Soil Carbon Dynamics in Residential Lawns Converted from Appalachian Mixed Oak Stands
Abstract
:1. Introduction
2. Experimental Section
2.1. Site Selection
| Subdivision (n) | Basal Area (m2/ha) | Tree Height (m) | Trees per Hectare | Basal Area of Most Common Tree Species (m2/ha) |
|---|---|---|---|---|
| Brush Mountain | ||||
| Forest (5) | 13 | 23 | 89 | Quercus alba (10.1); Q. prinus (1.2); Q. coccinea (1.0) |
| Lawn (5) | 2 | 20 | 36 | Q. prinus (3.4); Q. alba (0.9); N. sylvatica (0.1) |
| Cherokee Hills | ||||
| Forest (4) | 13 | 24 | 111 | Acer rubrum (3.2); Q. prinus (2.7); N. sylvatica (1.7) |
| Lawn (4) | 9 | 18 | 40 | Q. alba (14.1); Q. prinus (7.5); Carya alba (0.6) |
| DeerCroft | ||||
| Forest (5) | 15 | 24 | 69 | Q. prinus (8.8); Q. alba (2.7); C. alba (1.5) |
| Lawn (5) | 9 | 22 | 55 | Q. prinus (0.4); Q. alba (1.2); C. glabra (2.0) |
| Forest Hill | ||||
| Forest (2) | 14 | 25 | 53 | Q. alba (8.9); Q. rubra (2.2); Liriodendron tulipifera (2.6) |
| Lawn (6) | 9 | 25 | 41 | Q. alba (1.3); Q. rubra (0.2); L. tulipifera (0.9) |
| Glenvar Heights | ||||
| Forest (8) | 4 | 25 | 75 | A. rubrum (1.4); Q. alba (0.9); Q. coccinea (0.5) |
| Lawn (11) | 3 | 25 | 51 | Q. rubra (0.4); Q. alba (2.2); Q. prinus (0.2) |
| Laurel Ridge | ||||
| Forest (14) | 13 | 21 | 113 | Q. prinus (4.6); A.rubrum (2.3); Pinus rigida (2.0) |
| Lawn (14) | 10 | 19 | 86 | Q. prinus (3.5); Q. rubra (1.0); N. sylvatica (0.4) |
| Mossy Spring | ||||
| Forest (2) | 8 | 27 | 81 | Q. alba (2.5); C. alba (0.4); Q. rubra (0.2) |
