Forest Typification to Characterize the Structure and Composition of Old-growth Evergreen Forests on Chiloe Island, North Patagonia (Chile)
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area

| Species | Family | Abrev. | Shade tolerance | Position in vertical profile of old-growth forests |
|---|---|---|---|---|
| Embothrium coccineum J.R. et. G. Forster.* | Proteaceae | Ec | Intolerant1 | § |
| Nothofagus nitida (Phil) Krasser. | Fagaceae | Nn | Intolerant2 | Emergent |
| Semi-tolerant1 | ||||
| Tepualia stipularis (Hook et. Arn.) Griseb.* | Myrtaceae | Ts | Intolerant2 | Understory |
| Weinmannia trichosperma Cav. | Cunoniaceae | Wt | Intolerant1 | Emergent |
| Amomyrtus luma (Mol.) Legr. et. Kausel. | Myrtaceae | Al | Tolerant1 | Lower canopy |
| Laureliopsis philippiana Looser. | Monimiaceae | Lp | Tolerant1 | Canopy |
| Lomatia ferruginea (Cav.) R. Br.* | Proteaceae | Lf | Tolerant1 | Understory |
| Luma apiculata (D.C.) Burret.* | Myrtaceae | La | Tolerant1 | Understory |
| Podocarpus nubigena Lindl.* | Podocarpaceae | Pn | Tolerant1 | Canopy |
| Saxegothaea conspicua Lindl. | Podocarpaceae | Sc | Tolerant1 | Canopy |
| Caldcluvia panniculata (Cav.) D. Don.* | Cunoniaceae | Cp | Semi-tolerant1 | Understory |
| Drimys winteri J.R. et. G. Forster. | Winteraceae | Dw | Semi-tolerant1 | Canopy |
2.2. Experimental Design and Statistical Analysis


3. Results
3.1. Similarity between Evergreen Forests of the Region

3.2. Definition of Groups in Old-Growth Forests
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| n = 16 | n = 12 | n = 9 | n = 9 | |
| Density (trees per ha) | Mean (Sd) | Mean (Sd) | Mean (Sd) | Mean (Sd) |
| Drimys winteri | 104 (±62.3) | 133 (±85.5) | 214 (±105.5) | 106 (±56.8) |
| Amomyrtus luma | 313 (±148.6) | 295 (±165.9) | 207 (±150.4) | 190 (±185.1) |
| Laureliopsis philippiana | 218 (±121.4) | 170 (±103.6) | 62 (±58.5) | 43 (±65.8) |
