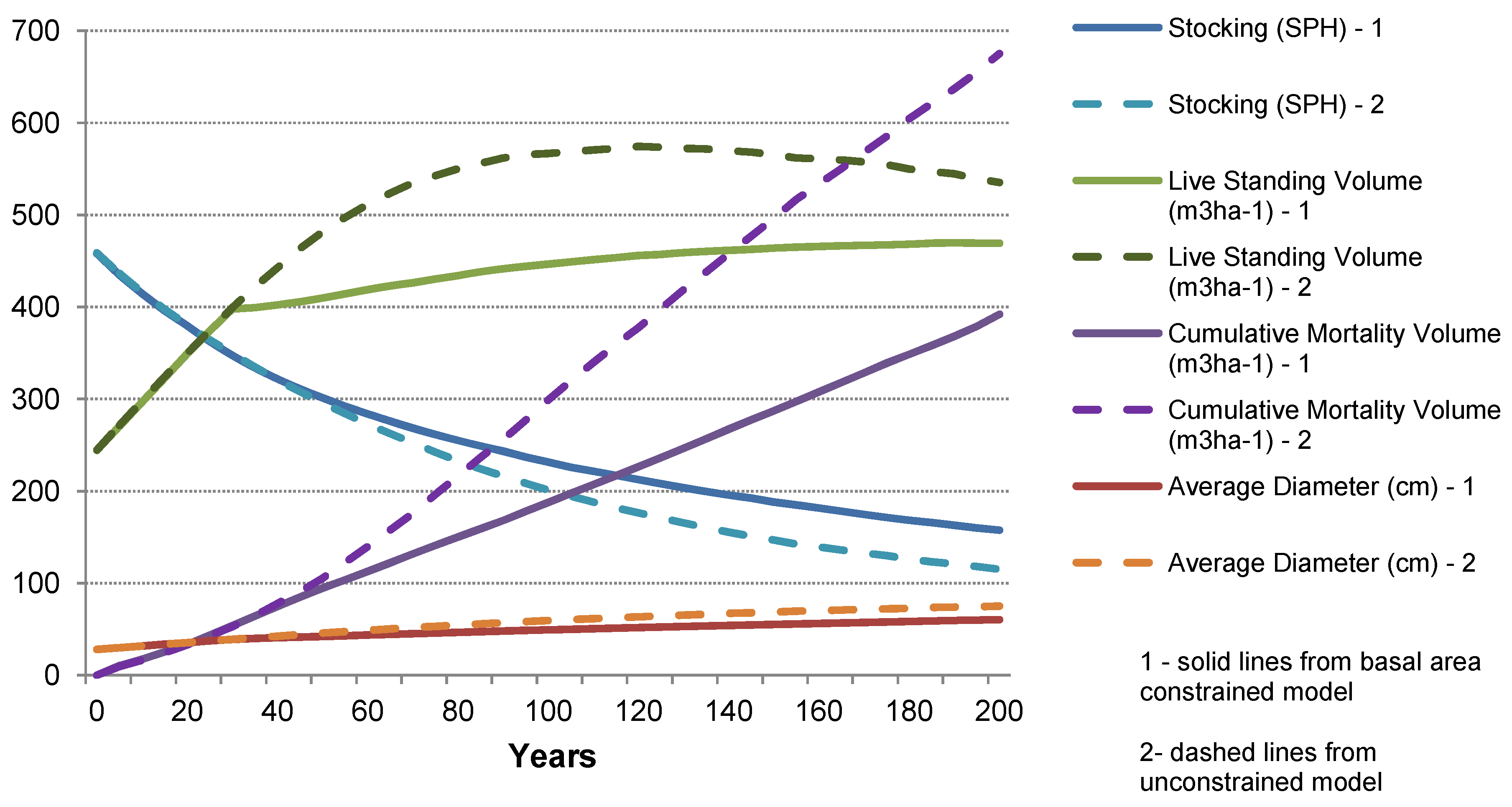
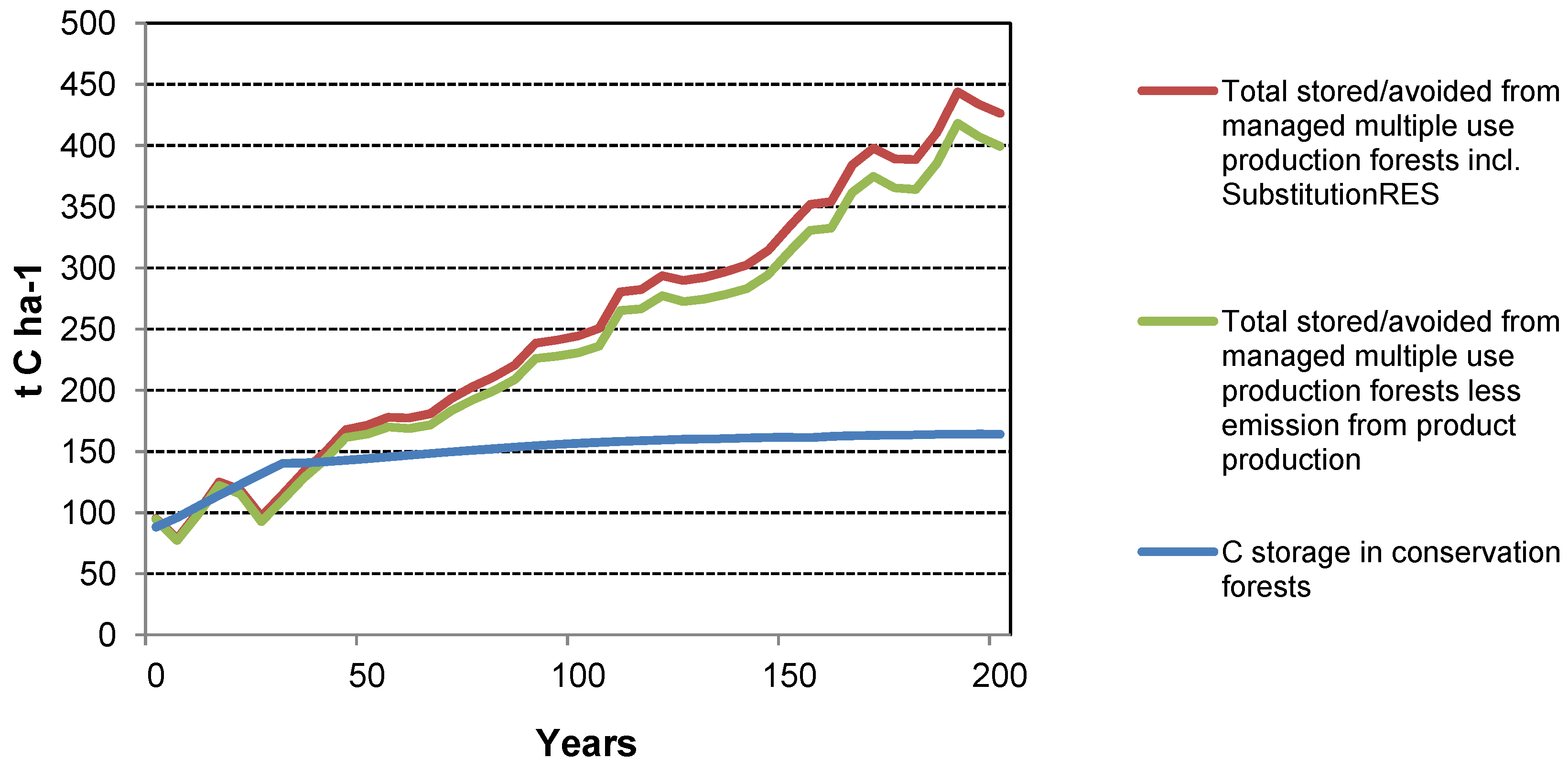
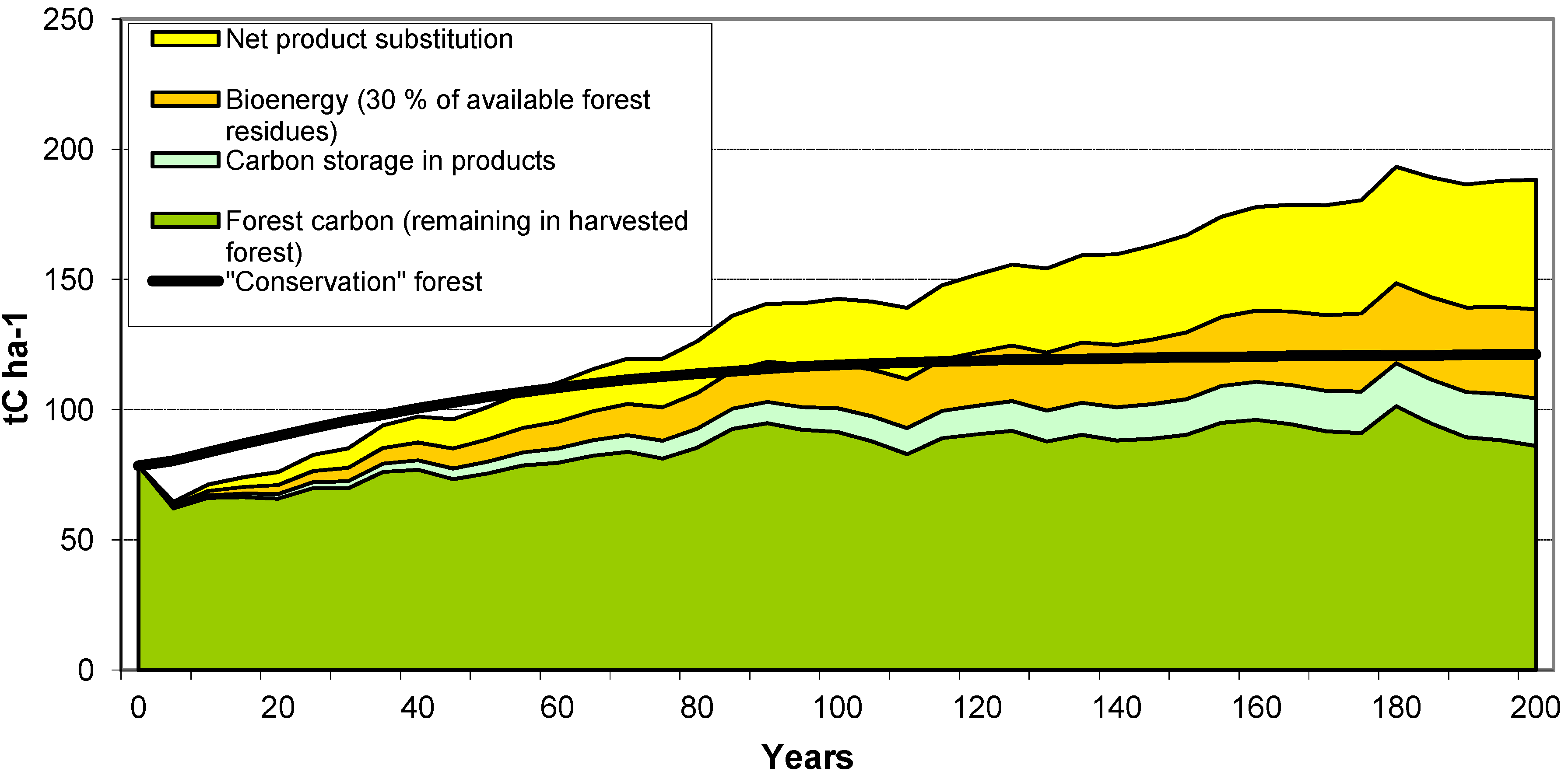
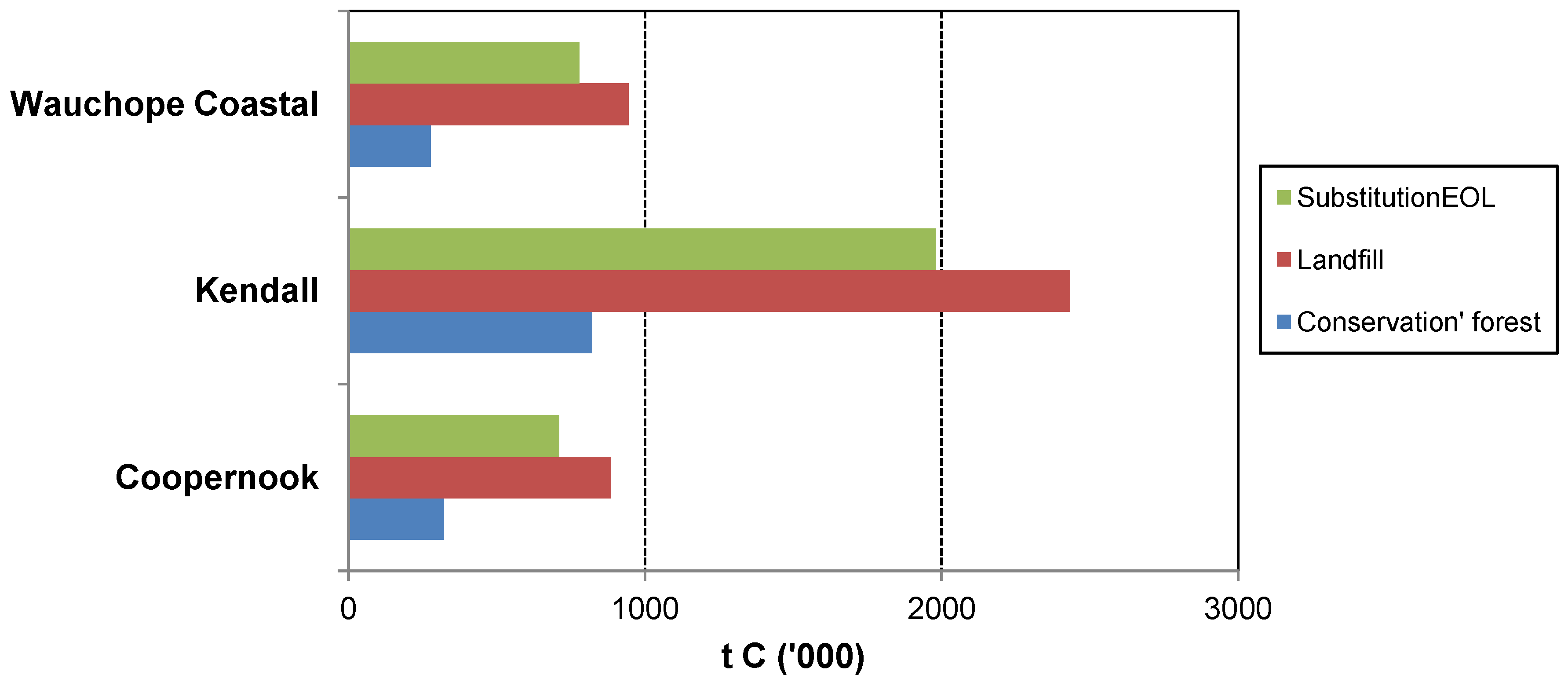
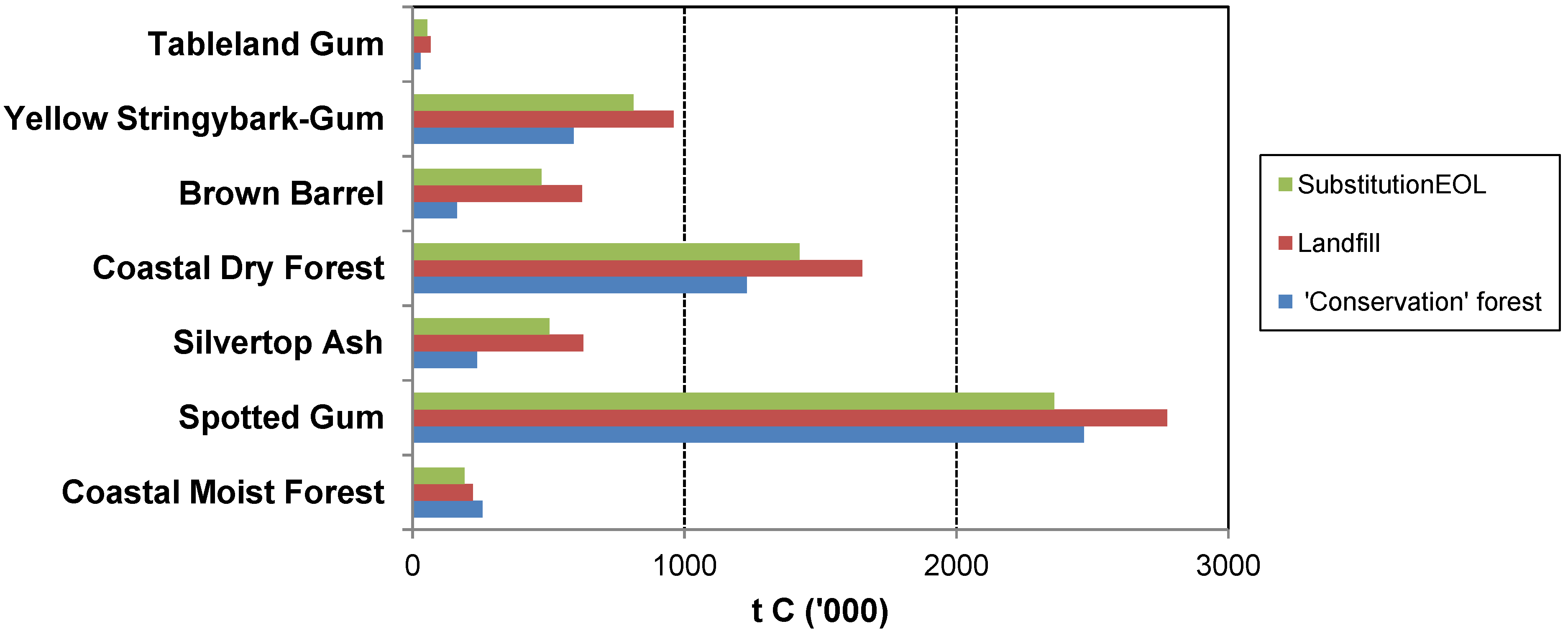
The case studies show that management of forests for wood production has the potential to generate a greater GHG mitigation benefit than managing for conservation alone. Similar conclusions have been drawn by others (e.g., [
