Foliage and Litter Chemistry, Decomposition, and Nutrient Release in Pinus taeda
Abstract
:1. Introduction
2. Experimental Section
2.1. Site Description
2.2. Sampling
2.2.1. Foliage and Litter Solubility Indices
2.2.2. Long-Term Forest Floor Decomposition
2.2.3. Simulated Disturbance Forest Floor Decomposition
2.3. Chemical Analysis
2.3.1. Foliage and Litter Solubility Indices
2.3.2. Simulated Disturbance Forest Floor Decomposition
2.4. Statistical Analysis
2.4.1. Foliage and Litter Solubility Indices

2.4.2. Long-Term Forest Floor Decomposition
2.4.3. Simulated Disturbance Forest Floor Decomposition


3. Results and Discussion
3.1. Results
3.1.1. Foliage and Litter Solubility Indices
3.1.1.1.Nitrogen
| Ct | I | F | FI | F | I | F × I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble Amino-N g·kg−1 | p-value | ||||||||||
| Foliage | 0.351 | (6.5) | 0.309 | (18.6) | 0.468 | (25.5) | 0.464 | (15.6) | 0.0085 | 0.5896 | 0.6524 |
| Litter | 0.267 | (24.2) | 0.275 | (27.5) | 0.279 | (7.7) | 0.279 | (19.3) | 0.7759 | 0.8866 | 0.8866 |
| Soluble Nucleic-N g·kg−1 | |||||||||||
| Foliage | 1.01 | (8.0) | 0.910 | (9.8) | 1.22 | (13.5) | 1.13 | (12.0) | <0.0001 | 0.0122 | 0.7828 |
| Litter † | 0.317 | (24.7) | 0.286 | (19.8) | 0.377 | (23.7) | 0.308 | (17.4) | 0.2231 | 0.1476 | 0.5507 |
| Residual N g·kg−1 | |||||||||||
| Foliage | 7.67 | (6.8) | 7.62 | (3.6) | 10.34 | (7.4) | 10.87 | (4.4) | <0.0001 | 0.3853 | 0.3078 |
| Litter | 2.55 | (9.5) | 2.74 | (6.1) | 3.99 | (4.9) | 3.46 | (15.1) | 0.0001 | 0.3407 | 0.0605 |
| Total-N g·kg−1 | |||||||||||
| Foliage | 9.03 | (5.8) | 8.84 | (4.0) | 12.03 | (12.5) | 12.37 | (3.8) | <0.0001 | 0.6779 | 0.3138 |
| Litter | 3.13 | (5.0) | 3.30 | (7.9) | 4.64 | (6.5) | 4.04 | (12.7) | 0.0002 | 0.2934 | 0.0741 |
3.1.1.2. Phosphorus
| Ct | I | F | FI | F | I | F × I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Soluble Inorganic-P mg·kg−1 | p-value | ||||||||||
| Foliage | 257.2 | (8.6) | 245.1 | (5.7) | 300.5 | (9.9) | 313.6 | (6.4) | 0.0012 | 0.9692 | 0.3200 |