| Nothofagus nitida | 5 (±12.1) | 27 (±21.0) | 60 (±77.3) | 44 (±31.3) |
| Saxegothaea conspicua | - | 7 (±20.2) | 3 (±7.1) | 141 (±94.7) |
| Weinmannia trichosperma | 4 (±8.9) | 1 (±2.9) | 64 (±59.8) | 63 (±65.4) |
| Other species* | 71 (±106.9) | 89 (±73.5) | 149 (±117.1) | 58 (±52.6) |
| Total | 715a (±208.4) | 722a (±195.4) | 760a (±251.3) | 646a (±183.0) |
| Basal area (m² per ha) | Mean (Sd) | Mean (Sd) | Mean (Sd) | Mean (Sd) |
| Drimys winteri | 12.9 (±8.7) | 10.7 (±6.6) | 15.9 (±8.0) | 9.6 (±8.7) |
| Amomyrtus luma | 10.6 (±6.3) | 8.1 (±3.4) | 6.6 (±4.3) | 2.9 (±3.5) |
| Laureliopsis philippiana | 25.1 (±17.3) | 13.5 (±8.4) | 6.4 (±9.2) | 2.6 (±5.6) |
| Nothofagus nitida | 1.1 (±4.2) | 12.7 (±10.8) | 9.1 (±12.6) | 12.7 (±8.5) |
| Saxegothaea conspicua | - | 1.6 (±4.1) | 0.9 (±2.6) | 32.3 (±15.2) |
| Weinmannia trichosperma | 2.1 (±4.0) | 0.5 (±2.0) | 18.0 (±13.9) | 16.7 (±13.7) |
| Other species* | 1.7 (±2.6) | 2.1 (±1.3) | 3.5 (±3.2) | 1.3 (±1.3) |
| Total | 53.8a (±16.6) | 49.6a (±10.3) | 60.8ab (±13.4) | 78.3b (±13.1) |
| Quadratic mean diameter (cm) | Mean (Sd) | Mean (Sd) | Mean (Sd) | Mean (Sd) |
| Drimys winteri | 38.9 (±10.7) | 33.6 (±13.9) | 31.5 (±8.9) | 36.3 (±17.2) |
| Amomyrtus luma | 20.8 (±4.6) | 19.3 (±3.1) | 21.2 (±6.0) | 14.4 (±4.3) |
| Laureliopsis philippiana | 38.2 (±8.6) | 35.6 (±13.7) | 33.2 (±10.4) | 21.6 (±7.7) |
| Nothofagus nitida | 47.6 (±37.3) | 83.3 (±27.9) | 49.7 (±29.9) | 60.4 (±15.6) |
| Saxegothaea conspicua | - | 72.7 (±34.4) | 51.4 (±27.4) | 64.3 (±29.7) |
| Weinmannia trichosperma | 83.8 (±20.9) | 94.0 (-) | 72.5 (±25.8) | 61.1 (±13.3) |
| Other species* | 17.2 (±4.0) | 20.7 (±12.8) | 18.8 (±6.1) | 17.8 (±5.6) |
| Total | 31.3a (±5.2) | 30.1a (±4.0) | 32.7ab (±4.9) | 39.9b (±5.1) |
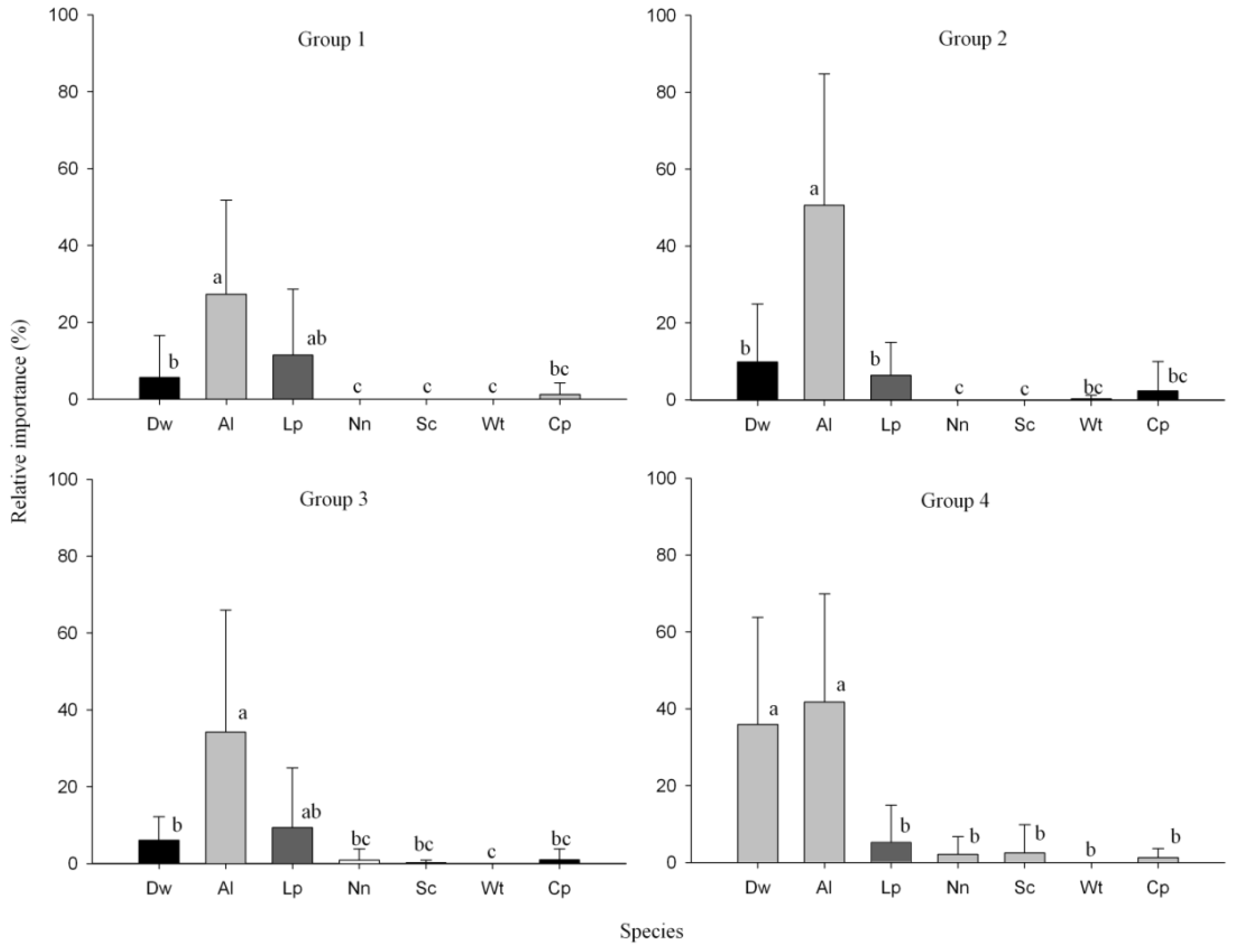
3.3. Structure and Composition of Forest Groups



