Leaf Physiological and Morphological Responses to Shade in Grass-Stage Seedlings and Young Trees of Longleaf Pine
Abstract
:1. Introduction
2. Methods
2.1. Seedling Study
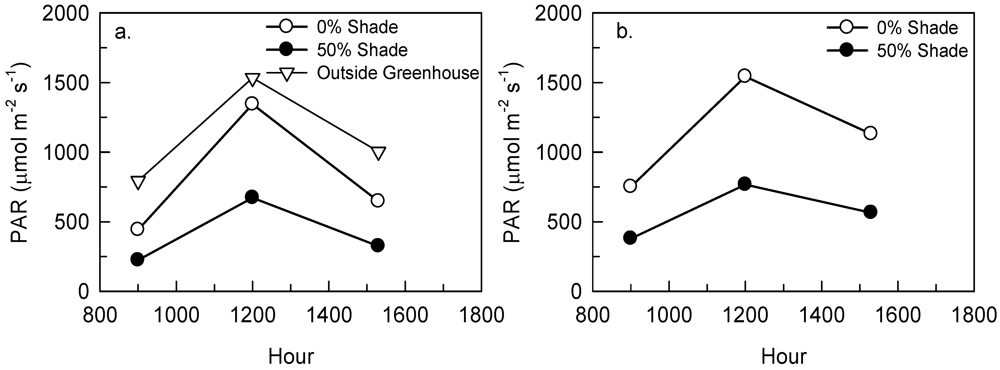
2.2. Tree Study
| Tree | Dbh (cm) | Height (m) | Shade | Branch height (m) | Branch diameter (cm) | Branch length (m) |
|---|---|---|---|---|---|---|
| 1 | 7.0 | 5.2 | 0% | 0.58 | 1.85 | 0.63 |
| 50% | 0.58 | 1.77 | 0.66 | |||
| 2 | 12.1 | 6.3 | 0% | 1.30 | 4.06 | 2.08 |
| 50% | 1.30 | 3.98 | 1.50 | |||
| 3 | 10.0 | 6.3 | 0% | 1.42 | 3.12 | 1.09 |
| 50% | 1.15 | 2.39 | 1.11 | |||
| 4 | 10.3 | 5.4 | 0% | 1.50 | 3.90 | 1.69 |
| 50% | 1.50 | 3.59 | 1.35 | |||
| 5 | 5.5 | 4.6 | 0% | 1.48 | 1.45 | 0.27 |
| 50% | 1.49 | 1.51 | 0.29 |
2.3. Leaf Physiology and Morphology
2.4. Chlorophyll
2.5. Data Analysis
3. Results
3.1. Seedlings

| Age | Month | Shade | LMA (g m−2) | Amax (µmol m−2 s−1) | gsmax (mmol m−2 s−1) | ΨPD (MPa) | ΨMD (MPa) |
|---|---|---|---|---|---|---|---|
| S | Jul. | 0% | 52.2 ± 1.1 | 5.1 ± 0.2 | 31.2 ± 1.3 | −0.71 ± 0.03 | −0.99 ± 0.05 |
| 50% | 43.6 ± 0.6 | 5.8 ± 0.4 | 36.9 ± 3.2 | −0.73 ± 0.08 | −0.92 ± 0.06 | ||
| P > F | 0.007 | 0.140 | 0.120 | 0.859 | 0.149 | ||
| Aug. | 0% | 49.9 ± 0.9 | 5.4 ± 0.4 | 34.2 ± 3.7 | −0.72 ± 0.02 | −0.90 ± 0.04 | |
| 50% | 38.5 ± 1.6 | 4.6 ± 0.2 | 34.1 ± 1.5 | −0.69 ± 0.04 | −0.78 ± 0.08 | ||
| P > F | 0.001 | 0.259 | 0.993 | 0.209 | 0.154 | ||
| T | Jul. | 0% | 80.2 ± 5.9 | 4.4 ± 0.8 | 44.4 ± 3.7 | −1.72 ± 0.09 | −1.80 ± 0.06 |
| 50% | 77.6 ± 4.7 | 3.3 ± 0.3 | 32.1 ± 5.4 | −1.76 ± 0.12 | −1.54 ± 0.16 | ||
| P > F | 0.583 | 0.172 | 0.147 | 0.456 | 0.213 | ||
| Aug. | 0% | 74.5 ± 3.7 | 4.9 ± 0.6 | 49.7 ± 9.4 | −1.34 ± 0.09 | −1.78 ± 0.14 | |
| 50% | 64.4 ± 3.7 | 3.5 ± 0.2 | 27.8 ± 1.8 | −1.18 ± 0.14 | −1.35 ± 0.04 | ||
| P > F | <0.001 | 0.126 | 0.074 | 0.099 | 0.002 |
| Age | Month | Shade | RD (µmol m−2 s−1) | Amax:RD | Φ (µmol CO2 µmol photon−1) | LCP (µmol m−2 s−1) |
|---|---|---|---|---|---|---|
| S | Jul. | 0% | 0.22 ± 0.02 | 31.4 ± 3.5 | 0.026 ± 0.001 | 8.5 ± 0.8 |
| 50% | 0.18 ± 0.02 | 34.6 ± 6.6 | 0.025 ± 0.001 | 7.4 ± 0.8 | ||
| P > F | 0.422 | 0.763 | 0.696 | 0.492 | ||
| Aug. | 0% | 0.32 ± 0.01 | 19.0 ± 1.9 | 0.025 ± 0.001 | 13.1 ± 0.4 | |
| 50% | 0.19 ± 0.02 | 34.0 ± 3.2 | 0.022 ± 0.002 | 8.9 ± 0.7 | ||
| P > F | 0.001 | 0.002 | 0.073 | 0.005 | ||
| T | Jul. | 0% | 0.29 ± 0.05 | 16.8 ± 3.4 | 0.016 ± 0.002 | 19.6 ± 4.2 |
| 50% | 0.24 ± 0.04 | 14.8 ± 1.9 | 0.015 ± 0.002 | 16.9 ± 1.3 | ||
| P > F | 0.568 | 0.704 | 0.288 | 0.577 | ||
| Aug. | 0% | 0.27 ± 0.04 | 19.8 ± 3.2 | 0.017 ± 0.003 | 17.2 ± 3.2 | |
| 50% | 0.34 ± 0.07 | 12.8 ± 3.4 | 0.025 ± 0.008 | 18.0 ± 4.2 | ||
| P > F | 0.310 | 0.148 | 0.470 | 0.912 |
| Age | Shade | NA (g m−2) | NW (mg g−1) |
|---|---|---|---|
| S | 0% | 1.3 ± 0.1 | 26.9 ± 0.4 |
| 50% | 0.9 ± 0.1 | 24.9 ± 0.8 | |
| P > F | <0.001 | 0.110 | |
| T | 0% | 0.6 ± 0.1 | 7.4 ± 0.4 |
| 50% | 0.6 ± 0.1 | 7.5 ± 0.6 | |
