Protective Effect of Nitric Oxide (NO) against Oxidative Damage in Larix gmelinii Seedlings under Ultraviolet-B Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Experimental Design
2.2. Methods
2.2.1. Measurement of Gas Exchange Parameters and Pigments
2.2.2. Antioxidant System
2.2.3. Flavonoids
2.2.4. Hydrogen Peroxide Content
2.2.5. Peroxidation of Leaf Membrane Lipid
2.2.6. Statistical Analyses
3. Results
3.1. Effect of SNP on Biological Characteristics
3.2. Adjustment of NO Levels in Leaves
3.3. Photosynthetic Characteristics
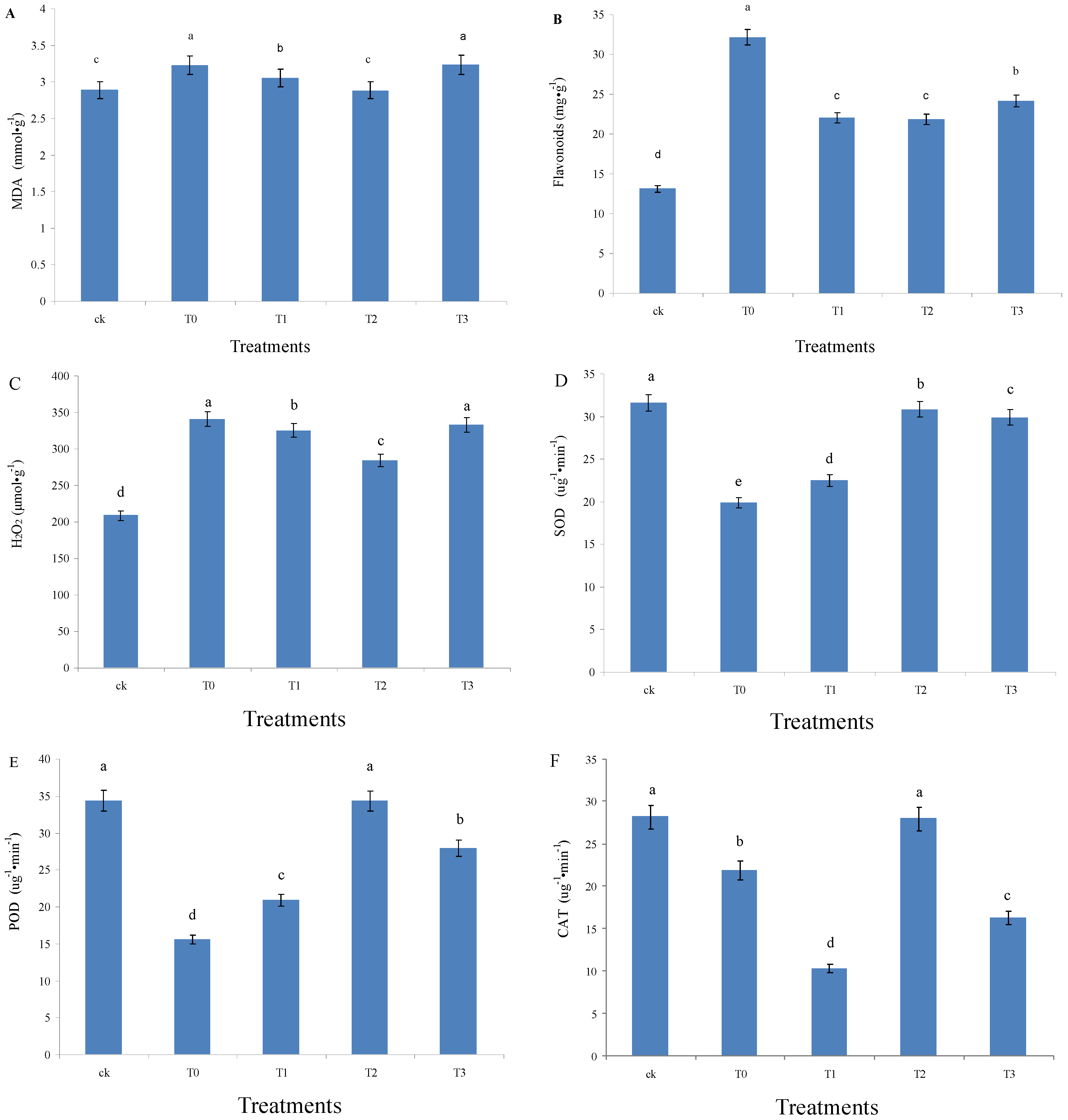
3.4. Peroxidation of the Leaf Membrane Lipid
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lv, Z.; Zhang, X.; Liu, L.; Guo, Y.; Fan, Y.; Yang, X.; Li, Y.; Zhang, W. Comparing intraspecific responses of 12 winter wheat cultivars to different doses of ultraviolet-B radiation. J. Photochem. Photobiol. B. 2013, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peykarestan, B.; Seify, M.; Fadaei, M.S.; Hatim, M. UV irradiation effects on seed germination and growth, protein content, peroxidase and protease activity in Portulaca grandiflora and Portulaca oleracea. World Appl. Sci. J. 2012, 17, 802–808. [Google Scholar]
- El Morchid, E.M.; Torres Londoño, P.; Papagiannopoulos, M.; Gobbo-Neto, L.; Müller, C. Variation in flavonoid pattern in leaves and flowers of Primula veris of different origin and impact of UV-B. Biochem. Syst. Ecol. 2014, 53, 81–88. [Google Scholar] [CrossRef]
- Aiamla-Or, S.; Shigyo, M.; Ito, S.; Yamauchi, N. Involvement of chloroplast peroxidase on chlorophyll degradation in postharvest broccoli florets and its control by UV-B treatment. Food Chem. 2014, 165, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.M.; Al Watban, A.A.; Al-Fughom, A.T. Effect of ultraviolet radiation on chlorophyll, carotenoid, protein and proline contents of some annual desert plants. Saudi J. Biol. Sci. 2011, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Han, R. He-Ne laser treatment improves the photosynthetic efficiency of wheat exposed to enhanced UV-B radiation. Laser Phys. 2014, 24, 1–7. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Björn, L.O.; Li, S.S. UV-B-induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips. Planta 2011, 233, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Lario, L.D.; Ramirez-Parra, E.; Gutierrez, C.; Spampinato, C.P.; Casati, P. ANTI-SILENCING FUNCTION1 proteins are involved in ultraviolet-induced DNA damage repair and are cell cycle regulated by E2F transcription factors in Arabidopsis. Plant Physiol. 2013, 162, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, R.; Rodriguez, R.E.; Debernardi, J.M.; Palatnik, J.F.; Casati, P. Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 2013, 25, 3570–3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaelewyn, L.; Prinsen, E.; van der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Radic. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, R.J.; Pan, Y.Z.; Ma, M.; Pan, J.; Zhao, Y.; Cheng, Q.; Wu, M.; Wang, M.; Zhang, L. Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicol. Environ. Saf. 2015, 119, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Singh, S.; Dubey, P.; Singh, A.; Singh, A.K. Nitric oxide mediated amelioration of arsenic toxicity which alters the alternative oxidase (Aox1) gene expression in Hordeum vulgare L. Ecotoxicol. Environ. Saf. 2015, 120, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.P.; Wu, X.L.; Zhong, Y. Exogenously applied nitric oxide enhances the drought tolerance in hulless barley. Plant Product. Sci. 2015, 18, 52–56. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, L.; Fu, G.F.; Yang, Y.; Zhu, C.; Tao, L. Drought-induced proline accumulation is uninvolves with increased nitric oxide, which alleviates drought stress by decreasing transpiration in rice. J. Plant Res. 2012, 125, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.J.; Zhou, Y.; Liu, M.J. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protolasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.; Cassia, R.; Bruzzone, S.; Zocchi, E.; Lamattina, L. ABA says NO to UV-B: A universal response? Trends Plant Sci. 2012, 17, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Krasylenko, Y.A.; Yemets, A.I.; Sheremet, Y.A.; Blume, Y.B. Nitric oxide as a critical factor for perception of UV-B irradiation by microtubules in Arabidopsis. Physiol. Plant 2012, 145, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.; Lamattina, L.; Jenkins, G.I.; Cassia, R.O. Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol. 2014, 164, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, E.W.; Kim, M.S. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentration of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Rem. Sen. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Ros-Barceló, A.; Gómez-Ros, L.V.; Ferrer, M.A.; Hernández, J.A. The apoplastic antioxidant enzymatic system in the wood-forming tissues of trees. Trees 2006, 20, 145–156. [Google Scholar] [CrossRef]
- Lizana, X.C.; Hess, S.; Calderini, D.F. Crop pH enology modifies wheat responses to increased UV-B radiation. Agric. For. Meteorol. 2009, 149, 1964–1974. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [PubMed]
- Pedroso, M.C.; Magalhaes, J.R.; Durzan, D.J. A nitric oxide burst precedes apoptosis in angiosperm and gymnosperm and foliar tissues. J. Exp. Bot. 2000, 51, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Vanacker, H.; Gomez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Review. Plant Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, X.F.; Yang, F.J.; Sun, J.Z.; Wei, M.; Shi, Q.H.; Wang, X.H. Effects of exogenous NO on the growth and antioxidant enzyme activities of cucumber seedlings under NO3− stress. Chin. J. Appl. Ecol. 2009, 20, 3009–3014. [Google Scholar]
- Belignim, V.; Lamattina, L. Is nitric oxide toxic or protective? Trends Plant Sci. 1999, 4, 299–300. [Google Scholar] [CrossRef]
- Giba, Z.; Grubisic, D.; Todorovic, S.; Sajc, L.; Stojakovic, D.; Konjevic, R. Effect of nitric oxide-releasing compounds on phytochrome-controlled germination of empress tree seeds. Plant Growth Res. 1998, 26, 175–181. [Google Scholar] [CrossRef]
