Aboveground Biomass of Glossy Buckthorn is Similar in Open and Understory Environments but Architectural Strategy Differs
Abstract
:1. Introduction
2. Methods
2.1. Study Site
2.2. Sampling, Regression Procedures and Data Analyses


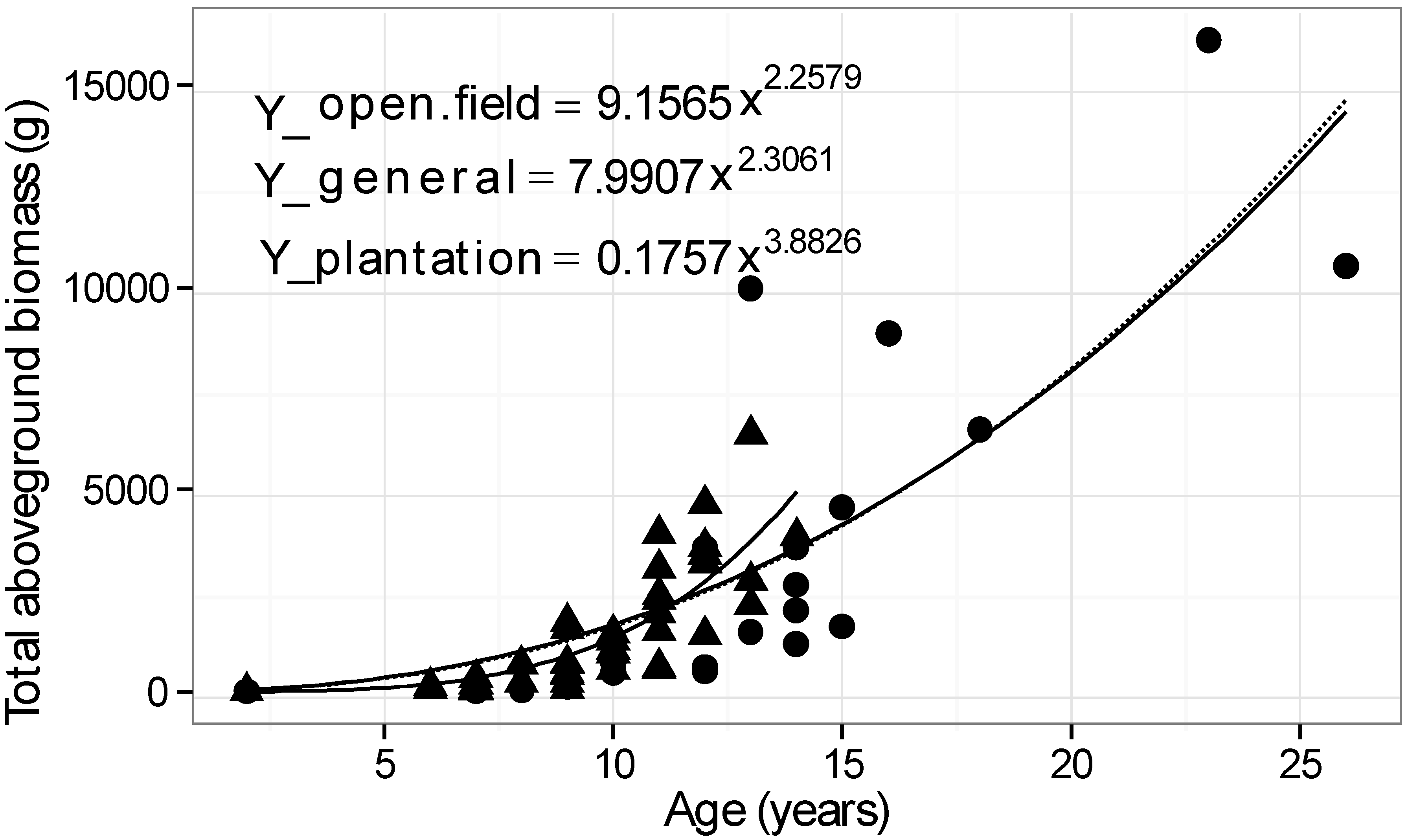
3. Results and Discussion
| Environment | Buckthorns harvested | Age range (years) | Basal diameter range (mm) | Height range (cm) | Model x = basal diam. (mm) Y = dry biomass (g) | R2 |
|---|---|---|---|---|---|---|
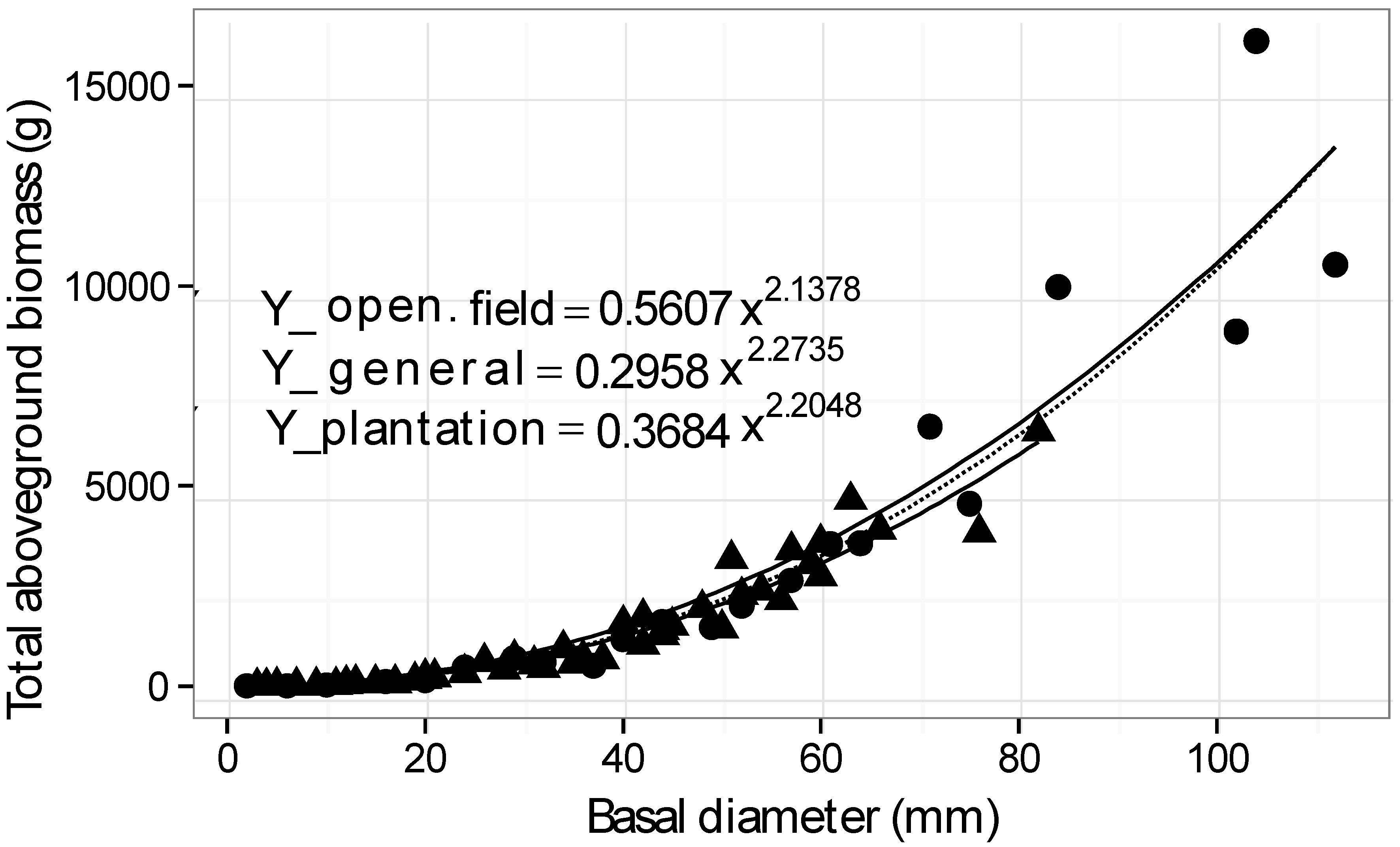
| Plantation | 44 | 2–14 | 2–82 | 54–620 | Y = 0.3684 x2.2048 | 0.93 |
| Open field | 22 | 2–26 | 2–112 | 53–695 | Y = 0.5607 x2.1378 | 0.88 |
| General | 66 | 2–26 | 2–112 | 53–695 | Y = 0.2958 x2.2735 | 0.90 |
| Response (Y) | Predictor (x) | ||
|---|---|---|---|
| Age (years) | Basal diameter (mm) | Height (cm) | |
| Aboveground biomass (g) | 0.5025 | 0.1244 | 0.0149 * |
| Height (cm) | 0.0100 * | 0.0149 * | |
| Basal diameter (mm) | 0.0199 * | ||



4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Catling, P.M.; Porebski, Z.S. The history of invasion and current status of glossy buckthorn, Rhamnus frangula, in Southern Ontario. Can. Field-Nat. 1994, 108, 305–310. [Google Scholar]
- Burnham, K.M.; Lee, T.D. Canopy gaps facilitate establishment, growth, and reproduction of invasive Frangula alnus in a Tsuga canadensis dominated forest. Biol. Invasions 2009, 12, 1509–1520. [Google Scholar] [CrossRef]
- Lee, T.D.; Thompson, J.H. Effects of logging history on invasion of eastern white pine forests by exotic glossy buckthorn (Frangula alnus P. Mill.). For. Ecol. Manag. 2012, 265, 201–210. [Google Scholar] [CrossRef]
- Converse, C.K. Element Stewardship Abstract for Rhamnus cathartica and Rhamnus Frangula (syn. Frangula alnus); The Nature Conservancy: Arlington, VA, USA, 1984. [Google Scholar]
- Frappier, B.; Eckert, R.T.; Lee, T.D. Potential impacts of the invasive exotic shrub Rhamnus frangula L. (Glossy Buckthorn) on forests of Southern New Hampshire. Northeast. Nat. 2003, 10, 277–296. [Google Scholar] [CrossRef]