| P > F | 0.737 | 0.953 |
| Age | Shade | Chl a (mg m−2) | Chl a (mg g−1) | Chl b (mg m−2) | Chl b (mg g−1) | ChlTotal (mg m−2) | ChlTotal (mg g−1) | Chl a/b | Chl N/Total N (%) |
|---|---|---|---|---|---|---|---|---|---|
| S | 0% | 190.5 ± 22.4 | 3.8 ± 0.5 | 106.9 ± 13.5 | 2.1 ± 0.3 | 297.4 ± 35.0 | 5.9 ± 0.8 | 1.9 ± 0.1 | 1.2 ± 0.2 |
| 50% | 188.8 ± 15.7 | 5.0 ± 0.7 | 110.9 ± 13.1 | 2.9 ± 0.5 | 299.7 ± 28.5 | 7.9 ± 1.2 | 1.8 ± 0.1 | 1.7 ± 0.3 | |
| P > F | 0.938 | 0.125 | 0.513 | 0.062 | 0.934 | 0.095 | 0.151 | 0.132 | |
| T | 0% | 71.0 ± 12.6 | 0.94 ± 0.2 | 21.3 ± 4.8 | 0.28 ± 0.1 | 92.3 ± 16.8 | 1.2 ± 0.2 | 3.5 ± 0.5 | 0.9 ± 0.2 |
| 50% | 88.0 ± 12.8 | 1.28 ± 0.1 | 29.7 ± 3.8 | 0.43 ± 0.1 | 117.7 ± 12.3 | 1.7 ± 0.2 | 3.0 ± 0.2 | 1.2 ± 0.1 | |
| P > F | 0.174 | 0.031 | 0.088 | 0.029 | 0.137 | 0.027 | 0.199 | 0.051 |
3.2. Trees
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Brockway, D.G.; Lewis, C.E. Long-term effects of dormant-season prescribed fire in plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. For. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Platt, W.J. Effects of long-term fire exclusion on tree species composition and stand structure in an old-growth Pinus palustris (longleaf pine) forest. Plant Ecol. 1999, 140, 15–26. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Collineau, S. The physical nature of solar radiation in heterogeneous canopies: Spatial and temporal attributes. In Exploitation of Environmental Heterogeneity by Plants, Ecophysiological Processes Above and Below Ground; Caldwell, M.M., Pearcy, R.W., Eds.; Academic Press: London, UK, 1994; pp. 21–71. [Google Scholar]
- Brockway, D.G.; Outcalt, K.W.; Boyer, W.D. Longleaf pine regeneration ecology and methods. In The Longleaf Pine Ecosystem, Ecology, Silviculture and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 95–134. [Google Scholar]
- Baker, F.S. A revised shade tolerance table. J. For. 1949, 47, 179–181. [Google Scholar]
- Brockway, D.G.; Outcalt, K.W. Gap-phase regeneration in longleaf pine—Wiregrass ecosystems. For. Ecol. Manag. 1998, 106, 125–139. [Google Scholar] [CrossRef]
- Rodriguez-Trejo, D.A.; Duryea, M.L.; White, T.L.; English, J.R.; McGuire, J. Artificially regenerating longleaf pine in canopy gaps: Initial survival and growth during a year of drought. For. Ecol. Manag. 2003, 180, 25–36. [Google Scholar] [CrossRef]
- McGuire, J.P.; Mitchell, R.J.; Moser, E.B.; Pecot, S.D.; Gjerstad, D.H.; Hedman, C.W. Gaps in a gappy forest: Plant resources, longleaf pine regeneration, and understory response to tree removal in longleaf pine savannas. Can. J. For. Res. 2001, 31, 765–778. [Google Scholar] [CrossRef]
- Bhuta, A.A.; Kenneday, R.L.M.; Copenheaver, C.A.; Sheridan, P.M.; Campbell, J.B. Boundary-line patterns to determine disturbance history of remnant longleaf pine (Pinus palustris P. Mill.) in mixed forests of southeastern Virginia. J. Torrey Bot. Soc. 2008, 135, 516–529. [Google Scholar]