- Liu, K.L.; Ling, T.F.; Liu, Z.B.; Hua, R.; Sun, Y.; Shen, W. Effects of soaking seeds in exogenous nitric oxide donor on growth of rice seedlings under salt stress. Plant Physiol. J. 2004, 40, 419–422. [Google Scholar]
- Correia, C.M.; Pereira, J.M.M.; Coutinho, J.F.; Björn, L.O.; Torres-Pereira, J.M.G. Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: A Mediterranean field study. Eur. J. Agron. 2005, 22, 337–347. [Google Scholar] [CrossRef]
- Zhang, L.G.; Zhou, S.; Xuan, Y.; Sun, M.; Zhao, L.Q. Protective effect of nitric oxide against oxidative damage in Arabidopsis leaves under ultraviolet-B irradiation. J. Plant Biol. 2009, 52, 135–140. [Google Scholar] [CrossRef]
- Qu, Y.; Feng, H.Y.; Wang, Y.B.; Zhang, M.X. Nitric oxide functions as a signal in ultraviolet-B induced inhibition of pea stems elongation. Plant Sci. 2006, 170, 994–1000. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, J.F.; Jin, H.H.; Sun, L.N.; Xu, M.J. Ultraviolet-B induced flavonoid accumulation in Betula pendula leaves is dependent upon nitrate reductase-mediated nitric oxide signaling. Tree Physiol. 2011, 31, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002, 53, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cantrel, C.; Vazquez, T.; Puyaubert, J.; Rezé, N.; Lesch, M.; Kaiser, W.M.; Dutilleul, C.; Guillas, I.; Zachowski, A.; Baudouin, E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011, 189, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.M.; Teramura, A.H. The role of flavonol glycoside and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993, 103, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Zheng, M.; Bian, X.; Liu, M.; Sun, Y.; Jiang, J.; Wang, F.; Li, S.; Cui, Y.; et al. Comparative Analysis of Growth and Photosynthetic Characteristics of (Populus simonii × P. nigra) × (P. nigra × P. simonii) Hybrid Clones of Different Ploidides. PLoS ONE 2015, 10, e0119259. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, 515 acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- García-Mata, C.; Lamattina, L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ulm, R.; Jenkins, G.I. Q & A: How do plants sense and respond to UV-B radiation? BMC Biol. 2015, 13, 45. [Google Scholar] [PubMed]
- Christie, J.M.; Arvai, A.S.; Baxter, K.J.; Heilmann, M.; Pratt, A.J.; O’Hara, A.; Kelly, S.M.; Hothorn, M.; Smith, B.O.; Hitomi, K.; et al. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 2012, 335, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Doupis, G.; Chartzoulakis, K.; Patakas, A. Differences in antioxidant mechanisms in grapevines subjected to drought and enhanced UV-B radiation. Emir J. Food Agric. 2012, 24, 607–613. [Google Scholar] [CrossRef]
- Zu, Y.G.; Pang, H.H.; Yu, J.H.; Li, D.W.; Wei, X.X.; Gao, Y.X.; Tong, L. Responses in the morphology, physiology and biochemistry of Taxuschinensis var. mairei grown under supplementary UV-B radiation. J. Photochem. Photobiol. B 2010, 98, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Ranjan, S.; Deeba, F.; Pandey, A.K.; Singh, R.; Shirke, P.A.; Pathre, U.V. Desiccation-induced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. J. Plant. Physiol. 2010, 167, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