| Lawn (3) | 6 | 25 | 58 | L. tulipifera (1.2); C. alba (0.4); Q. alba (0.8) |
| Oilwell Road | ||||
| Forest (8) | 8 | 23 | 109 | A. rubrum (1.9); Q. prinus (1.4); Q. alba (1.4) |
| Lawn (8) | 5 | 20 | 75 | Q. alba (1.2); A. rubrum (0.8); Q. rubra (0.2) |
| Preston Forest | ||||
| Forest (5) | 10 | 23 | 101 | Q. prinus (5.1); Q. coccinea (1.2); Q. alba (1.0) |
| Lawn (6) | 7 | 19 | 30 | Q. prinus (3.0); A. rubrum (1.8); Q. alba (1.8) |
| Entire Study Area | ||||
| Forest (57) | 11 | 24 | 89 | Q. alba (3.2); Q. prinus (2.4); A. rubrum (0.8) |
| Lawn (64) | 7 | 21 | 52 | Q. alba (1.6); Q. prinus (1.2); A. rubrum (0.2) |
2.2. Field Measurements
2.3. Laboratory Measurements
2.4. Survey of Lawn Management Practices
2.5. Data Analysis
3. Results and Discussion
3.1. Carbon Storage in Lawns and Adjacent Forest
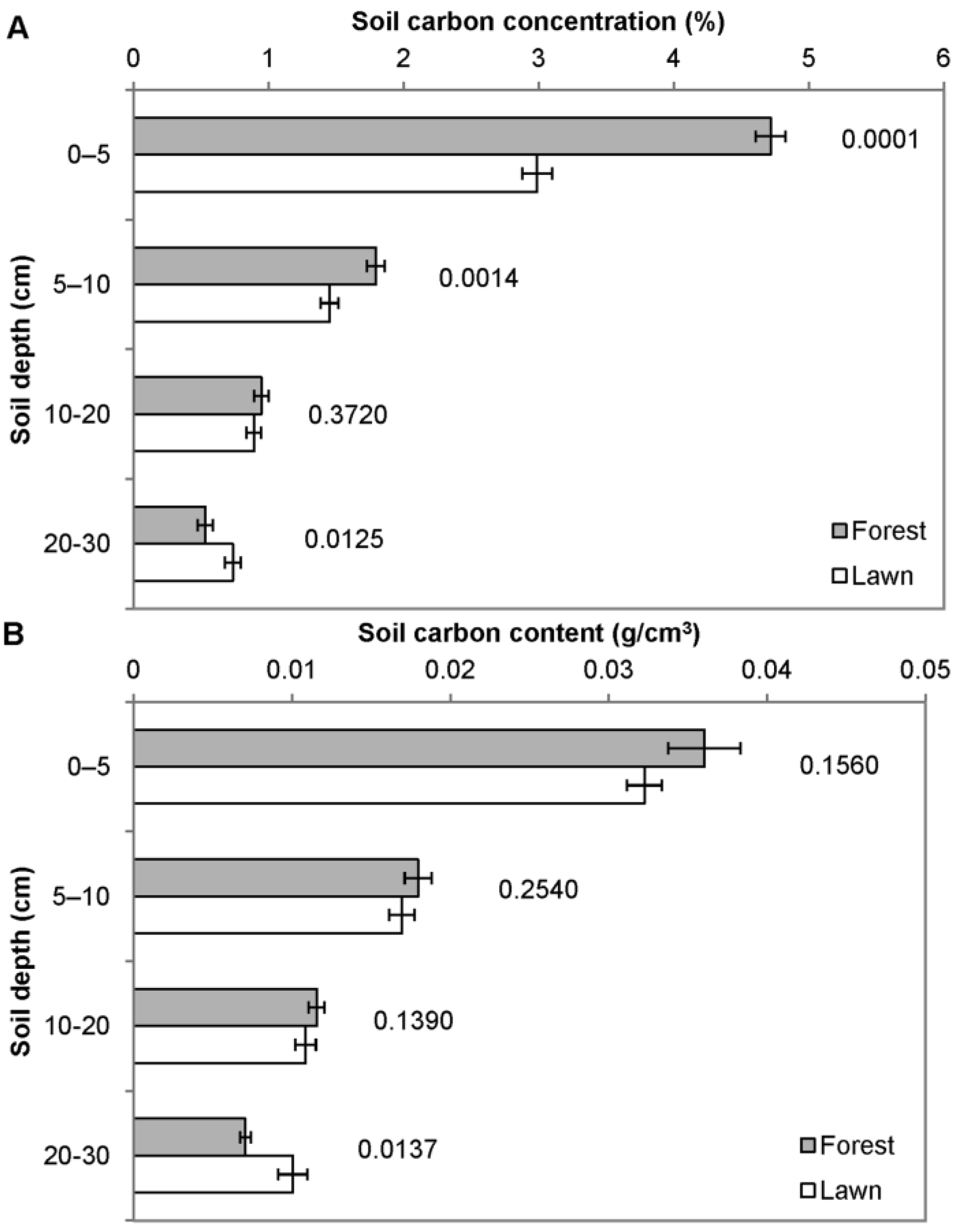

3.2. Other Soil and Turf Variables
| Carbon/Nitrogen Ratio | Bulk Density (g/cm3) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–5 | 62 | 21.8 | 13.6 | 0.0001 | 64 | 0.81 | 1.10 | 0.0001 |
| 5–10 | 62 | 22.4 | 14.9 | 0.0001 | 63 | 1.02 | 1.18 | 0.0001 |
| 10–20 | 61 | 18.4 | 14.5 | 0.0002 | 59 | 1.26 | 1.26 | 0.9733 |
| 20–30 | 55 | 14.3 | 13.5 | 0.3845 | 44 | 1.37 | 1.40 | 0.6505 |
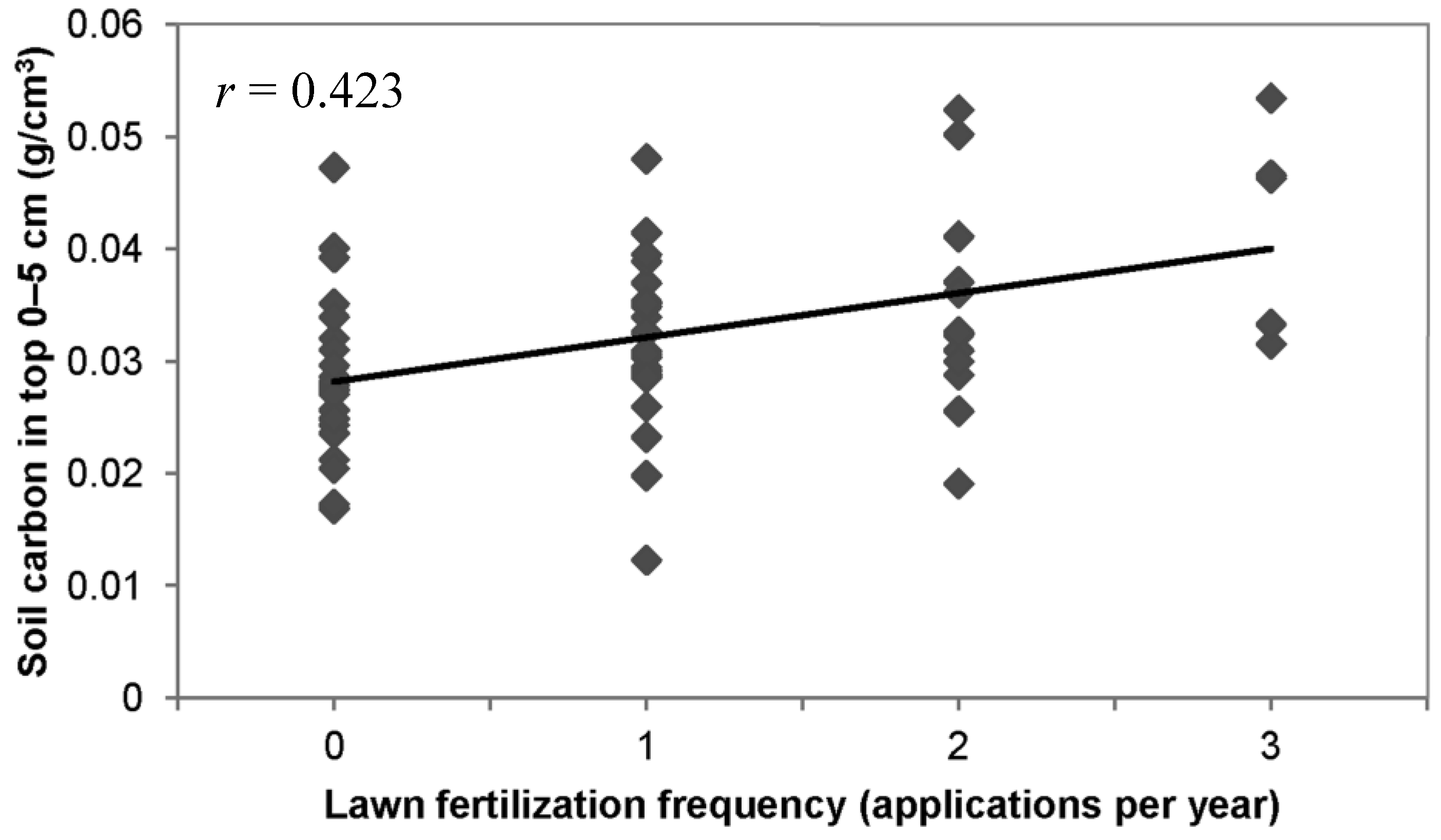
3.3. Lawn Management Practices, Owner Environmental Attitudes, and Soil Carbon
3.4. Discussion
4. Conclusions
Conflicts of Interest
References