- Keeley, J.E.; Zedler, P.H. Evolution of life histories in Pinus. In Ecology and Biogeography of Pinus; Richardson, D.M., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 219–250. [Google Scholar]
- Wahlenberg, W.G. Longleaf Pine: Its Use, Ecology, Regeneration, Protection, Growth, and Management; Charles Lathrop Pack Forestry Foundation: Washington, DC, USA, 1946. [Google Scholar]
- Platt, E.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat. 1998, 131, 491–525. [Google Scholar]
- Boyer, W.D. Pinus palustris Mill. longleaf pine. In Silvics of North America, Conifers; Burns, R.M., Honkala, B.H., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 405–412. [Google Scholar]
- Pessin, L.J. Annual ring formation in Pinus palustris seedlings. Am. J. Bot. 1934, 21, 599–601. [Google Scholar] [CrossRef]
- Pecot, S.D.; Mitchell, R.J.; Palik, B.J.; Moser, E.B.; Hiers, J.K. Competitive responses of seedlings and understory plants in longleaf pine woodlands: Separating canopy influences above and below ground. Can. J. For. Res. 2007, 37, 634–648. [Google Scholar] [CrossRef]
- Harlow, W.H.; Harrar, E.S.; White, F.M. Textbook of Dendrology; McGraw-Hill Co.: New York, NY, USA, 1979; pp. 87–90. [Google Scholar]
- Battaglia, M.A.; Mitchell, R.J.; Mou, P.P.; Pecot, S.D. Light transmittance estimates in a longleaf pine woodland. For. Sci. 2003, 49, 752–762. [Google Scholar]
- Givnish, T.J. Adaptation to sun and shade: A whole plant perspective. Funct. Plant Biol. 1998, 15, 63–92. [Google Scholar]
- Cregg, B.M.; Teskey, R.O.; Dougherty, P.M. Effect of shade stress on growth, morphology and carbon dynamics of loblolly pine branches. Trees 1993, 7, 208–213. [Google Scholar]
- Henriksson, J. Differential shading of branches or whole trees: Survival, growth and reproduction. Oecologia 2001, 126, 482–486. [Google Scholar] [CrossRef]
- Lacointe, A.; Deleens, E.; Ameglio, T.; Saint-Joanis, B.; Lelarge, C.; Vandame, M.; Song, G.C.; Daudet, F.A. Testing the branch autonomy theory: A 13C/14C double-labeling experiment on differentially shaded branches. Plant Cell Environ. 2004, 27, 1159–1168. [Google Scholar] [CrossRef]
- Brooks, J.R.; Shulte, P.J.; Bond, B.J.; Coulombe, R.; Domec, J.C.; Hinkley, T.M.; McDowell, N.; Phillips, N. Does foliage on the same branch compete for the same water? Experiments on Douglas-fir trees. Trees 2003, 17, 101–108. [Google Scholar]
- Hanson, P.J.; McRoberts, R.E.; Isebrands, J.G.; Dixon, R.K. An optimal strategy for determining CO2 exchange range rate as a function of photosynthetic photon flux density. Photosynthetica 1987, 21, 98–101. [Google Scholar]