| Treatments | Height (cm) | Base Diameter (cm) | Root Shoot Ratio | Relative Water Content (%) |
|---|---|---|---|---|
| CK | 74.2 ± 1.825 a | 0.759 ± 0.025 a | 0.49 ± 0.017 a | 68.72 ± 1.656 a |
| T0 | 71.9 ± 0.891 c | 0.751 ± 0.014 b | 0.40 ± 0.011 d | 58.48 ± 1.824 c |
| T1 | 72.1 ± 1.182 c | 0.744 ± 0.015 c | 0.47 ± 0.006 b | 63.27 ± 2.031 b |
| T2 | 73.6 ± 2.049 b | 0.757 ± 0.016 a | 0.43 ± 0.007 c | 62.58 ± 1.583 b |
| T3 | 70.3 ± 2.801 d | 0.740 ± 0.007 d | 0.41 ± 0.018 c | 56.91 ± 1.354 d |
| Treatments | NO (ng·g−1 FW) | NOS (μmol·g−1) | NR (μg·g−1·h−1) |
|---|---|---|---|
| CK | 24.310 c | 0.436 d | 25.556 c |
| T0 | 28.056 a | 0.614 a | 22.401 d |
| T1 | 26.413 b | 0.530 b | 31.032 a |
| T2 | 25.810 b | 0.5410 b | 28.902 b |
| T3 | 27.444 a | 0.504 c | 27.444 b |
| Treatments | CHl (a + b) (mg·g−1) | Car (mg·g−1) | Chlorophyll a/b | Car/CHl (a + b) |
|---|---|---|---|---|
| CK | 1.933 ± 0.069 a | 0.230 ± 0.008 a | 2.707 ± 0.087 d | 0.119 b |
| T0 | 1.366 ± 0.032 e | 0.171 ± 0.005 d | 2.791 ± 0.067 c | 0.125 a |
| T1 | 1.643 ± 0.059 c | 0.172 ± 0.006 d | 2.885 ± 0.102 b | 0.105 d |
| T2 | 1.762 ± 0.057 b | 0.195 ± 0.004 b | 2.808 ± 0.091 c | 0.111 c |
| T3 | 1.498 ± 0.047 d | 0.178 ± 0.003 c | 3.089 ± 0.039 a | 0.119 b |
| Treatments | Amax (µmol·m−2·s−1) | Tr (µmol·m−2·s−1) | Ci (µmol·mol−1) | AQY | Ic (µmol·m−2·s−1) | Im (µmol·m−2·s−1) |
|---|---|---|---|---|---|---|
| CK | 6.012 ± 0.180 a | 1.533 ± 0.046 a | 300.919 ± 15.046 d | 0.032 ± 0.0009 a | 42.951 ± 2.148 a | 1261.737 ± 63.087 a |
| T0 | 4.824 ± 0.145 d | 1.112 ± 0.033 d | 290.161 ± 14.508 e | 0.021 ± 0.0006 d | 19.628 ± 0.981 e | 934.596 ± 46.730 d |
| T1 | 4.876 ± 0.146 c | 1.285 ± 0.039 c | 308.806 ± 15.440 b | 0.024 ± 0.0007 c | 31.643 ± 1.582 c | 1194.757 ± 59.738 b |
| T2 | 5.947 ± 0.178 b | 1.461 ± 0.044 b | 309.711 ± 15.486 a | 0.025 ± 0.0007 b | 30.424 ± 1.521 d | 1092.483 ± 54.624 c |
| T3 | 4.271 ± 0.128 e | 0.923 ± 0.028 e | 306.724 ± 15.336 c | 0.019 ± 0.0006 e | 36.554 ± 1.828 b | 868.484 ± 53.424 e |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Zhou, Z.; Sun, X.; Zhang, Z.; Meng, Q. Protective Effect of Nitric Oxide (NO) against Oxidative Damage in Larix gmelinii Seedlings under Ultraviolet-B Irradiation. Forests 2016, 7, 251. https://doi.org/10.3390/f7110251
Hu H, Zhou Z, Sun X, Zhang Z, Meng Q. Protective Effect of Nitric Oxide (NO) against Oxidative Damage in Larix gmelinii Seedlings under Ultraviolet-B Irradiation. Forests. 2016; 7(11):251. https://doi.org/10.3390/f7110251
Chicago/Turabian StyleHu, Haiqing, Zhenbao Zhou, Xiaoxin Sun, Zhonghua Zhang, and Qinghuan Meng. 2016. "Protective Effect of Nitric Oxide (NO) against Oxidative Damage in Larix gmelinii Seedlings under Ultraviolet-B Irradiation" Forests 7, no. 11: 251. https://doi.org/10.3390/f7110251