- Pouyat, R.V.; Yesilonis, I.D.; Russel-Anelli, J.; Neerchal, N. Soil chemical and physical properties that differentiate urban land-use and cover types. Soil Sci. Soc. Am. J. 2006, 71, 1010–1019. [Google Scholar]
- Scharenbroch, B.C.; Lloyd, J.E.; Johnson-Maynard, J.L. Distinguishing urban soils with physical, chemical, and biological properties. Pedobiologia 2005, 49, 283–296. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biogeochemical C and N cycles in urban soils. Environ. Int. 2009, 35, 1–8. [Google Scholar] [CrossRef]
- Brown, D.G.; Johnson, K.M.; Loveland, T.R.; Theobald, D.M. Rural land use trends in the coterminus United States, 1950–2000. Ecol. Appl. 2005, 15, 1851–1863. [Google Scholar] [CrossRef]
- Beyer, L.; Blume, H.P.; Elsner, D.C.; Willnow, A. Soil organic matter and microbial activity in urban soils. Sci. Total Environ. 1995, 168, 267–278. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Pataki, D.E.; Alig, R.J.; Fung, A.S.; Golubiewski, N.E.; Kennedy, C.A.; McPherson, E.G.; Nowak, D.J.; Pouyat, R.V.; Lankoa, P.R. Urban ecosystems and the North American carbon cycle. Glob. Chang. Biol. 2006, 12, 1–11. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion impact on agronomic productivity and environment quality. Crit. Rev. Plant Sci. 1998, 17, 319–464. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Lubowski, R.; Vesterby, M.; Bucholtz, S.; Baez, A.; Roberts, M. Major Uses of Land in the United States, 2002. USDA ERS Economic Information Bulletin No. EIB-14. 2006. Available online: http://www.ers.usda.gov/Publications/EIB14 (accessed on 31 July 2012).