17,
50,
51]). Similar to a conservation forest, a multiple-use production forest takes CO
2 from the atmosphere and fixes the C within the tree biomass. However, when biomass is removed at harvest, the C is stored in HWPs while the forest grows more biomass, allowing removal of more biomass in more HWPs in future harvests. After several harvest cycles, more C is stored in the forest plus through the use of HWPs than if the forest had not been harvested. These benefits are realized regardless of the waste management strategy assumed for the case studies (
i.e., whether HWPs are disposed off in landfills or incinerated with energy recovery). Although the most recent research in the area of decomposition of HWPs in landfills strongly suggests that solid HWPs and products such as particleboard and MDF (medium-density fiberboard) do not decay to any significant extent [
8], in this paper it was conservatively assumed that some decomposition has occurred [
6]. In addition, it is assumed that none of the methane generated is either flared or captured to generate electricity-despite the fact that most of the waste produced in Australia is deposited in modern landfills, which typically capture 50%–75% of methane generated to produce electricity [
38]. The assumptions for Substitution
EOL adopted here were based on international industry-average incinerator technology producing electricity alone [
46] as no waste-to-energy plants are currently operating in Australia. Modern plants using gasification, or combined heat and power, could have an even greater efficiency and therefore an increased net GHG mitigation benefit.