- Samuelson, L.J.; Seiler, J.R.; Feret, P.P. Gas exchange and canopy structure of 9-year-old loblolly pine, pitch pine and pitch x loblolly hybrids. Trees 1992, 6, 28–31. [Google Scholar]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Lichtenthaler, J.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Zhang, S.; Hennesssey, T.C.; Heinemann, R.A. Acclimation of loblolly pine (Pinus taeda) foliage to light intensity as related to leaf nitrogen availability. Can. J. For. Res. 1997, 27, 1032–1040. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Lieffers, V.J. Photosynthesis and carbon allocation of six boreal species grown in understory and open conditions. Tree Physiol. 2001, 21, 243–250. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ű.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Wyka, T.; Robakowski, P.; Zytkowiak, R. Acclimation of leaves to contrasting irradiance in juvenile trees differing in shade tolerance. Tree Physiol. 2007, 27, 1293–1306. [Google Scholar] [CrossRef]
- Greenwood, M.S.; Day, M.E.; Berlyn, G.P. Regulation of foliar plasticity in conifers: Developmental and environmental factors. J. Sustain. For. 2009, 28, 48–62. [Google Scholar] [CrossRef]
- Funk, J.L.; McDaniel, S. Altering light availability to restore invaded forest: The predictive role of plant traits. Restor. Ecol. 2010, 18, 865–872. [Google Scholar] [CrossRef]
- Jose, S.; Merritt, S.; Ramsey, C.L. Growth, nutrition, photosynthesis and transpiration responses of longleaf pine seedlings to light, water and nitrogen. For. Ecol. Manage. 2003, 180, 335–344. [Google Scholar] [CrossRef]
- Groninger, J.W.; Seiler, J.R.; Peterson, J.A.; Kreh, R.E. Growth and photosynthetic responses of four Virginia Piedmont trees species to shade. Tree Physiol. 1996, 16, 773–778. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Lukjanova, A.; Tobias, M. Dependence of needle architecture and chemical composition on canopy light availability in three North American Pinus species with contrasting needle length. Tree Physiol. 2002, 22, 747–761. [Google Scholar] [CrossRef]
- Niinemets, Ű. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Saldaña, A.; Gianoli, E.; Lusk, C.H. Ecophysiological responses to light availability and three Blechem species (Pteridophyta, Blechnaceae) of different ecological breadth. Oecologia 2005, 145, 252–257. [Google Scholar]
- Walters, M.B.; Field, C.B. Photosynthetic light acclimation in two rainforest piper species with different ecological amplitudes. Oecologia 1987, 72, 449–456. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology; Springer: New York, NY, USA, 1996. [Google Scholar]
- Samuelson, L.; Stokes, T.; Cooksey, T.; McLemore, P., III. Production efficiency of loblolly pine and sweetgum in response to four years of intensive management. Tree Physiol. 2001, 21, 369–376. [Google Scholar] [CrossRef]
- Teskey, R.O.; Shrestha, R.B. A relationship between carbon dioxide, photosynthetic efficiency and shade tolerance. Physiol. Plant 1985, 63, 126–132. [Google Scholar] [CrossRef]