- Nagel, L.M.; Corace, R.G., III; Storer, A.J. An experimental approach to testing the efficacy of management treatments for glossy buckthorn at Seney National Wildlife Refuge, Upper Michigan. Ecol. Restor. 2008, 26, 136–142. [Google Scholar] [CrossRef]
- Sanford, N.L.; Harrington, R.A.; Fownes, J.H. Survival and growth of native and alien woody seedlings in open and understory environments. For. Ecol. Manag. 2003, 183, 377–385. [Google Scholar] [CrossRef]
- Godwin, H. Studies in the Ecology of Wicken Fen: III . The establishment and development of fen scrub (carr). J. Ecol. 1936, 24, 82–116. [Google Scholar] [CrossRef]
- Godwin, H. Frangula alnus Miller. J. Ecol. 1943, 31, 77–92. [Google Scholar] [CrossRef]
- Mills, J.E.; Meyer, G.A.; Reinartz, J.A.; Young, E.B. An exotic invasive shrub has greater recruitment than native shrub species within a large undisturbed wetland. Plant Ecol. 2012, 213, 1425–1436. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Botany 2011, 98, 539–548. [Google Scholar] [CrossRef]
- Lines, E.R.; Zavala, M.A.; Purves, D.W.; Coomes, D.A. Predictable changes in aboveground allometry of trees along gradients of temperature, aridity and competition. Global Ecol. Biogeogr. 2012, 21, 1017–1028. [Google Scholar] [CrossRef]
- Gosselin, J. Guide de Reconnaissance des Types Écologiques-Région Écologique 2c-Coteaux de l’Estrie; Ministère des Ressources naturelles et de la Faune, Forêt Québec, Direction des inventaires forestiers, Division de la classification écologique et productivité des stations: Québec, QC, Canada, 2007; p. 186. [Google Scholar]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Yield in 8 year-old hybrid poplar plantations on abandoned farmland along climatic and soil fertility gradients. For. Ecol. Manag. 2012, 267, 228–239. [Google Scholar] [CrossRef]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Biomass and volume yield in mature hybrid poplar plantations on temperate abandoned farmland. Forests 2014, 5, 3107–3130. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA ), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Institute of Ecosystem Studies: Millbrook, NY, USA, 1999; p. 36. [Google Scholar]