Furthermore, the case studies show that Substitution
HWP provides greater GHG mitigation benefit than the value of C storage in products, as also reported by other researchers (e.g., [
17,
52,
53]). Care should be exercised though when calculating Substitution
HWP to ensure that the increased use of wood will not result in a net loss of forest area due to unsustainable levels of harvest; and that realistic substitution scenarios are used [
54]. It is possible that in some instances the use of HWPs will displace the use of less GHG-intensive materials. In this paper the GHG impacts of reduced availability of wood products are demonstrated—not the GHG implications of increased use of wood products. Thus, the risk of a decrease in harvest or wood production elsewhere is not relevant for this discussion. The role of green building schemes in achieving real product substitution would be especially important in the scenario where an increased production of HWPs is assessed.
The GHG implications of not producing paper products from the “production” forests were not taken into account. It is possible that a proportion of the displaced paper products, that would need to be sourced elsewhere if harvest of native forests decreased significantly, would be sourced from areas where unsustainable forestry practices are adopted. This would lead to increased GHG emissions associated with the “conservation” scenario.
The case studies illustrate that the abatement benefit would be enhanced by using a portion of the harvest residues for energy (Substitution
RES), as previously demonstrated in other studies [
17,
50]. The estimates of emissions offset due to displacement of fossil energy emissions are made for a case where policies or programs result in full displacement of fossil energy (rather than just increasing the total primary energy use). In this context, policies that support increased use of renewable energy (e.g., [
19]) are essential to achieve real fossil fuel displacement. If a proportion of harvest residues are used for bioenergy generation to replace fossil fuels, Substitution
RES needs to take into account associated GHG emissions due to fuel usage in the extraction and transport of biomass. These same residues if left in the forest result in gradual emissions of biogenic C over time, and a “lost opportunity” of fossil fuel displacement. Sathre and Gustavsson [