- Nowak, D.J. Institutionalizing urban forestry as a “biotechnology” to improve environmental quality. Urban For. Urban Green. 2006, 5, 93–100. [Google Scholar] [CrossRef]
- Curtis, P.S.; Hanson, P.J.; Bolstad, P.; Barford, C.; Randolph, J.C.; Schmid, H.P.; Wilson, K.B. Biometric and eddy-covariance based estimates of annual carbon. Agric. For. Meterology 2002, 113, 3–19. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Pataki, D.E.; Belt, K.T.; Groffman, P.M.; Hom, J.; Band, L.E. Effects of Urban Land-use Change on Biogeochemical Cycles. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 45–58. [Google Scholar]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Groffman, P.M.; Band, L.E.; Boone, C.G.; Burch, W.R., Jr.; Grimmond, S.B.; Hom, J.; Jenkins, J.C.; et al. Beyond urban legends: An emerging framework of urban ecology, as illustrated by the Baltimore Ecosystem Study. BioScience 2008, 58, 139–150. [Google Scholar] [CrossRef]
- Golubiewski, N.E. Urbanization increases grassland carbon pools: Effects of landscaping in Colorado’s front range. Ecol. Appl. 2006, 16, 555–571. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Golubiewski, N.E. A comparison of soil organic carbon between residential turf grass and native soil. Urban Ecosyst. 2009, 12, 45–62. [Google Scholar] [CrossRef]
- Milesi, C.; Running, S.W.; Elvidge, C.E.; Dietz, J.B.; Tuttle, B.T.; Nemani, R.R. Mapping and modeling the biogeochemical cycling of turf grasses in the United States. Environ. Manag. 2005, 36, 426–438. [Google Scholar] [CrossRef]
- Bormann, F.H.; Balmori, D.; Geballe, G.T. Redesigning the American Lawn: A Search for Environmental Harmony, 2nd ed.; Yale University Press: New Haven, CT, USA, 2001. [Google Scholar]
- Qian, Y.; Follett, R.F. Assessing soil carbon sequestration in turfgrass systems using long-term soil testing data. Agron. J. 2002, 94, 930–935. [Google Scholar] [CrossRef]
- Kaye, J.P.; Groffman, P.M.; Grimm, N.B.; Baker, L.A.; Pouyat, R.V. A distinct urban biogeochemistry? Trends Ecol. Evol. 2006, 21, 192–199. [Google Scholar] [CrossRef]
- Jo, H.-K.; McPherson, E.G. Carbon storage and flux in urban residential greenspace. J. Environ. Manag. 1995, 45, 109–133. [Google Scholar] [CrossRef]
- Bandaranayake, W.; Qian, Y.L.; Parton, W.J.; Ojima, D.S.; Follett, R.F. Estimation of soil organic carbon changes in turfgrass systems using the century model. Agron. J. 2002, 95, 558–563. [Google Scholar]
- Qian, Y.L.; Bandaranayake, W.; Parton, W.J.; Mecham, B.; Harivandi, M.A.; Mosier, A.R. Long-term effects of clipping and nitrogen management in turfgrass on soil organic carbon and nitrogen dynamics—The CENTURY model simulation. J. Environ. Qual. 2003, 32, 1694–1700. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Groffman, D; Yesilonis, I.D.; Hernandez, L. Soil carbon pools and fluxes in urban ecosystems. Environ. Pollut. 2002, 116, S107–S118. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Russell-Anell, J.; Yesilonis, I.D.; Groffman, P.M. Soil Carbon in Urban Forest Ecosystem. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; Kimble, J.K., Lal, R., Birdsey, R.A., Heath, L.S., Eds.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Nikon, C.H.; Pouyat, E.V.; Zipperer, W.C.; Constanza, B. Urban ecological systems. Linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Revi. Ecol. Syst. 2001, 32, 127–157. [Google Scholar] [CrossRef]
- Web Soil Survey. USDA Natural Resources Conservation Service. Available online: http://websoilsurvey.nrcs.usda.gov/ (accessed on 5 March 2011).