| Litter | 66.90 | (8.0) | 71.35 | (2.3) | 84.08 | (6.3) | 91.50 | (8.9) | <0.0001 | 0.0139 | 0.4650 |
| Total Soluble Organic-P mg·kg−1 | |||||||||||
| Foliage | 130.2 | (21.1) | 115.7 | (29.1) | 386.3 | (39.6) | 284.3 | (15.2) | 0.0006 | 0.1921 | 0.3170 |
| Litter | 47.55 | (28.4) | 28.65 | (61.4) | 73.70 | (29.3) | 71.90 | (45.2) | 0.0068 | 0.3247 | 0.4118 |
| Residual-P mg·kg−1 | |||||||||||
| Foliage | 368.6 | (13.6) | 324.9 | (21.6) | 269.9 | (65.6) | 409.5 | (7.8) | 0.8969 | 0.3869 | 0.1161 |
| Litter | 51.15 | (33.5) | 60.63 | (30.7) | 98.93 | (13.0) | 75.50 | (8.7) | 0.0009 | 0.3065 | 0.0309 |
| Total-P mg·kg−1 | |||||||||||
| Foliage | 755.9 | (4.0) | 685.6 | (9.4) | 956.7 | (7.9) | 1007.3 | (5.4) | <0.0001 | 0.7272 | 0.0542 |
| Litter | 165.6 | (6.4) | 160.6 | (8.5) | 256.7 | (5.2) | 238.9 | (12.5) | <0.0001 | 0.2542 | 0.5129 |
3.1.1.3. Soluble Carbohydrate
| Ct | I | F | FI | F | I | F × I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble Sugar g·kg−1 | p-value | ||||||||||
| Foliage | 58.65 | (13.0) | 61.30 | (5.9) | 67.55 | (7.3) | 65.60 | (12.9) | 0.0972 | 0.9240 | 0.5350 |
| Litter | 34.58 | (5.2) | 36.20 | (6.0) | 33.75 | (7.2) | 40.73 | (12.1) | 0.1787 | 0.0080 | 0.0642 |
| Soluble Starch g·kg−1 | |||||||||||
| Foliage | 52.88 | (5.2) | 55.68 | (5.4) | 55.35 | (19.4) | 50.98 | (3.9) | 0.7002 | 0.7848 | 0.2319 |
| Litter | 41.00 | (8.2) | 40.03 | (5.8) | 37.38 | (8.5) | 39.93 | (1.5) | 0.2398 | 0.6075 | 0.2641 |
| Total Soluble Carbohydrates g·kg−1 | |||||||||||
| Foliage | 111.45 | (8.6) | 116.98 | (2.8) | 122.90 | (10.4) | 116.53 | (8.5) | 0.3365 | 0.9392 | 0.3006 |
| Litter | 75.60 | (5.8) | 76.20 | (5.1) | 71.10 | (3.8) | 80.68 | (5.8) | 0.9950 | 0.0279 | 0.0463 |
3.1.1.4. Soluble Phenol and Tannin
| Ct | I | F | FI | F | I | F × I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble Phenol g·kg−1 | p-value | ||||||||||
| Foliage | 8.75 | (16.6) | 9.20 | (14.4) | 7.95 | (14.9) | 7.68 | (8.7) | 0.0555 | 0.8723 | 0.5104 |
| Litter † | 7.25 | (9.9) | 7.23 | (13.6) | 5.15 | (16.9) | 6.90 | (11.7) | 0.0138 | 0.0501 | 0.0429 |
| Soluble Tannin g·kg−1 | |||||||||||