55] examined the radiative forcing impact in their analysis of the climate impacts of using forest biomass as biofuel, in order to account for this temporal pattern of C emissions and uptakes. Using this approach, they demonstrated that use of residues as biofuel results in a significant GHG mitigation benefit when compared to natural decay in the forest. Of all the factors impacting on the climate benefits of using harvest residues for bioenergy, the quantity of biomass produced and recovered and the type of fossil fuel replaced, are the most important. In Australia, where currently a large volume of harvest residue is underutilized and where coal is the main source of energy, the GHG mitigation benefit of utilizing harvest residues for bioenergy generation is especially significant.
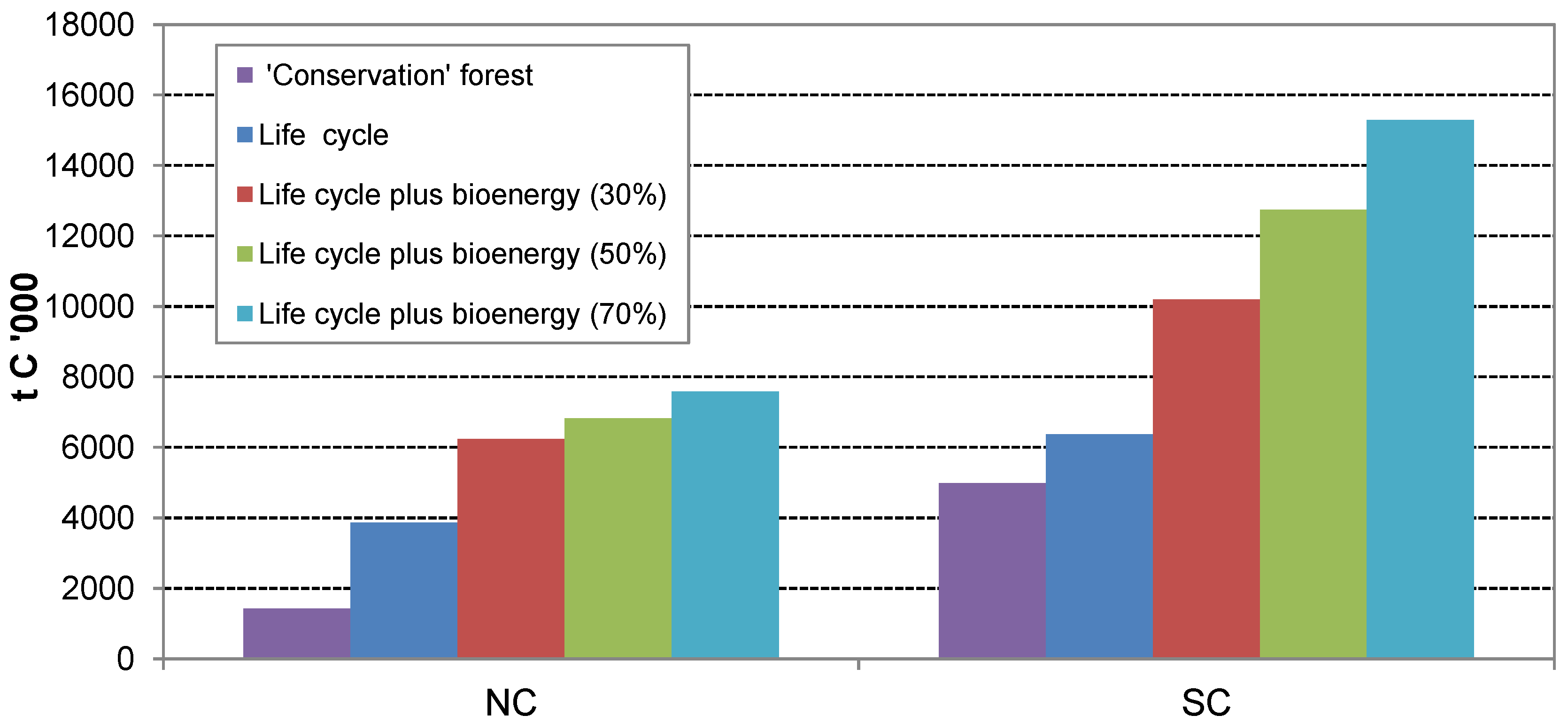
The C stocks for the NC and SC “
conservation” forests were considerably lower than the mean value predicted by Mackey
et al. [
56] for SE Australian forests undisturbed by harvesting, but within the typical range of values reported for mature native forests in Australia [
35,
57,
58,
59,
60,
61,
62]. The estimated C stocks for mature native forests will likely have little impact on the relative difference between the “
conservation” and “
production” options. A higher C stock in the “
conservation” forest at year 200 implies greater forest productivity than that of the forests used for the case studies—this would in most cases also apply to the harvested forest scenario. As a result, both the forest C stocks and off-site GHG mitigation benefits, such as HWPs, would also increase.
Inclusion of C in coarse woody debris (CWD), dead standing wood and fine litter would increase the C stocks for the “
conservation” forest scenario by approximately 25 t C ha
−1, assuming that published figures for forest types similar to those included the SC study areas [
60] can be applied here (similar published data was not found for forests comparable to those included in the NC case study area). Although this would reduce the combined forest and offset C GHG balance for the SC forests by approximately 25% (
Table 8), the overall GHG outcome of the SC “
production” forest is still significantly better (75 t C ha
−1) than that of the SC “
conservation” forests. The magnitude of the difference in the GHG balance between NC “
production” and “
conservation” forests was such (249.5 t C ha
−1) that inclusion of C in CWD would result in, proportionally, even less significant changes to the overall GHG outcome.