- Beers, T.W.; Dress, P.E.; Wensel, L.C. Aspect transformation in site productivity research. J. For. 1966, 64, 691–692. [Google Scholar]
- Maguire, R.; Heckendorn, S. Explanation of Soil Tests. Virginia Cooperative Extension Publication 452–701. Available online: http://pubs.ext.vt.edu/452/452-701/452-701.html (accessed on 13 March 2014).
- Davis, J.L.; Green, J.D.; Reed, A. Interdependence with the environment: Commitment, interconnectedness, and environmental behavior. J. Environ. Psychol. 2009, 29, 173–180. [Google Scholar] [CrossRef]
- Raciti, S.M.; Groffman, P.M.; Jenkins, J.C.; Pouyat, R.V.; Fahey, T.J.; Pickett, S.T.A.; Cadenasso, M.L. Accumulation of carbon and nitrogen in residential soils with different land use histories. Ecosystems 2011, 14, 287–297. [Google Scholar] [CrossRef]
- Jobaggy, E.G.; Jackson, B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Singh, M.H. Soil Organic Carbon Pools in Turfgrass Systems of Ohio. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2007. [Google Scholar]
- Gough, C.M.; Seiler, J.R. Below ground carbon dynamics in loblolly pine (Pinus taeda) immediately following diammonium phosphate fertilization. Tree Physiol. 2004, 24, 845–851. [Google Scholar] [CrossRef]
- Tyree, M.C.; Seiler, J.R.; Fox, T.R. The effects of fertilization on soil respiration in two-year-old Pinus taeda L. clones. For. Sci. 2008, 54, 21–30. [Google Scholar]
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Johnson, D.; Leake, J.R.; Read, D.J. Liming and nitrogen fertilization affects phosphatase activities, microbial biomass and mycorrhizal colonization in upland grassland. Plant Soil 2005, 271, 157–164. [Google Scholar] [CrossRef]
- Trumbore, S.E.; Czimczik, C.I. An uncertain future for soil carbon. Science 2008, 321, 1455–1456. [Google Scholar] [CrossRef]
- Janzen, H.H. The soil carbon dilemma: Shall we hoard it or use it? Soil Biol. Biochem. 2006, 38, 419–424. [Google Scholar] [CrossRef]
- Mangiafico, S.S.; Obropta, C.C.; Rossi-Griffin, E. Demographic factors influence environmental values: A lawn-care survey of homeowners in New Jersey. J. Ext. 2012. Available online: http://www.joe.org/joe/2012february/rb6.php (accessed on 13 March 2014).
- Sewell, S.; McCallister, D.; Gaussoin, R.; Wortmann, C. Lawn management practices and perceptions of residents in 14 sandpit lakes of Nebraska. J. Ext. 2010. Available online: http://www.joe.org/joe/2010april/rb4.php (accessed on 13 March 2014).
- Hefner, S.G.; Robertson, C.; Coulter, A.; Stevens, G. Engaging citizens to urban nutrient planning of lawns within a nutrient sensitive watershed. J. Ext. 2009. Available online: http://www.joe.org/joe/2009august/iw5.php (accessedon 13 March 2014).
- Birdsey, R.A. Carbon Storage and Accumulation in United States Forest Ecosystems; USDA Forest Service: Washington, DC, USA, 1992. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Campbell, C.D.; Seiler, J.R.; Wiseman, P.E.; Strahm, B.D.; Munsell, J.F. Soil Carbon Dynamics in Residential Lawns Converted from Appalachian Mixed Oak Stands. Forests 2014, 5, 425-438. https://doi.org/10.3390/f5030425
Campbell CD, Seiler JR, Wiseman PE, Strahm BD, Munsell JF. Soil Carbon Dynamics in Residential Lawns Converted from Appalachian Mixed Oak Stands. Forests. 2014; 5(3):425-438. https://doi.org/10.3390/f5030425
Chicago/Turabian StyleCampbell, Chad D., John R. Seiler, P. Eric Wiseman, Brian D. Strahm, and John F. Munsell. 2014. "Soil Carbon Dynamics in Residential Lawns Converted from Appalachian Mixed Oak Stands" Forests 5, no. 3: 425-438. https://doi.org/10.3390/f5030425