| Foliage | 16.25 | (6.8) | 17.15 | (9.8) | 12.68 | (6.8) | 13.15 | (4.0) | 0.0001 | 0.2634 | 0.7208 |
| Litter | 10.03 | (12.6) | 9.83 | (13.6) | 6.80 | (9.4) | 8.30 | (16.1) | 0.0025 | 0.2869 | 0.1729 |
| Total Soluble Phenol + Tannin g·kg−1 | |||||||||||
| Foliage | 25.00 | (9.7) | 26.35 | (10.4) | 20.63 | (8.6) | 20.80 | (1.9) | 0.0004 | 0.4291 | 0.5394 |
| Litter | 17.30 | (10.7) | 17.05 | (13.5) | 11.90 | (10.7) | 15.18 | (13.3) | 0.0042 | 0.1476 | 0.0980 |
3.1.2. Long-Term Forest Floor Decomposition
3.1.3. Simulated Disturbance Forest Floor Decomposition
| Effect | Proportion Mass Remaining | |
|---|---|---|
| F-Statistic | p-Value | |
| F | 0.73 | 0.3973 |
| I | 0.12 | 0.7356 |
| Tm | 637.43 | <0.0001 |
| Tm × F | 0.01 | 0.9348 |
| Tm × I | 0.22 | 0.6451 |
| Tm × I | 0.85 | 0.3616 |
| Tm × F × I | 0.01 | 0.9058 |
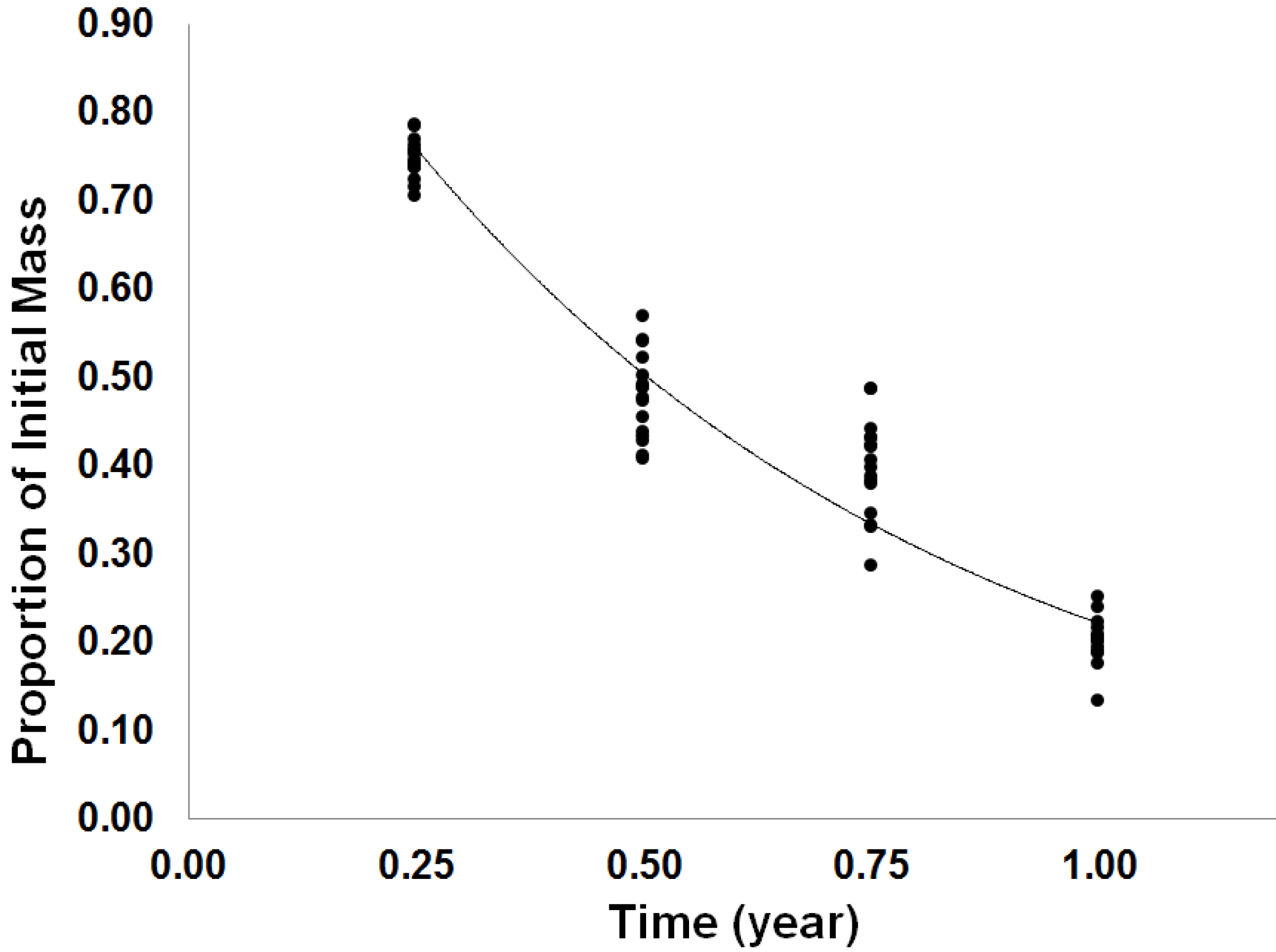
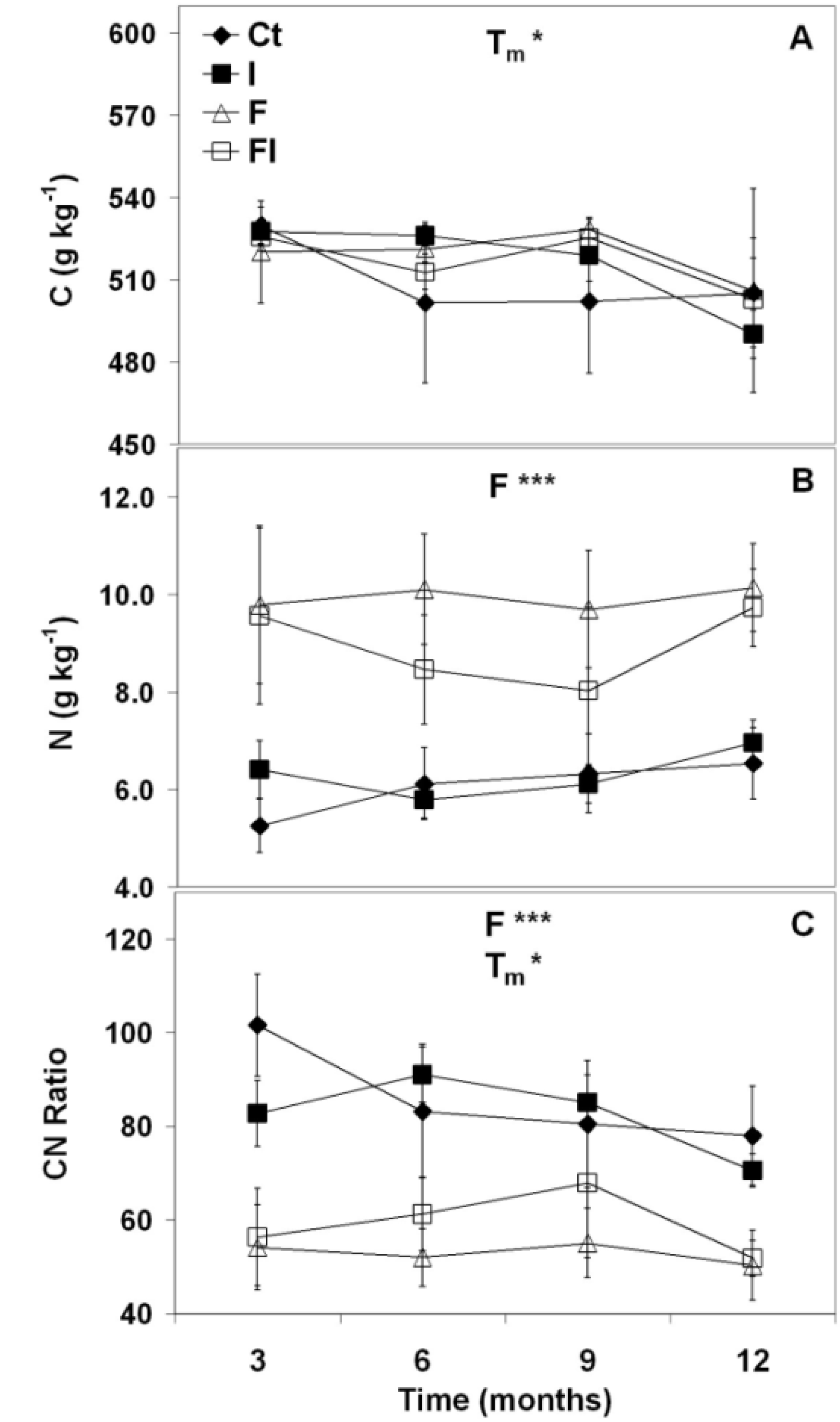
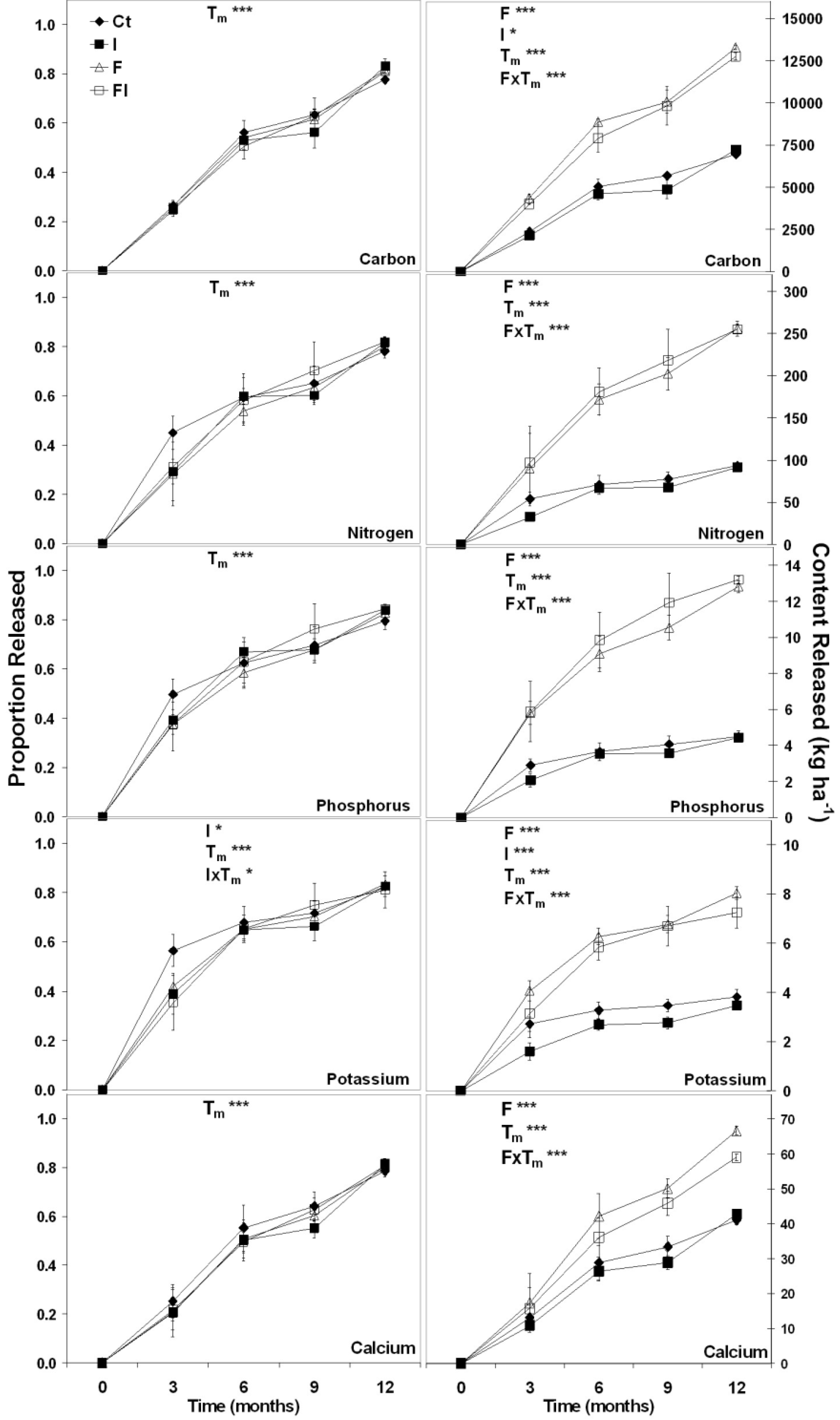
3.2. Discussion
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Allen, H.L. Forest fertilizers: Nutrient amendment, stand productivity, and environmental impact. J. For. 1987, 85, 37–46. [Google Scholar]
- Fox, T.R.; Jokela, E.J.; Allen, H.L. The development of pine plantation silviculture in the southern US. J. For. 2007, 105, 337–347. [Google Scholar]
- Albaugh, T.J.; Allen, H.L.; Dougherty, P.M.; Kress, L.W.; King, J.S. Leaf-area and above- and belowground growth responses of loblolly pine to nutrient and water additions. For. Sci. 1998, 44, 317–328. [Google Scholar]
- Miller, H.G. Forest fertilization: Some guiding concepts. Forestry 1981, 54, 157–167. [Google Scholar] [CrossRef]
- Kiser, L.C.; Fox, T.R. Soil accumulation of nitrogen and phosphorus following annual fertilization of loblolly pine and sweetgum on sandy sites. Soil Sci. Soc. Am. J. 2012, 76, 2278–2288. [Google Scholar] [CrossRef]
- Polglase, P.J.; Comerford, N.B.; Jokela, E.J. Nitrogen and phosphorus release from decomposing needles of southern pine plantations. Soil Sci. Soc. Am. J. 1992, 56, 914–920. [Google Scholar] [CrossRef]