- Rebbeck, J.; Scherzer, A.; Gottschalk, K. Do chestnut, northern red, and white oak germinant seedlings respond similarly to light treatments? II. Gas exchange and chlorophyll responses. Can. J. For. Res. 2012, 42, 1025–1037. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, J.H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Terashima, I.; Hikosaka, K. Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ. 1995, 18, 111–1128. [Google Scholar]
- Christensen, N.L. Fire and soil-plant nutrient relations in a pine-wiregrass savanna on the coastal plain of North Carolina. Oecologia 1977, 31, 27–44. [Google Scholar] [CrossRef]
- Blevins, D.; Allen, H.L.; Colbert, S.; Gardner, W. Nutrition Management for Longleaf Pinestraw; Woodland Owner Notes-30; North Carolina (State University) Cooperative Extension Service: Raleigh, NC, USA, 1996. [Google Scholar]
- Samuelson, L.J.; McLemore, P.C., III.; Somers, G.L. Relationship between foliar δ13C and hydraulic pathway length in Pinus palustris. For. Sci. 2003, 49, 790–798. [Google Scholar]
- Jackson, D.P.; Dumroese, R.K.; Barnett, J.P. Nursery response of container Pinus palustris seedlings to nitrogen supply and subsequent effects on outplanting performance. For. Ecol. Manage. 2012, 265, 1–12. [Google Scholar] [CrossRef]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Seedling nutrition and irrigation. In The Container Tree Nursery Manual; Landis, T.D., Tinus, R.W., McDonald, S.E., Barnett, J.P., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1989; Volume 4, pp. 1–67. [Google Scholar]
- Portsmuth, A.; Niinemets, Ű. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2006, 57, 343–354. [Google Scholar]
- Fownes, J.H.; Harrington, R.A. Seedling response to gaps; separating effects of light and nitrogen. For. Ecol. Manage. 2004, 203, 297–310. [Google Scholar] [CrossRef]
- Gulden, J.M. Uneven-aged silviculture of longleaf pine. In The Longleaf Pine Ecosystem, Ecology, Silviculture and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 217–241. [Google Scholar]
- Gagnon, J.L.; Jokela, E.J.; Moser, W.K.; Huber, D.A. Dynamics of artificial regeneration in gaps within a longleaf pine flatwoods ecosystem. For. Ecol. Manag. 2003, 172, 133–144. [Google Scholar] [CrossRef]
- Lusk, C.H. Leaf area and growth of juvenile temperate evergreens in low light, species of contrasting shade tolerance change rank during ontogeny. Funct. Ecol. 2004, 18, 820–828. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Samuelson, L.J.; Stokes, T.A. Leaf Physiological and Morphological Responses to Shade in Grass-Stage Seedlings and Young Trees of Longleaf Pine. Forests 2012, 3, 684-699. https://doi.org/10.3390/f3030684
Samuelson LJ, Stokes TA. Leaf Physiological and Morphological Responses to Shade in Grass-Stage Seedlings and Young Trees of Longleaf Pine. Forests. 2012; 3(3):684-699. https://doi.org/10.3390/f3030684
Chicago/Turabian StyleSamuelson, Lisa J., and Tom A. Stokes. 2012. "Leaf Physiological and Morphological Responses to Shade in Grass-Stage Seedlings and Young Trees of Longleaf Pine" Forests 3, no. 3: 684-699. https://doi.org/10.3390/f3030684