Although the GHG impact of fires is large over time (even discounting biogenic CO
2 emissions), their effect is more pronounced for “
conservation” forests, as the proportion of fire events represented by wildfires was greater for those forests. This resulted in higher estimates of GHG emissions. The impact of including non-CO
2 GHG emissions is significant—the net GHG outcome for NC “
conservation” forests at year 200 is nullified, and it becomes negative for SC “
conservation” forests. A more accurate calculation of the impact of fire on carbon balance requires better field data as well as modeling—using average fuel consumption rates will reduce the reliability of estimates of GHG emissions from fire [
23].
Internationally the focus of Reducing Emissions from Deforestation and Forest Degradation (REDD) projects is justifiably on achieving emission reductions through avoided deforestation and increased forest protection. However, this focus is not applicable in countries where sustainable forest management is adopted, such as Australia. The perception that cessation of harvest in native forests in Australia will provide substantial GHG abatement has led to pressure to convert production forests to conservation forests. Policy being enacted through the Australian Federal Parliament, that potentially will provide credit for cessation of harvest [
63] and does not recognise native forest biomass as eligible to earn renewable energy credits [
64], does not adequately recognise the nature of C flows in managed forests and hence undervalues their role in GHG mitigation. These policies fail to recognise the potential mitigation benefit of forests harvested for timber and biomass for energy. Lack of recognition of the GHG mitigation benefit of sustainable forest management will limit the potential mitigation that could be achieved by policies that acknowledge and support production of HWPs and use of forest biomass for renewable energy (ideally through mechanisms such as green building certification programs and renewable energy standards). Abatement provided by forest sequestration, HWPs and bioenergy has low cost compared with the cost of abatement through many other measures [
65]. Cessation of harvest in some native forests will give no additional mitigation benefits over business as usual (BAU), and will represent a missed opportunity to maintain and potentially enhance forests positive mitigation role and also to support socio-economic development in regional Australia. The findings of the case studies will apply equally to plantations: management of plantations for production of HWPs and bioenergy will deliver a greater GHG mitigation benefit than unharvested plantings.
It has been suggested [
66] that HWPs from native forests in Australia could be replaced with products from the existing plantation estate, which would avoid the use of GHG-intensive non-wood products. However, the existing NSW plantation estate has not expanded at the anticipated rate, and the species grown are not suitable for replacing the products such as flooring and external decking, for which native forest timbers are used. Therefore, if other HWPs are to replace native forest timbers these are likely to be imported, with a significant risk of “leakage” through increased emissions from forests harvested (often unsustainably) outside Australia. Much of Australia’s hardwood imports are derived from south-east Asia, predominantly Indonesia [
67], where the rate of deforestation (primarily due to logging) is about 1.1 M ha per year, and this is anticipated to increase [
67]. Indonesia’s emissions due to deforestation, excluding emissions from peatland fire and oxidation, averaged about 850 Mt per year in the period 2000–2004 [
68]. While the Indonesian Government is taking action to promote sustainable forest management [
68] increased imports of tropical hardwood timber by Australia are likely to be supplied at least partially from deforestation. Thus, the current GHG policy direction in Australia in relation to forests has the potential to result in increased net global emissions, due to the need for GHG-intensive alternative products and/or the import of HWPs from unsustainably managed forests. This risk is clearly identified by Kastner
et al. [
69], who state that “policies aiming at increasing national forest stocks, should include careful assessments if and to what extent this forest return will be facilitated by increasing risks and vulnerabilities in distant places”.
The case study findings demonstrate the importance of considering the entire forestry system, from a life cycle perspective. Upstream, downstream and indirect effects need to be accounted for when assessing the GHG impacts of forest management decisions. While it may be efficient to address environmental objectives such as water management, biodiversity conservation and GHG management simultaneously, striving for synergistic outcomes, there are inevitably tradeoffs [
70]. These should be made transparent and explicit. In devising policy measures to meet environmental and production objectives, governments should be mindful of the need to provide clear and consistent policy, to encourage industry to develop low GHG products and energy systems including bioenergy [
71].