- Piatek, K.B.; Allen, H.L. Are forest floors in mid-rotation loblolly pine stands a sink for nitrogen and phosphorus? Can. J. For. Res. 2001, 31, 1164–1174. [Google Scholar] [CrossRef]
- Sanchez, F. Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. For. Ecol. Manag. 2001, 152, 85–96. [Google Scholar] [CrossRef]
- Polglase, P.J.; Jokela, E.J.; Comerford, N.B. Phosphorus, nitrogen, and carbon fractions in litter and soil of southern pine plantations. Soil Sci. Soc. Am. J. 1992, 56, 566–572. [Google Scholar] [CrossRef]
- Polglase, P.J.; Comerford, N.B.; Jokela, E.J. Leaching of inorganic phosphorus from litter of southern pine plantations. Soil Sci. Soc. Am. J. 1992, 56, 573–577. [Google Scholar] [CrossRef]
- Prescott, C.E. Does N availability control rates of litter decomposition in forests? Plant Soil 1995, 168–169, 83–88. [Google Scholar] [CrossRef]
- Osono, T.; Ono, Y.; Takeda, H. Fungal ingrowth on forest floor and decomposing needle litter of Chamaecyparis obtusa in relation to resource availability and moisture condition. Soil Biol. Biochem. 2003, 35, 1423–1431. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Kalbitz, K.; Meyer, A.; Yang, R.; Gerstberger, P. Response of dissolved organic matter in the forest floor to long-term manipulation of litter and throughfall inputs. Biogeochemistry 2007, 86, 301–318. [Google Scholar] [CrossRef]
- Klotzbucher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef]
- Adams, M.B.; Allen, H.L.; Davey, C.B. Accumulation of starch in roots and foliage of loblolly pine (Pinus taeda L.): Effects of season, site and fertilization. Tree Physiol. 1986, 2, 35–46. [Google Scholar] [CrossRef]
- Balsberg-Pahlsson, A. Influence of nitrogen fertilization on minerals, carbohydrates, amino acids, and phenolic compounds in beech (Fagus sylvatica L.) leaves. Tree Physiol. 1992, 10, 93–100. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Allen, H.L.; Dougherty, P.M.; Johnsen, K.H. Long term growth responses of loblolly pine to optimal nutrient and water resource availability. For. Ecol. Manag. 2004, 192, 3–19. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Allen, H.L.; Fox, T.R. Nutrient use and uptake in Pinus taeda. Tree Physiol. 2008, 28, 1083–1098. [Google Scholar] [CrossRef]