- Charles-Dominique, T.; Edelin, C.; Brisson, J.; Bouchard, A. Architectural strategies of Rhamnus cathartica (Rhamnaceae) in relation to canopy openness. Botany 2012, 90, 976–989. [Google Scholar] [CrossRef]
- Heuret, P.; Barthélémy, D.; Nicolini, E.; Atger, C. Analyse des composantes de la croissance en hauteur et de la formation du tronc chez le chêne sessile, Quercus petraea (Matt.) Liebl. (Fagaceae) en sylviculture dynamique. Can. J. Botany 2000, 78, 361–373. [Google Scholar]
- Boothroyd-Roberts, K.; Gagnon, D.; Truax, B. Can hybrid poplar plantations accelerate the restoration of forest understory attributes on abandoned fields? For. Ecol. Manag. 2013, 287, 77–89. [Google Scholar] [CrossRef]
- Luken, J.O.; Goessling, N. Seedling distribution and potential persistence of the exotic shrub Lonicera maackii in fragmented forests. Am. Midl. Nat. 1995, 133, 124–130. [Google Scholar] [CrossRef]
- Malicky, H.; Sobhian, R.; Zwolfer, H. Investigations on the possibilities of a biological control of Rhamnus cathartica L. in Canada: Host Ranges, Feeding Sites, and Phenology of Insects Associated with European Rhamnaceae. J. Appl. Entomol. 1970, 65, 77–97. [Google Scholar]
- Harrington, R.A.; Brown, B.J.; Reich, P.B. Ecophysiology of exotic and native shrubs in Southern Wisconsin and phenology to seasonal patterns of carbon gain. Oecologia 1989, 80, 356–367. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamelin, C.; Gagnon, D.; Truax, B. Aboveground Biomass of Glossy Buckthorn is Similar in Open and Understory Environments but Architectural Strategy Differs. Forests 2015, 6, 1083-1093. https://doi.org/10.3390/f6041083
Hamelin C, Gagnon D, Truax B. Aboveground Biomass of Glossy Buckthorn is Similar in Open and Understory Environments but Architectural Strategy Differs. Forests. 2015; 6(4):1083-1093. https://doi.org/10.3390/f6041083
Chicago/Turabian StyleHamelin, Caroline, Daniel Gagnon, and Benoit Truax. 2015. "Aboveground Biomass of Glossy Buckthorn is Similar in Open and Understory Environments but Architectural Strategy Differs" Forests 6, no. 4: 1083-1093. https://doi.org/10.3390/f6041083