4. Discussion
4.1. Valdivian or North-Patagonian Forests?
4.2. Forest Groups in the Evergreen Forests of Southern Chiloé
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rowe, J.S.; Sheard, J.W. Ecological land classification: A survey approach. Environ. Manage. 1981, 5, 451–464. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Goebel, P.C.; Wigley, T.B. Evaluating forestland classification schemes as tools for maintaining biodiversity. J. For. 2001, 99, 33–40. [Google Scholar]
- Barnes, B.V.; Zak, D.R.; Denton, S.R.; Spurr, S.H. Forest Ecology, Fourth Edition. ed; John Wiley & Sons: New York, NY, USA, 1999; p. 774. [Google Scholar]
- Ministère des Ressources Naturelles. Available online: http://www.mrn.gouv.qc.ca/espanol/inicio.jsp (accessed on 10 July 2013).
- Tappenier, J.C.; Maguire, D.A.; Harrington, T.B. Silviculture and Ecology of Western U.S. Forests; Oregon State University Press: Corvallis, Oregon, OR, USA, 2007; p. 440. [Google Scholar]
- Donoso, C. Tipos forestales de los bosques nativos de Chile; Investigación y Desarrollo Forestal; CONAF/PNUD/FAO.; Food and Agriculture Organization of the United States: Chile, 1981; p. 70. [Google Scholar]
- Corporación Nacional Forestal; Comisión Nacional de Medio Ambiente; Universidad Austral de Chile. Pontificia Universidad Católica de Chile; Universidad Católica de Temuco. In Catastro y evaluación de los recursos vegetacionales nativos de Chile. Informe nacional con variables ambientales; Corporación Nacional Forestal: Santiago, Chile, 1999; p. 87. [Google Scholar]
- Donoso, C.; Grez, R.; Escobar, B. Estructura y dinámica de bosques del tipo forestal siempreverde en un sector de Chiloé Insular. Bosque 1984, 5, 82–104. [Google Scholar]
- Donoso, C.; Escobar, P.; Urrutia, J. Estructura y estrategias regenerativas de un bosque virgen de Ulmo (Eucryphia cordifolia Cav.)—Tepa (Laurelia philippiana Phil.) Looser en Chiloé, Chile. Rev. Chil. Hist. Nat. 1985, 58, 171–186. [Google Scholar]
- Armesto, J.J.; Figueroa, J. Stand Structure and dynamics in the temperate rain forests of Chiloé Archipelago, Chile. J. Biogeogr. 1987, 14, 367–376. [Google Scholar] [CrossRef]
- Gutierrez, A.G.; Armesto, J.J.; Aravena, J.C. Disturbance and regeneration dynamics of an old-growth North Patagonian rain forest in Chiloé Island, Chile. J. Ecol. 2004, 92, 598–608. [Google Scholar] [CrossRef]
- Gutierrez, A.G.; Aravena, J.C.; Carrasco-Farías, N.V.; Christie, D.A.; Fuentes, M.; Armesto, J.J. Gap-phase dynamics and coexistence of a long-lived pioneer and shade-tolerant tree species in the canopy of an old-growth coastal temperate rain forest of Chiloé Island, Chile. J. Biogeogr. 2008, 35, 1674–1687. [Google Scholar] [CrossRef]
- Gutierrez, A.G.; Armesto, J.J.; Aravena, J.C.; Carmona, M.; Carrasco, N.V.; Christie, D.A.; Peña, M.P.; Pérez, C.; Huth, A. Structural and environmental characterization of old-growth temperate rainforests of northern Chiloé Island, Chile: Regional and global relevance. For. Ecol. Manag. 2009, 258, 376–388. [Google Scholar]
- Donoso, P.J.; Soto, D.P.; Bertín, R.A. Size-density relationships in Drimys winteri secondary forests of the Chiloé Island, Chile: Effects of physiography and species composition. For. Ecol. Manag. 2007, 239, 120–127. [Google Scholar] [CrossRef]
- Veblen, T.T.; Schlegel, F.; Oltremari, J. Temperate broad-leaved evergreen forests of South America. In Temperate Broad-Leaved Evergreen Forests; Elsevier Science Publishers: Amsterdam, The Netherlands, 1983; pp. 5–31. [Google Scholar]
- Veblen, T.T. Forest development in tree-fall gaps in the temperate rain forests of Chile. Natl. Geogr. Res. 1985, 1, 161–184. [Google Scholar]