- Albaugh, T.J.; North Carolina State University, Raleigh, NC, USA. Personal Communication, 2008.
- Kedrowski, R.A. Extraction and analysis of nitrogen, phosphorus, and carbon fractions in plant material. J. Plant Nutr. 1983, 6, 989–1011. [Google Scholar] [CrossRef]
- Keeny, D.R.; Nelson, D.W. Nitrogen–Inorganic Forms. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Ed.; Agronomy Monograph: Madison, WI, USA, 1982; Volume 9, pp. 643–698. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Dubois, M.; Giles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Price, M.L.; Butler, L.G. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J. Agric. Food Chem. 1977, 25, 1268–1273. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Hasey, M.M. Decomposition of chaparral shrub foliage: Losses of organic and inorganic constituents from deciduous and evergreen leaves. Ecology 1981, 62, 762–774. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS Procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar]
- Ballard, R. Effect of first rotation phosphorus applications on fertilizer requirements of second rotation pine. N. Z. J. For. Sci. 1978, 8, 135–145. [Google Scholar]
- Gentle, S.W.; Humphreys, F.R.; Lambert, M.J. Continuing response of Pinus radiata to phosphate fertilizers over two rotations. For. Sci. 1986, 32, 822–829. [Google Scholar]
- Comerford, N.B.; McLeod, M.; Skinner, M. Phosphorus form and bioavailability in the pine rotation following fertilization: P fertilization influences P form and potential bioavailability to pine in the subsequent rotation. For. Ecol. Manag. 2002, 169, 203–211. [Google Scholar] [CrossRef]
- Crous, J.W.; Morris, A.R.; Schole, M.C. The significance of residual phosphorus and potassium fertilization in countering yield decline in a fourth rotation of Pinus patula in Swaziland. South. Hemisph. For. J. 2007, 69, 1–8. [Google Scholar] [CrossRef]
- Everett, C.J.; Palm-Leis, H. Availability of residual phosphorus fertilizer for loblolly pine. For. Ecol. Manag. 2009, 258, 2207–2213. [Google Scholar] [CrossRef]
- Switzer, G.L.; Nelson, L.E. Nutrient accumulation and cycling in loblolly pine (Pinus taeda L.) plantation ecosystems: The first twenty years. Soil Sci. Soc. Am. J. 1972, 36, 143–147. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballare, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- Booker, F.L.; Maier, C.A. Atmospheric carbon dioxide, irrigation, and fertilization effects on phenolic and nitrogen concentrations in loblolly pine (Pinus taeda) needles. Tree Physiol. 2001, 21, 609–616. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Hobbie, S.E.; Milchunas, D.G.; Sinsabaugh, R.L. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 2010, 13, 765–781. [Google Scholar] [CrossRef]
- Brandt, L.A.; Bohnet, C.; King, J.Y. Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Vogt, K.A.; Grier, C.C.; Meier, C.E.; Keyes, M.R. Organic matter and nutrient dynamics in forest floors of young and mature Abies amabilis stands in western Washington, as affected by fine-root input. Ecol. Monogr. 1983, 53, 139–157. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Decomposition rate and chemical changes of Scots pine needle litter. II. Influence of chemical composition. Ecol. Bull. 1980, 32, 373–390. [Google Scholar]
- Melillo, J.M.; Aber, J.D.; Linkins, A.E.; Ricca, A.; Fry, B.; Nadelhoffer, K.J. Carbon and nitrogen dynamics along the decay continuum: Plant litter to soil organic matter. Plant Soil 1989, 115, 189–198. [Google Scholar] [CrossRef]
- Berg, B.; Soderstrom, B. Fungal biomass and nitrogen in decomposing Scots pine needle litter. Soil Biol. Biochem. 1979, 11, 339–341. [Google Scholar] [CrossRef]
- Tian, X.J.; Sun, S.C.; Ma, K.P.; An, S.Q. Behavior of carbon and nutrients within two types of leaf litter during 3.5 year decomposition. Acta Bot. Sin. 2003, 45, 1413–1420. [Google Scholar]