- Armesto, J.J.; Fuentes, E.R. Tree species regeneration in a mid-elevation, temperate rain forest in Isla de Chiloé, Chile. Vegetatio 1988, 74, 151–159. [Google Scholar] [CrossRef]
- Innes, J.L. Structure of evergreen temperate rain forests on the Taitao Peninsula, Southern Chile. J. Biogeogr. 1992, 19, 555–562. [Google Scholar] [CrossRef]
- Ther-Ríos, F. Prácticas cotidianas e imaginarios en sociedades litorales. El sector de Cucao, Isla Grande de Chiloé. Rev. Antropol. Chil. 2008, 40, 67–80. [Google Scholar]
- Echeverria, C.; Coomes, D.A.; Hall, M.; Newton, A.C. Spatially explicit models to analyze forest loss and fragmentation between 1976 and 2020 in southern Chile. Ecol. Model. 2008, 212, 439–449. [Google Scholar] [CrossRef]
- Donoso, C.; Donoso, P.J.; González, M.E.; Sandoval, V. Los bosques siempreverdes. In Silvicultura de los bosques nativos de Chile; Editorial Universitaria: Santiago, Chile, 1999; pp. 297–339. [Google Scholar]
- Donoso, C. Regeneración y crecimiento en el tipo forestal siempreverde costero y andino tras distintos tratamientos silviculturales. Bosque 1989, 10, 69–83. [Google Scholar]
- Di Castri, F.; Hajek, E. Bioclimatología de Chile; Vicerrectoría Académica de la Universidad Católica de Chile: Santiago, Chile, 1976; p. 163. [Google Scholar]
- Watters, W.A.; Fleming, C.A. Contributions to the geology and palaeontology of Chiloé Island, Southern Chile. Philos. Trans. R. Soc. B Biol. Sci. 1972, 263, 369–408. [Google Scholar] [CrossRef]
- Bannister, J.R.; Donoso, P.J.; Bauhus, J. Persistence of the slow growing conifer Pilgerodendron uviferum in old-growth and fire-disturbed southern bog forests. Ecosystems 2012, 15, 1158–1172. [Google Scholar] [CrossRef]
- Donoso, C. Antecedentes básicos para la silvicultura del tipo forestal siempreverde. Bosque 1989, 10, 37–53. [Google Scholar]
- Aravena, J.C.; Carmona, M.R.; Perez, C.A.; Armesto, J.J. Changes in tree species richness, stand structure and soil properties in a successional chronosequence in northern Chiloé Island, Chile. Rev. Chil. Hist. Nat. 2002, 75, 339–360. [Google Scholar]
- Kreft, H.; Jetz, W. A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 2010, 37, 2029–2053. [Google Scholar] [CrossRef]
- Donoso, C. Bosques Templados de Chile y Argentina: Variación, Estructura y Dinámica; Universitaria: Santiago, Chile, 1993; p. 484. [Google Scholar]
- Bailey, R.; Dell, T.R. Quantifying diameter distributions with the Weibull function. For. Sci. 1973, 19, 97–104. [Google Scholar]
- Cohen, A.C. Maximum likelihood estimation in the Weibull distribution based on complete and on censored samples. Technometrics 1965, 7, 579–588. [Google Scholar] [CrossRef]
- Veblen, T.T.; Alaback, P.B. A comparative review of forest dynamics and disturbance in the temperate rainforests of North and South America. In High-Latitude Rainforests and Associated Ecosystems of the West Coast of the Americas. Climate, Hydrology, Ecology and Conservation; Springer Verlag: New York, NY, USA, 1995; pp. 173–215. [Google Scholar]
- Bannister, J.R.; Vidal, O.J.; Teneb, E.; Sandoval, V. Latitudinal patterns and regionalization of plant diversity along a 4270-km gradient in continental Chile. Austral Ecol. 2012, 37, 500–509. [Google Scholar] [CrossRef]
- Donoso, P.J.; Lusk, C.H. Differential effects of emergent Nothofagus dombeyi on growth and basal area of canopy species in an old-growth temperate rainforest. J. Veg. Sci. 2007, 18, 675–684. [Google Scholar] [CrossRef]
- Goff, F.G.; West, D. Canopy-understory interaction effects on forest population structure. Forest Sci. 1975, 21, 98–108. [Google Scholar]