- Jerabkova, L.; Prescott, C.E.; Kishchuk, B.E. Effect of variable-retention harvesting on soil nitrogen availability in boreal mixedwood forests. Can. J. For. Res. 2006, 36, 3029–3038. [Google Scholar] [CrossRef]
- Martin, W.L.; Bradley, R.L.; Kimmins, J.P. Post-clearcutting chronosequence in the BCcoastal western hemlock zone: I. Changes in forest floor mass and N storage. J. Sustain. For. 2002, 14, 1–22. [Google Scholar]
- Titus, B.D.; Prescott, C.E.; Maynard, D.G.; Mitchell, A.K.; Bradley, R.L.; Feller, M.C.; Beese, W.J.; Seely, B.A.; Benton, R.A.; Senyk, J.P.; et al. Post-harvest nitrogen cycling in clearcut and alternative silvicultural systems in a montane forest in coastal British Columbia. For. Chron. 2006, 82, 844–859. [Google Scholar]
- Kim, C.; Sharik, T.L.; Jurgensen, M.F. Canopy cover effects on mass loss, and nitrogen and phosphorus dynamics from decomposing litter in oak and pine stands in northern Lower Michigan. For. Ecol. Manag. 1996, 80, 13–20. [Google Scholar] [CrossRef]
- Prescott, C.E. The influence of the forest canopy on nutrient cycling. Tree Physiol. 2002, 22, 1193–1200. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Matson, P.A. Mechanisms of nitrogen retention in forest ecosystems: A field experiment. Science 1984, 225, 51–52. [Google Scholar]
- Fox, T.R.; Burger, J.A.; Kreh, R.E. Effects of site preparation on nitrogen dynamics in the southern Piedmont. For. Ecol. Manag. 1986, 15, 241–256. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kiser, L.C.; Fox, T.R.; Carlson, C.A. Foliage and Litter Chemistry, Decomposition, and Nutrient Release in Pinus taeda. Forests 2013, 4, 595-612. https://doi.org/10.3390/f4030595
Kiser LC, Fox TR, Carlson CA. Foliage and Litter Chemistry, Decomposition, and Nutrient Release in Pinus taeda. Forests. 2013; 4(3):595-612. https://doi.org/10.3390/f4030595
Chicago/Turabian StyleKiser, L. Chris, Thomas R. Fox, and Colleen A. Carlson. 2013. "Foliage and Litter Chemistry, Decomposition, and Nutrient Release in Pinus taeda" Forests 4, no. 3: 595-612. https://doi.org/10.3390/f4030595