- Veblen, T.T.; Schlegel, F.; Escobar, B. Structure and dynamics of old-growth Nothofagus forests in the Valdivian Andes. J. Ecol. 1980, 68, 1–31. [Google Scholar] [CrossRef]
- Donoso, P.J. Structure and Growth in Coastal Evergreen Forests as the Bases for Uneven-Aged Silviculture in Chile; State University of New York: New York, NY, USA, 2002; p. 314. [Google Scholar]
- Lorimer, C.G.; Krug, A.G. Diameter distributions in even-aged stands of shade-tolerant and midtolerant tree species. Am. Midl. Nat. 1983, 109, 331–345. [Google Scholar] [CrossRef]
- Tyrrel, L.E.; Crow, T. Structural characteristics of old-growth hemlock-hardwood forests in relation to age. Ecology 1994, 75, 370–386. [Google Scholar] [CrossRef]
- Westphal, C.; Tremer, N.; Von Oheimb, G.; Hansen, J.; Von Gadow, K.; Hardtle, W. Is the reverse J-shaped diameter distribution universally applicable in European virgin beech forests? For. Ecol. Manag. 2006, 223, 75–83. [Google Scholar] [CrossRef]
- Neira, E.; Bertín, R.A. Hábitos del uso de la leña en Castro, Isla de Chiloé. Bosque Nativo 2009, 45, 3–8. [Google Scholar]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Nyland, R.D. Silviculture: Concepts and Applications.; McGraw-Hill: New York, NY, USA, 2002; p. 682. [Google Scholar]
- Nyland, R.D. Even- to uneven-aged: The challenges of conversion. For. Ecol. Manag. 2003, 172, 291–300. [Google Scholar] [CrossRef]
| Species name | Family |
|---|---|
| Aextoxicon punctatum R. Et Pav. | Aextoxicaceae |
| Amomyrtus luma (Mol.) Legr. et. Kausel. | Myrtaceae |
| Amomyrtus meli(Phil) Legr. et Kausel | Myrtaceae |
| Aristotelia chilensis (Mol) Stuntz | Elaeocarpaceae |
| Caldcluvia panniculata (Cav.) D. Don. | Cunoniaceae |
| Dassiphylum diacanthoides (Less.) Cabrera | Asteraceae |
| Drimys winteri J.R. et. G. Forster. | Winteraceae |
| Embothrium coccineum J.R. et.G. Forster. | Proteaceae |
| Eucryphia cordifoliaCav. | Eucryphiaceae |
| Gevuina avellanaMol. | Proteaceae |
| Laureliopsis philippiana Looser. | Monimiaceae |
| Lomatia ferruginea (Cav.) R. Br. | Proteaceae |
| Luma apiculata (D.C.) Burret. | Myrtaceae |
| Myrceugenia chrysocarpa (O. Berg) Kausel | Myrtaceae |
| Myrceugenia ovata(Hooker et Arntt) Berg | Myrtaceae |
| Myrceugenia parviflora (DC) Kausel. | Myrtaceae |
| Myrceugenia planipes(H et A.) Berg | Myrtaceae |
| Nothofagus dombeyi(Mirb.) Oerst. | Fagaceae |
| Nothofagus nitida (Phil) Krasser. | Fagaceae |
| Ovidia pillo pillo (C.Gray) Hohen. ex. C.F.W.Meissn. | Thymelaeaceae |
| Podocarpus nubigena Lindl. | Podocarpaceae |
| Raphithamnus spinosus(Juss.) Moldenke | Verbenaceae |
| Saxegothaea conspicua Lindl. | Podocarpaceae |
| Sophora microphylla Aiton | Papilionaceae |
| Tepualia stipularis (Hook et. Arn.) Grises. | Myrtaceae |
| Weinmannia trichosperma Cav. | Cunoniaceae |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bannister, J.R.; Donoso, P.J. Forest Typification to Characterize the Structure and Composition of Old-growth Evergreen Forests on Chiloe Island, North Patagonia (Chile). Forests 2013, 4, 1087-1105. https://doi.org/10.3390/f4041087
Bannister JR, Donoso PJ. Forest Typification to Characterize the Structure and Composition of Old-growth Evergreen Forests on Chiloe Island, North Patagonia (Chile). Forests. 2013; 4(4):1087-1105. https://doi.org/10.3390/f4041087
Chicago/Turabian StyleBannister, Jan R., and Pablo J. Donoso. 2013. "Forest Typification to Characterize the Structure and Composition of Old-growth Evergreen Forests on Chiloe Island, North Patagonia (Chile)" Forests 4, no. 4: 1087-1105. https://doi.org/10.3390/f4041087




